- 1Department of Woman, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, Naples, Italy
1意大利那不勒斯坎帕尼亚大学 “Luigi Vanvitelli” 妇女、儿童和普通外科和专业外科 - 2Department of Translational Medical Sciences, Section of Pediatric, University of Naples Federico II, Naples, Italy
阿拉伯数字意大利那不勒斯费德里科二世大学儿科转化医学科学系 - 3Maternal and Child Department, IRCCS Foundation Policlinico “S. Matteo” di Pavia, Pavia, Italy
3IRCCS 基金会妇幼部 Policlinico “S. Matteo” di Pavia, 帕维亚, 意大利 - 4Department of Pediatrics, University of Perugia, Perugia, Italy
4佩鲁贾大学儿科系,意大利佩鲁贾 - 5Department of Pediatrics, University of L'Aquila, L'Aquila, Italy
5意大利拉奎拉大学儿科 - 6CEINGE Biotecnologie Avanzate S. C. A R. L., Naples, Italy
6CEINGE Biotecnologie Avanzate S. C. A R. L., 那不勒斯, 意大利 - 7Department of Human Sciences and Promotion of the Quality of Life, San Raffaele Open University, Rome, Italy
7意大利罗马圣拉斐尔开放大学人文科学与生活质量促进系
Background: Polyethylene glycol (PEG) is recommended as first-line treatment of pediatric functional constipation. However, the oral route of administration is often poorly feasible in children mostly due to poor palatability. Promelaxin microenemas exert a topical evacuative action and may offer a valuable option in pediatric FC.
背景:聚乙二醇 (PEG) 被推荐作为儿童功能性便秘的一线治疗。然而,口服给药途径在儿童中通常不太可行,主要是由于适口性差。普罗美拉辛微灌肠具有局部排便作用,可能在儿科 FC 中提供有价值的选择。
Aim: To assess whether Promelaxin microenemas would be non-inferior to PEG 4000 in young children with FC.
目的:评估 Promelaxin 微灌肠是否不劣于 PEG 4000 治疗 FC 幼儿。
Methods: This is a randomized, open-label, multi-centric, non-inferiority trial enrolling infants and young children aged 6–48 months, with FC according to Rome III criteria. After 1 week of run in, children were randomized to 2 weeks of Promelaxin or PEG, followed by a 6-week on-demand treatment period. Primary endpoint was response rate to randomized treatment, with “response” defined as at least 3 evacuations per week and an average increase of at least one evacuation per week as compared to baseline. Safety, stool consistency and the analysis of fecal microbiota were secondary endpoints.
方法:这是一项随机、开放标签、多中心、非劣效性试验,根据罗马 III 标准招募了 6-48 个月大的 FC 婴幼儿。磨合期 1 周后,儿童被随机分配到 2 周的普罗美拉辛或 PEG,然后是 6 周的按需治疗期。主要终点是对随机治疗的反应率,“反应”定义为每周至少 3 次排空,与基线相比,每周平均增加至少 1 次排空。安全性、粪便稠度和粪便微生物群分析是次要终点。
Results: Out of the 158 patients who entered the trial, 153 patients were treated (77 and 76, PEG and Promelaxin arm, respectively). In the primary analysis, the 95% confidence interval (CI) for the treatment's effect lay entirely above the non-inferiority margin in both Full Set (FAS) and Per Protocol (PP) analyses, providing evidence of the non-inferiority of Promelaxin vs. PEG 4000 [response rate difference: 16.5% (CI 1.55–31.49%) and 11.03% (CI −5.58 to 27.64%), FAS and PP analyses, respectively]. Mean compliance to the randomized treatment was >80% in both arms. Secondary endpoints did not significantly differ between the two arms, except for the average number of total days of on-demand treatment that was significantly lower in the Promelaxin arm [14.6 (12.7) vs. 9.8 (9.1), mean (SD); primary endpoint responders in PEG and Promelaxin arm, respectively; p = 0.027]. Microbiota evenness significantly increased in the PEG 4000 arm at V4 as compared to the Promelaxin arm (p < 0.05). In addition, at V5, patients treated with PEG showed a significantly decreased microbiota density as compared to patients treated with Promelaxin (p = 0.036).
结果:在进入试验的 158 名患者中,153 名患者接受了治疗 (分别为 77 名和 76 名,PEG 组和 Promelaxin 组)。在初步分析中,在全套 (FAS) 和按方案 (PP) 分析中,治疗效果的 95% 置信区间 (CI) 完全高于非劣效性边缘,提供了 Promelaxin 与 PEG 4000 的非劣效性的证据 [反应率差异:16.5% (CI 1.55-31.49%) 和 11.03% (CI -5.58 至 27.64%),FAS 和 PP 分析, 分别]。两组对随机治疗的平均依从性均为 >80%。两组之间的次要终点没有显著差异,除了按需治疗的平均总天数显著降低,普罗美拉辛组 [14.6 (12.7) vs. 9.8 (9.1),平均值 (SD);PEG 组和 Promelaxin 组的主要终点反应者分别为;p = 0.027]。与普罗美拉辛组相比,PEG 4000 组在 V4 时的微生物群均匀度显著增加 (p < 0.05)。此外,在 V5 时,与普罗美拉辛治疗的患者相比,PEG 治疗的患者表现出微生物群密度显著降低 (p = 0.036)。
Conclusions: Promelaxin microenemas are non-inferior to oral PEG in children with FC.
结论:普罗美拉辛微灌肠在 FC 患儿中不劣于口服 PEG。
Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT02751411.
临床试验注册号:www.ClinicalTrials.gov,标识符:NCT02751411。
Introduction 介绍
Functional constipation (FC), defined as the infrequent (<2/week) and painful passage of stools associated with stool retention, is a common problem in childhood (1, 2). A 2018 systematic review and meta-analysis reported the worldwide pooled prevalence of FC in children to be 9.5% (95% CI 7.5–12.1%) (2, 3). Overall, 1 in 10 children may suffer from FC. The transition to solid food, toilet training and school entry are usually precipitating events associated with the onset of FC (3, 4).
功能性便秘 (FC),定义为与大便潴留相关的不频繁 (<2/周) 和疼痛排便,是儿童期的常见问题 (1, 2)。2018 年的一项系统评价和荟萃分析报告称,全球儿童 FC 的总体患病率为 9.5% (95% CI 7.5-12.1%) (2, 3)。总体而言,每 10 名儿童中就有 1 名可能患有 FC。过渡到固体食物、如厕训练和入学通常是与 FC 发作相关的诱发事件 (3, 4)。
In infants and toddlers, FC usually appears to originate from an acquired behavior of stool withholding after experiencing painful defecation (4). This makes the rectal fecal mass difficult to eliminate, thus amplifying the persistence of constipation. In order to avoid long-term FC, a successful integrated treatment strategy should be implemented at an early stage, combining pharmacological and non-pharmacological approaches (4). In fact, it is recommended to integrate early pharmacological treatment with non-pharmacological treatment, i.e., behavioral, psychological, dietary interventions (2, 5, 6), to interrupt the loop leading to the persistence of FC, fecal impaction, psychological problems, and a significant burden on children and parents (7). Considering that the median age for the onset of this condition is around 2 years (8), acting before or around that age becomes crucial, in order to interrupt the vicious loop leading to persistent FC and to avoid its complications, such as fecal impaction.
在婴幼儿中,FC 通常似乎起源于排便疼痛后后憋大便的后天行为 (4)。这使得直肠粪便肿块难以消除,从而放大了便秘的持续性。为了避免长期 FC,应在早期实施成功的综合治疗策略,结合药物和非药物方法 (4)。事实上,建议将早期药物治疗与非药物治疗相结合,即行为、心理、饮食干预 (2, 5, 6),以中断导致 FC 持续存在的循环、粪便嵌塞、心理问题以及儿童和父母的重大负担 (7)。考虑到这种情况发作的中位年龄约为 2 岁 (8),因此在该年龄之前或前后采取行动变得至关重要,以中断导致持续性 FC 的恶性循环并避免其并发症,例如粪便嵌塞。
Oral polyethylene glycol (PEG) is currently recommended as the first-line treatment of pediatric FC by the major international Societies (9). However, adherence to PEG can be sub-optimal especially in infants, often due to poor palatability (10) as reported by parents. Consistently, adherence as low as 37% has been reported in children on long-term treatment with PEG for persistent FC (11). Therefore, other treatment options with an efficacy comparable to PEG could be relevant in optimizing the treatment of FC, especially in young children.
口服聚乙二醇 (PEG) 目前被主要国际学会推荐为儿科 FC 的一线治疗 (9)。然而,对 PEG 的依从性可能不是最佳的,尤其是在婴儿中,通常是由于父母报告的适口性差 (10)。据报道,长期接受 PEG 治疗持续性 FC 的儿童依从性低至 37% (11)。因此,其他疗效与 PEG 相当的治疗选择可能与优化 FC 的治疗相关,尤其是在幼儿中。
Enemas are used in pediatric patients, with volumes adapted to the function (local or systemic effect) and to the age of the child (7, 12). A randomized trial in children suffering from fecal impaction, a condition that, triggers or complicates FC, compared oral PEG vs. enemas (60 ml of dioctyl sulfo-succinate-sodium, once-daily for 6 days) in children aged 4–6 years. The trial showed that enemas were as effective as high-dose oral PEG (13).
灌肠剂用于儿科患者,其体积适应功能(局部或全身效应)和儿童的年龄 (7, 12)。一项针对患有粪便嵌塞(一种诱发 FC 或使 FC 复杂化的疾病)的儿童的随机试验,比较了 4-6 岁儿童的口服 PEG 与灌肠剂(60 毫升丁磺基琥珀酸二辛酯钠,每天一次,持续 6 天)。试验表明,灌肠剂与高剂量口服 PEG 一样有效 (13)。
Therefore, we tested whether also in the case of FC, an early and short-term treatment with microenemas (4 ml volume) might be as effective as oral PEG in infants and toddlers, and thereby possibly become a treatment option. In addition, since osmotic laxatives have been shown to be associated with changes in the gut microbiota in studies in humans as well as in animal models (14–18), we also tested whether microenemas, through their topical effect, may have a lesser effect on the gastrointestinal microbiota. Thus, we performed a randomized comparison between oral PEG and microenemas containing honeys and polysaccharides.
因此,我们测试了在 FC 的情况下,早期和短期使用微灌肠 (4 ml 体积) 治疗是否可能与婴儿和幼儿口服 PEG 一样有效,从而可能成为一种治疗选择。此外,由于在人类和动物模型中的研究显示渗透性泻药与肠道微生物群的变化有关 (14-18),我们还测试了微灌肠是否通过其局部作用对胃肠道微生物群的影响较小。因此,我们对口服 PEG 与含有蜂蜜和多糖的微灌肠进行了随机比较。
Materials and Methods 材料和方法
Study Design and Patients
研究设计和患者
This is a randomized, controlled, open label, multicenter, non-inferiority trial aimed at evaluating the efficacy and safety of Promelaxin microenemas (Melilax Pediatric, 4 ml/5 g, volume/weight, Aboca, Sansepolcro, Italy), vs. PEG 4000 (Paxabel 4 g, Ipsen Consumer Healthcare S.r.l., Milano, Italy) in the short-term treatment of FC. Promelaxin is a CE marked class IIb medical device made of 100% natural substances, which exerts a local evacuative action. Promelaxin microenemas are marketed in Europe. The study was carried out in four primary care hospitals in Italy between April 2016 and March 2020. The study protocol was approved by the local Ethical Committees of each participating center (coordinating center approval date: 09/09/2015, approval number 190/15) and was conducted in accordance with the Declaration of Helsinki. The written Informed Consent to participation in the study was obtained from the parents of all patients prior to their enrollment. The study was registered in Clinicaltrial.gov (NCT02751411) and EudraCT Database (2015-005111-32).
这是一项随机、对照、开放标签、多中心、非劣效性试验,旨在评估 Promelaxin 微灌肠(Melilax Pediatric,4 ml/5 g,体积/重量,Aboca,Sansepolcro,意大利)与 PEG 4000(Paxabel 4 g,Ipsen Consumer Healthcare S.r.l.,意大利米兰)在 FC 短期治疗中的疗效和安全性。Promelaxin 是一种带有 CE 标志的 IIb 类医疗器械,由 100% 天然物质制成,可发挥局部疏散作用。普罗美拉辛微灌肠药在欧洲销售。该研究于 2016 年 4 月至 2020 年 3 月期间在意大利的四家初级保健医院进行。研究方案由每个参与中心的当地伦理委员会批准(协调中心批准日期:2015 年 9 月 9 日,批准号 190/15),并根据赫尔辛基宣言进行。参与研究的书面知情同意书是在入组前从所有患者的父母那里获得的。该研究在 Clinicaltrial.gov (NCT02751411) 和 EudraCT 数据库 (2015-005111-32) 中注册。
Inclusion criteria were male and female children aged from 6 to 48 months with a diagnosis of FC according to the Rome III criteria (19). Exclusion criteria were: suspicion or diagnosis of organic diseases causing constipation such as inflammatory bowel disease, motility disorders, neurological disorders, inherited and metabolic disorders, surgical disorders, anal fissures and Hirschsprung's disease. Treatments with fecal softeners or prokinetics, probiotics, prebiotics, herbal dietary supplements or other herbal products and psychiatric drugs were all forbidden during the trial, starting from the run-in. The 7-day run-in phase was instrumental in ruling out the presence or development of fecal impaction prior to randomization. Parents were provided with dietary recommendations regarding fiber intake at enrollment, as well as recommendations on appropriate toilet training (9).
纳入标准是根据罗马 III 标准诊断为 FC 的 6 至 48 个月龄的男性和女性儿童 (19)。排除标准是:怀疑或诊断导致便秘的器质性疾病,例如炎症性肠病、动力障碍、神经系统疾病、遗传和代谢紊乱、外科疾病、肛裂和先天性巨结肠。从磨合期开始,试验期间禁止使用粪便软化剂或促动力剂、益生菌、益生元、草药膳食补充剂或其他草药产品和精神科药物进行治疗。7 天的磨合期有助于在随机分组之前排除粪便嵌塞的存在或发展。向家长提供有关入学时纤维摄入量的饮食建议,以及适当如厕训练的建议 (9)。
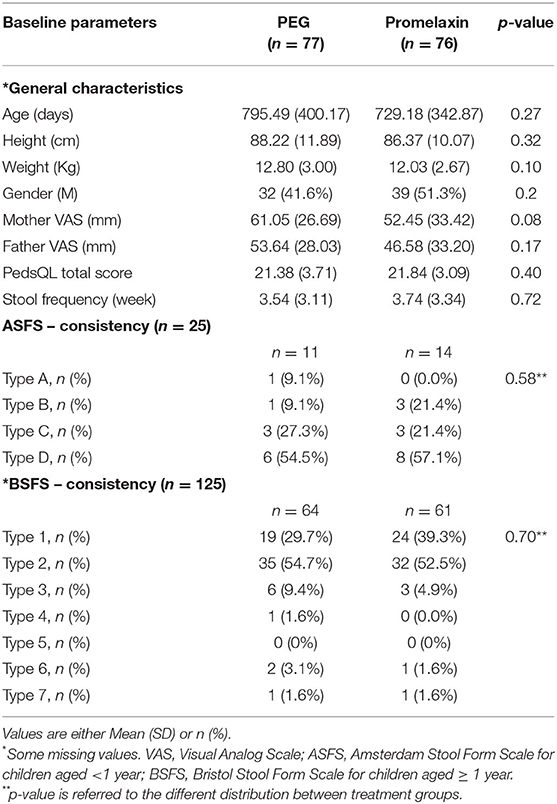
The study design is shown in Figure 1 and included: a 1-week run-in, a 2-week randomized treatment, followed by a 6-week observational period during which randomized treatment could be repeated on-demand, with 5 study visits in total. At visit 2 (V2), eligible patients were randomly assigned (1:1) to PEG or Promelaxin, according to a predefined block randomization list. Each center opened the randomization letters in sequential order to randomize patients. On demand treatment was defined as the need for PEG or Promelaxin (according to the randomized arm) after 48 h without a fecal evacuation. After V3, when primary endpoints were evaluated, two more visits were scheduled at day 21 (V4) and day 56 (V5 and end of study visit).
研究设计如图 1 所示,包括:1 周的磨合期,2 周的随机治疗,然后是 6 周的观察期,在此期间可以按需重复随机治疗,总共 5 次研究访问。在第 2 次就诊 (V2) 时,根据预定义的区组随机化列表,符合条件的患者被随机分配 (1:1) 至 PEG 或普罗美拉辛组。每个中心按顺序打开随机化字母以随机化患者。按需治疗定义为 48 小时后无粪便排空需要 PEG 或普罗美拉辛 (根据随机组)。V3 后,当评估主要终点时,计划在第 21 天 (V4) 和第 56 天 (V5 和研究访视结束) 再进行两次访视。
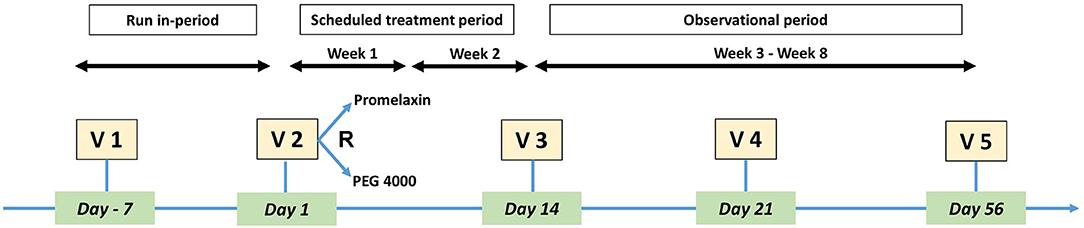
Figure 1. Study Design. The figure depicts the design of the study, including study Visits.
图 1.研究设计。该图描述了研究的设计,包括研究访问。
For each study period (run-in, treatment, on-demand period) a daily diary was supplied to parents to record the number of evacuations, the consistency score of stools, the administration frequency and dosage of the Investigational Product (IP), any associated gastrointestinal symptom (regurgitation, vomiting, flatulence, lack of appetite, diarrhea), any other symptom they deemed relevant, and any concomitant medication, meaning any medication other than the treatment in the study. Stool consistency was scored according to the Amsterdam Stool Form Scale (ASFS) (20) for children under 1 year of age and according to the Bristol Stool Form Scale (BSFS) (21) for children over 1 year of age.
对于每个研究期(磨合期、治疗期、按需期),向父母提供每日日记,以记录排便次数、粪便的一致性评分、研究产品 (IP) 的给药频率和剂量、任何相关的胃肠道症状(反流、呕吐、胀气、食欲不振、腹泻)、他们认为相关的任何其他症状,以及任何伴随药物, 是指研究中治疗以外的任何药物。1 岁以下儿童根据阿姆斯特丹大便形式量表 (ASFS) (20) 对粪便稠度进行评分,1 岁以上儿童根据布里斯托尔大便形式量表 (BSFS) (21) 进行评分。
A quality of life (QoL) questionnaire (extrapolated from PedsQL) (22) and parent QoL (Visual Analog Scale, VAS) were also filled in at study visits, to assess safety and treatment compliance and the most recent stool consistency score was registered by the Investigators.
在研究访问时还填写了生活质量 (QoL) 问卷(从 PedsQL 推断)(22)和父母 QoL(视觉模拟量表,VAS),以评估安全性和治疗依从性,研究人员记录了最新的粪便一致性评分。
The daily dosage of Promelaxin was 2.5 g (2 ml, half a microenema) for children 6–12 months old, 5 g (4 ml) for children aged 12–48 months; children 36–48 months were allowed a maximum of 10 g (8 ml). The daily dosage of PEG 4000 was decided by the Investigator based on the child's body weight and approved posology (i.e., 4 g/day for children 6–12 months old and 4–8 g/day for children 12–48 months old). During the 2-week randomized period (between V2 and V3), IPs were administered every day for the first week and every second day in week 2. During the on demand period (weeks 3–8), the same daily dosage was administered but on-demand. No other laxative/microenemas other than the IPs were allowed during the entire study.
普罗美拉辛的每日剂量为 6-12 个月大儿童 2.5 克(2 毫升,半个微肠),12-48 个月儿童 5 克(4 毫升);36-48 个月的儿童最多允许 10 克(8 毫升)。PEG 4000 的每日剂量由研究者根据儿童的体重和批准的剂量(即 4-6 个月大的儿童 12 克/天,4-8 个月大的儿童 12-48 克/天)决定。在 2 周的随机化期间 (V2 和 V3 之间),第一周每天给药,第 2 周每两天给药一次。在按需期间(第 3-8 周),每日剂量相同,但按需给药。在整个研究过程中,除 IP 外,不允许使用其他泻药/微灌肠剂。
Primary and Secondary Endpoints
主要和次要终点
The primary endpoint was stool frequency expressed as response rate assessed at V3, with “response” defined as 3 or more evacuations per week and an average increase of at least one evacuation per week as compared to the run in (baseline) in case of ≥3 evacuations at baseline. as reported on the patients' daily diary.
主要终点是大便频率,表示为 V3 时评估的反应率,“反应”定义为每周 3 次或更多次排空,与基线 ≥3 次排空相比,与磨合期(基线)相比,每周平均增加至少 1 次排空。正如患者每日日记中报告的那样。
Secondary endpoints included: response assessed as stool consistency, with responders defined as patients who experienced an increase, as compared to baseline, of one or more points on the ASFS or BSFS; “normalization” of bowel habits as defined by a composite response of stool frequency and consistency; days with gastrointestinal symptoms; intestinal microbiota profile; QoL scores, measured through a VAS scale ranging between 0 mm (Very good) and 100 mm (Very bad). A reduction from baseline of at least 1 point was considered as a QoL improvement.
次要终点包括:反应评估为粪便一致性,反应者定义为与基线相比,ASFS 或 BSFS 上一个或多个点增加的患者;排便习惯的“正常化”,由大便频率和稠度的综合反应定义;出现胃肠道症状的天数;肠道微生物群概况;QoL 分数,通过 0 毫米(非常好)和 100 毫米(非常差)之间的 VAS 量表测量。从基线减少至少 1 分被认为是 QoL 改善。
Microbiota Sub-study 微生物群子研究
Instructions for fecal sample collection were given to the parents at V1. Fecal samples were collected at V2, V4, and V5 and stored at −80°C until analyzed.
在 V1 向父母提供了粪便样本采集的说明。在 V2 、 V4 和 V5 收集粪便样本,并储存在 -80°C 直至分析。
Microbiota analysis was performed targeting the 16S rRNA gene variable region V4-V6 (23). DNA extraction, library preparation and sequencing were performed according to the protocol described by Nardelli et al. (24) 16S rRNA sequencing was performed using MiSeq Illumina sequencing (PE 2 × 300 cycles). The resulting 16S rRNA fastq files were analyzed using a standard procedure with Qiime 2.0 platform (25). After the overlapping and quality control of forward and reverse sequencing reads, the forward fastq files were used for further analysis (24). The feature table was constructed using the DADA2 algorithm integrated in Qiime 2.0 pipeline, keeping an average of ~59,000 reads per sample. The Pielou index was used to measure bacterial evenness, rarefacting the feature table at 24,000 reads per sample. Pielou's index measures diversity along with species richness. While species richness is the number of different species in a given area, evenness is the count of individuals of each species in an area. A calculated value of Pielou's index ranges from 0 (no evenness) to 1 (complete evenness). DNA concentration (ngDNA/100 mg fecal sample) was used to identify microbiota density (14).
针对 16S rRNA 基因可变区 V4-V6 进行微生物群分析 (23)。根据 Nardelli 等人描述的方案进行 DNA 提取、文库制备和测序。(24) 使用 MiSeq Illumina 测序 (PE 2 × 300 个循环) 进行 16S rRNA 测序。使用 Qiime 2.0 平台的标准程序分析得到的 16S rRNA fastq 文件 (25)。在对正向和反向测序读数进行重叠和质量控制后,使用正向 fastq 文件进行进一步分析 (24)。特征表是使用 Qiime 2.0 管道中集成的 DADA2 算法构建的,每个样本平均保持 ~59,000 个读数。Pielou 指数用于测量细菌均匀度,特征表很少见,每个样本 24,000 个读数。Pielou 指数衡量多样性和物种丰富度。物种丰富度是给定区域中不同物种的数量,而均匀度是一个区域中每个物种的个体计数。Pielou 指数的计算值范围从 0(无均匀度)到 1(完全均匀度)。DNA 浓度(ngDNA/100 mg 粪便样本)用于鉴定微生物群密度 (14)。
Sample Size Calculation 样本量计算
The sample size was calculated assuming a non-inferiority margin of 15% for the difference of the response rate for stool frequency between the two treatments assessed at V3. A response rate to PEG 4000 up to 93% was anticipated based on the available literature. Considering a drop-out rate of 25%, 80 patients per arm would achieve 80% power to detect non-inferiority with a one side significance level (alpha) of 0.025.
样本量的计算假设在 V3 评估的两种治疗之间大便频率反应率差异的非劣效性边际为 15%。根据现有文献,预计对 PEG 4000 的反应率高达 93%。考虑到 25% 的退出率,每组 80 名患者将达到 80% 的检测非劣效性功效,单侧显着性水平 (alpha) 为 0.025。
Statistical Analysis 统计分析
The following analyses were considered: Full Analysis Set (FAS) which considered all randomized subjects who received at least one dose of the IP; per protocol (PP) analysis that considered all subjects in the FAS population with no major protocol deviation until V3 with an evaluable primary endpoint. Microbiota analysis included patients in the FAS population with available fecal samples. The safety analysis included all randomized subjects who received at least one dose of the IP.
考虑了以下分析: 完整分析集 (FAS),考虑了所有接受至少一剂 IP 的随机受试者;根据方案 (PP) 分析,考虑了 FAS 人群中的所有受试者,在 V3 之前没有重大方案偏差,主要终点可评估。微生物群分析包括具有可用粪便样本的 FAS 人群患者。安全性分析包括所有接受至少一剂 IP 的随机受试者。
In the FAS analysis, patients with no data for primary endpoint assessment (i.e., baseline data missing, patient diary not returned) were considered non-responders. For the primary endpoint assessment, missing patient diary data were imputed using the worst-case approach. Comparison between arms was performed using T-test for continuous variables. Frequencies of Responders/Improved patients were compared using the Chi-square test. The difference was considered significant when p < 0.05, unless otherwise indicated. Differences in “counts” (e.g., no. of days of treatment use) were compared by using Poisson regression, adjusting for clinical site as a possible confounding factor. Kruskal-Wallis and T-Test were used to identify differences between arms of microbiota evenness and microbiota density, respectively.
在 FAS 分析中,没有主要终点评估数据 (即基线数据缺失、患者日记未返回) 的患者被视为无反应者。对于主要终点评估,使用最坏情况方法估算缺失的患者日记数据。使用连续变量的 T 检验进行组间比较。使用卡方检验比较反应者/改善患者的频率。除非另有说明,否则当 p < 0.05 时,差异被认为是显着的。通过使用泊松回归比较“计数”(例如,治疗使用天数)的差异,调整临床地点作为可能的混杂因素。Kruskal-Wallis 和 T 检验分别用于确定微生物群均匀度和微生物群密度的组间差异。
The compliance to the IP was assessed using the information recorded in the patient's diary and was calculated as the ratio between treatment administered vs. planned. Compliance <70% was considered as a major deviation.
使用患者日记中记录的信息评估对 IP 的依从性,并计算为已实施的治疗与计划的治疗之间的比率。依从性 <70% 被认为是主要偏差。
Descriptive statistics were used for demographics and baseline measurements.
描述性统计用于人口统计学和基线测量。
Results 结果
One hundred and sixty-one patients were screened, and 158 patients were randomized. The consort diagram is shown in Figure 2. Patient's baseline demographics and clinical characteristics are summarized in Table 1, and no statistically significant differences were observed between the two treatment arms. Nearly 90% of the patients were studied at V3.
筛选了 161 例患者,随机分配了 158 例患者。组合图如图 2 所示。患者的基线人口统计学和临床特征总结于表 1 中,两个治疗组之间未观察到统计学显着差异。近 90% 的患者在 V3 上进行了研究。
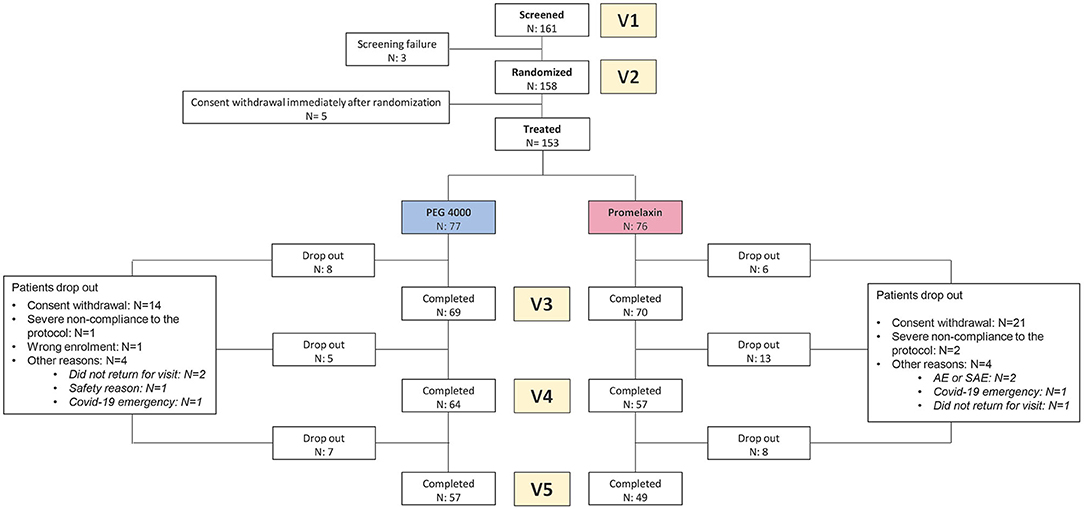
Figure 2. CONSORT diagram. The figure shows the Consort Diagram of the randomized trial.
图 2.CONSORT 图。该图显示了随机试验的 Consort Diagram。
During the 2-week randomized phase, compliance was 84.32 ± 29.10% for PEG and 85.07 ± 25.23% for Promelaxin (mean ± SD, p = 0.87). As shown in Figure 3, the 95% CIs for the treatment effect lay entirely above the non-inferiority margin in both FAS and PP analyses, providing evidence of the significant non-inferiority of Promelaxin vs. PEG 4000 [response rate difference: 16.5% (CI 1.55–31.49%) and 11.03% (CI −5.58 to 27.64%), FAS and PP populations, respectively]. In addition, in the FAS, the proportion of responders was significantly higher in the Promelaxin arm as compared to PEG (72.4 vs. 55.8%, respectively; p = 0.033).
Figure 3. Primary endpoint. The differences in response rates between Promelaxin and PEG and relative 95% confidence interval (CI) are shown according to the full analysis set (FAS) and the PP analyses.
Figure 4 displays composite response for stool frequency and consistency and the average days of treatment use, during and at the end of the observational period. At V4, 52.8% patients responded to Promelaxin compared to 39.5% who responded to PEG (Figure 4A, p = 0.25). This difference increased at V5, in favor of Promelaxin, although with a non-significant trend (Figure 4C, p = 0.14). The composite response rate showed the same trend when restricting the analysis to the responders to the primary endpoint (Figure 4, right columns).
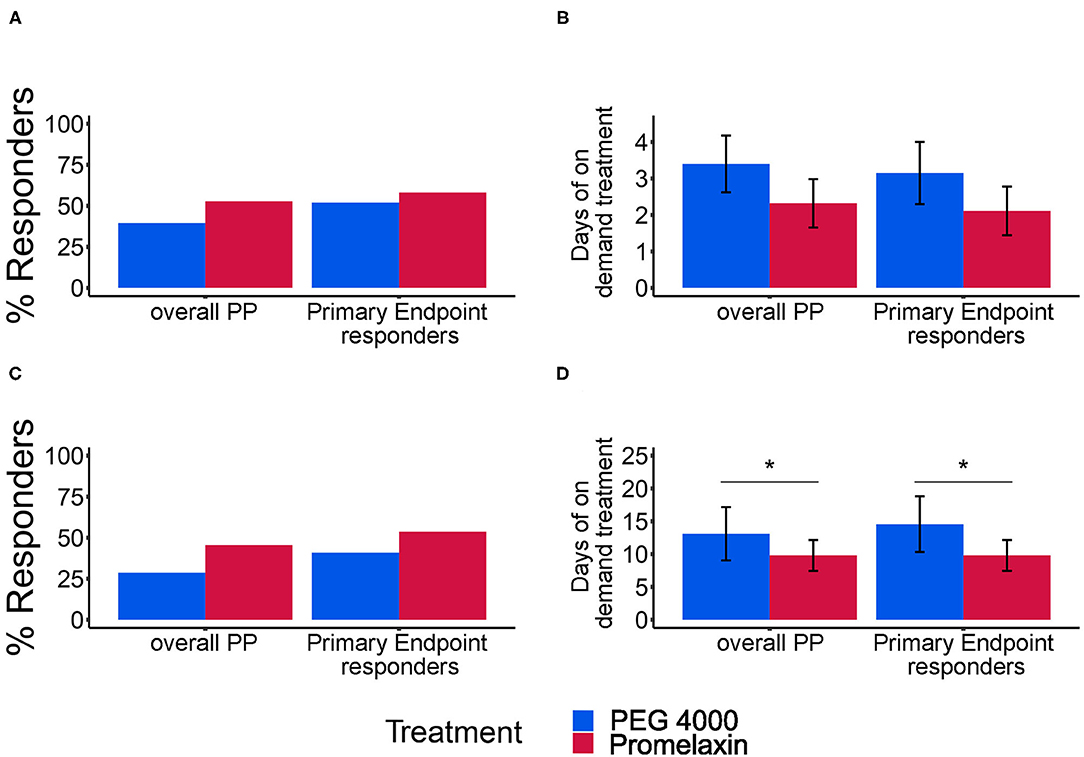
Figure 4. Response rate for combined stool frequency and consistency and average days of treatment. (A,C) show the response rate (%) for the combined stool frequency and consistency secondary endpoints at V4 and V5, respectively. (B,D) show the average days of treatment (mean ± SE) at V4 and V5, respectively. In each panel, results of separate analyses either according to the PP population or to the subgroup of PP patients evaluated as responders to the primary endpoint (Primary Endpoint Responders) are depicted. Error bars represent Standard Error. (A) Overall PP: PEG, n = 38; Promelaxin, n = 36; Primary Endpoint responders: PEG, n = 25; Promelaxin, n = 31. (B) Overall PP: PEG, n = 15; Promelaxin, n = 19; Primary Endpoint responders: PEG, n = 13; Promelaxin, n = 18. (C) Overall PP: PEG, n = 35; Promelaxin, n = 33; Primary Endpoint responders: PEG, n = 22; Promelaxin, n = 28. (D) Overall PP: PEG, n = 10; Promelaxin, n = 15; Primary Endpoint responders: PEG, n = 9; Promelaxin, n = 15. *p < 0.05.
At V4, the mean number of on-demand treatment days was lower in PP analysis of patients treated with Promelaxin (Figure 4B, p = 0.17). This difference became significant at V5 (Figure 4D; p = 0.02). Again, similar differences were observed when restricting the analysis to responders to the primary endpoint (right columns). In this latter subset, Promelaxin was used in 33% fewer days of the on-demand treatment period (p = 0.02, Figure 4). Stool frequency was also similar in the observation period, at V4 and V5 (p = 0.8 and p = 0.5).
There was no significant percentage difference in improved stool consistency (PEG 4000 vs. the Promelaxin group 71.1 vs. 62.5%, p = 0.36; 73.8 vs. 75.6%, p = 0.85; 67.6 vs. 78.9%, p = 0.26; V3, V4, and V5, respectively; Figure 5A).
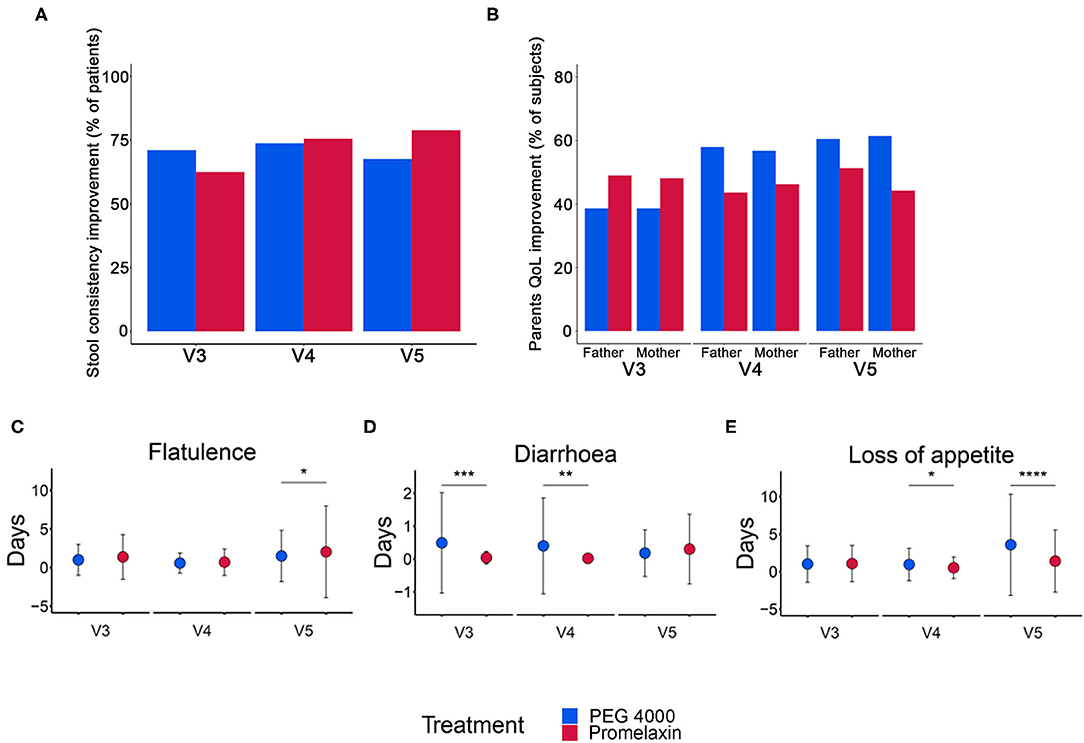
Figure 5. Stool consistency, QoL improvement and gastrointestinal symptoms in the randomized arms. (A) Percentage of patients with improved stool consistency at V3, V4, and V5. (B) Percentage of parents with improved QoL at V3, V4, and V5. (C–E) Number of days (mean ± SD) with gastrointestinal symptoms at V3, V4, and V5. Data are reported as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. (A) V3: PEG, n = 45; Promelaxin, n = 56; V4: PEG, n = 42; Promelaxin, n = 45; V5: PEG, n = 37; Promelaxin, n = 38; (B) V3 (Father): PEG, n = 44; Promelaxin, n = 49. V4 (Father): PEG, n = 38; Promelaxin, n = 39. V5 (Father). PEG, n = 38; Promelaxin, n = 39; (B) V3 (Mother): PEG, n = 44; Promelaxin, n = 52. V4 (Mother): PEG, n = 44; Promelaxin, n = 52. V5 (Mother): PEG, n = 44; Promelaxin, n = 52. (C–E) V3: PEG, n = 45; Promelaxin, n = 56. V4: PEG, n = 40; Promelaxin, n = 47. V5: PEG, n = 40; Promelaxin, n = 47.
QoL was similar between the treatment arms at all time points (Figure 5B). The total number of days with gastrointestinal symptoms was low in both treatment arms (Figures 5C–E). Patients receiving PEG 4000 experienced significantly more days with diarrhea at both V3 (p < 0.001) and V4 (p < 0.01), and more days with loss of appetite at V4 (p = 0.02) and V5 (p < 0.001). Patients on Promelaxin experienced more days with flatulence at V5 (p = 0.02).
Safety
There were 183 Treatment Emergent Adverse Events (TEAEs), defined as any event recorded by patients who took at least one IP dose (107 in the PEG and 76 in the Promelaxin arm). No TEAE was found to be causally related to the IP in each randomized arm.
Microbiota Results
At V2, microbiota evenness and density were similar between the two groups (Figures 6A,B). Microbiota evenness significantly increased in the PEG 4000 arm at V4 as compared to the Promelaxin arm (p < 0.05; Figure 6A). In addition, at V5, patients treated with PEG showed a significantly decreased microbiota density as compared to patients treated with Promelaxin (p = 0.036; Figure 6B). Taxonomy data showed no major differences among the two groups of treatment (data not shown and Supplementary Material). Detailed results on microbiota indicators are reported in the Supplementary Material.
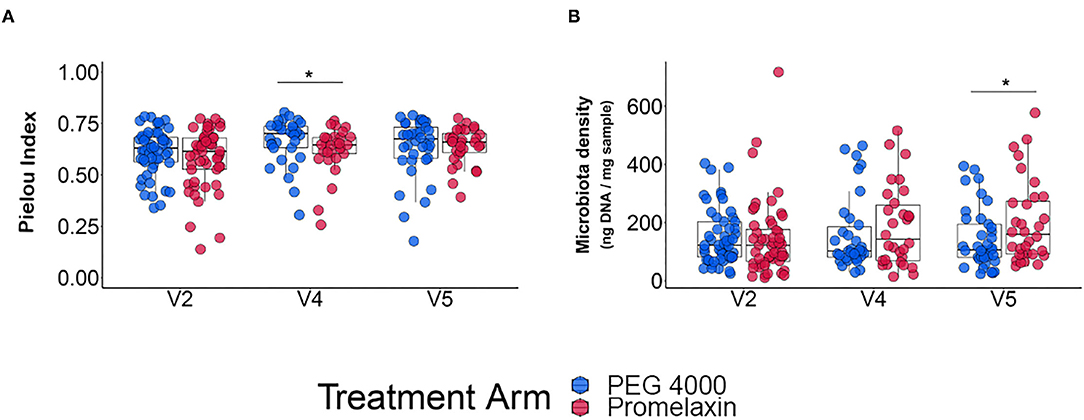
Figure 6. Microbiota analysis. The figure shows individual data and box-plots as median and IQR. (A) Microbiota evenness. (B) Microbiota density. Kruskal-Wallis-test and Student T-test were used for testing differences in microbiota evenness (Pielou's index) and microbiota density, respectively. *p < 0.05.
Discussion
To the best of our knowledge, this is the first randomized, controlled, non-inferiority trial comparing a micro-enema containing Promelaxin vs. an oral osmotic laxative, i.e., PEG 4000, in 158 young children (infant and toddlers) suffering from FC. A valuable aspect of this randomized controlled trial (RCT) is the age range of the recruited population (6–48 months), since it is known that RCTs in this age range are complex (26, 27). Thus there is a considerable unmet need for treatment and a poor quality of evidence in this age range (26, 27).
Based on a standard response criterion for treatment of FC, namely the number of evacuations per week, Promelaxin microenemas were non-inferior to PEG 4000, the current reference treatment for FC (2), over a 2-week exposure. Moreover, the results of the 6-week extension of this trial (the “on-demand” phase) as well as the secondary endpoints regarding stool characteristics, were all consistent with the main result of the trial.
Since PEG is already highly effective in FC, with a reported response rate between 77 and >90% (up to 97% response rate has been reported) (28) we have run a non-inferiority trial to provide evidence that Promelaxin microenemas can be at least similarly effective and safe compared to PEG 4000, thus becoming a suitable and feasible alternative to PEG 4000. The rationale stems from reports as well as common parental experience that the oral route of drug administration, especially in toddlers and infants, is hampered by palatability of whichever formulation, and, in case of liquid formulations, also by the volume to be administered (29). Taste-related problems between 50 and 60% have also been reported for lactulose and macrogol 3550 formulations (10). Moreover, a small fraction of subjects who are treated with oral osmotic laxatives experience gastrointestinal disturbances (abdominal pain, flatulence, vomiting) which limit their usage (4). The above characteristics generate an unmet therapeutic need in a significant fraction of young children with FC, which cannot be resolved by simply changing the type of oral laxative. Finally, polymers along the gastrointestinal tract may modify the microbiota, especially upon prolonged exposure (16). Therefore, under different circumstances, equally effective and safe alternatives to PEG are needed.
FC is a functional disorder with an early onset, reported to be on average around 24 months. Moreover, early and integrated (pharmacological and non-pharmacological) interventions are needed to promptly and effectively correct FC, avoiding its persistence, complications and thus the need for chronic treatment(s). These pivotal aspects are well-reflected in the design of the present study. Indeed, the study recruited 158 children aged up to 48 months, with a median age of 24 months, thus reflecting the average age for the onset of FC, when timely interventions are crucial in interrupting the vicious loop of fecal retention (2, 6, 9, 30). In children not affected by a long-lasting constipation, a condition which is mainly attributable to defecation disorder, the use of enemas is also expected to be more effective than oral fecal softeners by virtue of a direct action on the rectum: the results of the present study are consistent with this. Moreover, the short duration of this study also reflects the importance of intervening on the functional disturbance in the shortest possible time frame, in order to rapidly resolve the symptoms and avoid unpleasant repeated experiences for the child (and possibly for the parents as well), which are a major trigger for perpetuating FC. The choice of a relatively short time for the primary analysis was supported by the finding that over a 6-day treatment course, enemas and PEG were equally effective in treating rectal fecal impaction in children (13). Moreover, it is worth noting that the trial on fecal impaction used an enema's volume of 60 ml in children aged between 4 and 6 years. Our trial used microenemas, with a far lower volume, up to 8 ml in children aged 3–4 years. Therefore, microenemas in FC may increase the feasibility and acceptability of the treatment, minimizing the unpleasant experience of the use of enemas.
Another drawback of osmotic laxatives can be their effect on the microbiota, especially upon prolonged exposure. Microbiota is particularly important in growing children, since its perturbation has been reported to potentially affect metabolic disposition in growing bodies through adulthood (31). Moreover, the gut microbiota of children seems to be much less resilient than that of adults (32, 33). A reduced density of microbiota has been reported in mice exposed to PEG (14). We observed at V5 a reduced density of microbiota in PEG vs. Promelaxin, albeit the relative reduction was small (Figure 6), which may be consistent with experimental animal data. On the other hand, the Pielou's index was decreased at V4 in the Promelaxin group, indicating a reduction of evenness index of alpha diversity. Although these data may suggest a potential lower impact of microenemas on the microbiota as compared to PEG 4000, they remain hypothesis-generating and await further studies to understand their clinical relevance.
Thus, our study shows that microenemas with Promelaxin, a completely biodegradable substance system, being non-inferior to PEG 4000 in efficacy and with no major concerns on safety, are a valuable alternative to PEG, especially for those children for whom PEG administration can be problematic, i.e., in very young children with low compliance or low acceptance of an oral formulation due to poor palatability. An additional value of microenema treatment option is suggested by the present data comparing the on-demand use and the response rates throughout the observational period. The lower on-demand use of microenemas occurred in the context of comparable response for stool frequency and consistency, suggesting that Promelaxin may somehow help restore the physiological bowel function.
Despite the fact that non-pharmacological strategies are relevant for FC (2), head-to-head comparisons with the standard of care so far have been too limited (5) to provide sound evidence that can guide physicians and inform guidelines. A head-to-head comparison of enemas and PEG has only been performed with regard to fecal impaction (13), a disorder that is related, though different, to FC and that requires a fast intervention for a prompt resolution (4). Our randomized head-to-head comparison of a pharmacological vs. a non-pharmacological product (a medical device made of substances) in 158 young children can provide sound evidence-based, as well as useful, information to address an unmet therapeutic need. Thus, Promelaxin can offer a personalized treatment of FC in young children.
Our study does have some limitations. First, this is a relatively short-term study, thus we can only hypothesize that this treatment may be also useful in chronic FC when longer exposure is needed, even though the “on-demand” phase lasted for 6 weeks and the overall duration was 8 weeks. Moreover, the results of the “on demand” phase, where Promelaxin was used for 33% fewer days are also indicative of a possible positive effect in the long term. Importantly, it is key to quickly interrupt the vicious loop of FC in order to avoid prolonging the disorder and making it chronic. Another limitation is the age range of the patients (up to 48 months), which means that we can only infer similar results beyond this age range. However, it should be emphasized that the age range of this study closely reflects the age for the onset of FC. Moreover, in this age range oral drug administration can be more problematic as compared to older children, thus likely reflecting an unmet need in infants and toddlers.
In conclusions, Promelaxin microenemas have a similar efficacy, tolerability and safety compared to oral PEG, thus representing a valuable option for the treatment of FC in young children, and they may also help to individualize and optimize the treatment of FC in the early stages.
Data Availability Statement
The datasets presented in this article are not readily available because of the following reason related to the application of European data protection laws (specifically GDPR): meta-genomic data-files should be deposit in a public repository, whose country code Top-Level Domain is UK, that is abroad the EEA. Since the unique identifier of the interested person is included, these data do not qualify as anonymous data, but as (pseudonymized) personal data, and, for this reason, they are not transferable to the requested repository, that is outside the European Union, due to the limitations indicated in the text of the informed consent provided to enrolled subjects, which specifies that Personal data will not be transferred abroad. Requests to access the datasets should be directed to the Promoter of the study (who owns data property), at the following e-mail address: ClinicalTrials@aboca.it.
Ethics Statement
The study protocol was approved by the local ethical committees of each participating center (coordinating center approval date: 09/09/2015, approval number 190/15). The written informed consent to participation in the study was obtained from the parents of all patients prior to their enrollment.
Author Contributions
CS, VC, and MR: conception and design, acquisition, analysis and interpretation of data, drafting the article, and final approval of the version to be published. CT, GM, AV, SC, CC, VD'A, LS, and AS: conception and design, acquisition, drafting the article, and final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Funding
This study was sponsored by Aboca, the manufacturer of the medical device (i.e., the test product; brand name: Melilax Pediatric). The sponsor was not involved in the collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of Interest
AS is clinical investigator for Janssen Biologics B.V. and consultant for Angelini; she was clinical investigator for Aboca and PAREXEL International Srl; she was consultant for Aboca, for D.M.G. Italy and Nestlé, she was data safety monitoring board member for Sucampo AG and speaker for Aboca, Angelini, D.M.G. Italy and Valeas.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Dott. Kieran Tuohy (Fondazione Edmund Mach-Centro Ricerca ed Innovazione, S. Michele all'Adige, Trento. IT) and NBM (Natural Bio-Medicine Arezzo. IT) for the microbiota data analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.753938/full#supplementary-material
References
1. van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. (2006) 101:2401–9. doi: 10.1111/j.1572-0241.2006.00771.x
2. Vriesman MH, Koppen IJN, Camilleri M, Di Lorenzo C, Benninga MA. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol. (2020) 17:21–39. doi: 10.1038/s41575-019-0222-y
3. Koppen IJN, Vriesman MH, Saps M, Rajindrajith S, Shi X, van Etten-Jamaludin FS, et al. Prevalence of functional defecation disorders in children: a systematic review and meta-analysis. J Pediatr. (2018) 198:121–30.e6. doi: 10.1016/j.jpeds.2018.02.029
4. Leung AKC, Hon KL. Paediatrics: how to manage functional constipation. Drugs Context. (2021) 10:2020-11-2. doi: 10.7573/dic.2020-11-2
5. Tang J, Li H, Tang W. Efficacy of non-pharmacologic auxiliary treatments in improving defecation function in children with chronic idiopathic constipation: a systematic review and network meta-analysis. Front Pediatr. (2021) 9:667225. doi: 10.3389/fped.2021.667225
6. Koppen IJ, Lammers LA, Benninga MA, Tabbers MM. Management of functional constipation in children: therapy in practice. Paediatr Drugs. (2015) 17:349–60. doi: 10.1007/s40272-015-0142-4
7. Jannin V, Lemagnen G, Gueroult P, Larrouture D, Tuleu C. Rectal route in the 21st century to treat children. Adv Drug Deliv Rev. (2014) 73:34–49. doi: 10.1016/j.addr.2014.05.012
9. Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. (2014) 58:258–74. doi: 10.1097/MPG.0000000000000266
10. Venables R, Batchelor H, Hodson J, Stirling H, Marriott J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int J Pharm. (2015) 480:55–62. doi: 10.1016/j.ijpharm.2015.01.023
11. Koppen IJN, van Wassenaer EA, Barendsen RW, Brand PL, Benninga MA. Adherence to polyethylene glycol treatment in children with functional constipation is associated with parental illness perceptions, satisfaction with treatment, and perceived treatment convenience. J Pediatr. (2018) 199:132–9.e1. doi: 10.1016/j.jpeds.2018.03.066
12. Reflection Paper: Formulations of Choice for the Paediatric Population EMEA/CHMP/PEG/194810/2005.
13. Bekkali NL, van den Berg MM, Dijkgraaf MG, van Wijk MP, Bongers ME, Liem O, et al. Rectal fecal impaction treatment in childhood constipation: enemas versus high doses oral PEG. Pediatrics. (2009) 124:e1108–15. doi: 10.1542/peds.2009-0022
14. Contijoch EJ, Britton GJ, Yang C, Mogno I, Li Z, Ng R, et al. Gut microbiota density influences host physiology and is shaped by host and microbial factors. Elife. (2019) 8:e40553. doi: 10.7554/eLife.40553
15. van der Wulp MY, Derrien M, Stellaard F, Wolters H, Kleerebezem M, Dekker J, et al. Laxative treatment with polyethylene glycol decreases microbial primary bile salt dehydroxylation and lipid metabolism in the intestine of rats. Am J Physiol Gastrointest Liver Physiol. (2013) 305:G474–82. doi: 10.1152/ajpgi.00375.2012
16. Tropini C, Moss EL, Merrill BD, Ng KM, Higginbottom SK, Casavant EP, et al. Transient osmotic perturbation causes long-term alteration to the gut microbiota. Cell. (2018) 173:1742–54.e17. doi: 10.1016/j.cell.2018.05.008
17. Gorkiewicz G, Thallinger GG, Trajanoski S, Lackner S, Stocker G, Hinterleitner T, et al. Alterations in the colonic microbiota in response to osmotic diarrhea. PLoS ONE. (2013) 8:e55817. doi: 10.1371/journal.pone.0055817
18. Vich Vila A, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR, et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. (2020) 11:362. doi: 10.1038/s41467-019-14177-z
19. Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. (2006) 130:1519–26. doi: 10.1053/j.gastro.2005.11.065
20. Bekkali N, Hamers SL, Reitsma JB, Van Toledo L, Benninga MA. Infant stool form scale: development and results. J Pediatr. (2009) 154:521–6.e1. doi: 10.1016/j.jpeds.2008.10.010
21. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. (1997) 32:920–4. doi: 10.3109/00365529709011203
22. Varni JW, Seid M. Kurtin PS. PedsQL 40: reliability and validity of the Pediatric Quality of Life Inventory version 40 generic core scales in healthy and patient populations. Med Care. (2001) 39:800–12. doi: 10.1097/00005650-200108000-00006
23. Iaffaldano L, Granata I, Pagliuca C, Esposito MV, Casaburi G, Salerno G, et al. Oropharyngeal microbiome evaluation highlights Neisseria abundance in active celiac patients. Sci Rep. (2018) 8:11047. doi: 10.1038/s41598-018-29443-1
24. Nardelli C, Granata I, D'Argenio V, Tramontano S, Compare D, Guarracino MR, et al. Characterization of the duodenal mucosal microbiome in obese adult subjects by 16S rRNA sequencing. Microorganisms. (2020) 8:485. doi: 10.3390/microorganisms8040485
25. Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. (2019) 37:852–7. doi: 10.1038/s41587-019-0209-9
26. Kern SE. Challenges in conducting clinical trials in children: approaches for improving performance. Expert Rev Clin Pharmacol. (2009) 2:609–17. doi: 10.1586/ecp.09.40
27. Joseph PD, Craig JC, Caldwell PH. Clinical trials in children. Br J Clin Pharmacol. (2015) 79:357–69. doi: 10.1111/bcp.12305
28. Dziechciarz P, Horvath A, Szajewska H. Polyethylene glycol 4000 for treatment of functional constipation in children. J Pediatr Gastroenterol Nutr. (2015) 60:65–8. doi: 10.1097/MPG.0000000000000543
29. Walsh J, Schaufelberger D, Iurian S, Klein S, Batchelor H, Turner R, et al. Path towards efficient paediatric formulation development based on partnering with clinical pharmacologists and clinicians, a c4c expert group White paper. Br J Clin Pharmacol. (2021). doi: 10.1111/bcp.14989. [Epub ahead of print].
30. Benninga MA, Faure C, Hyman PE, St James Roberts I, Schechter NL, Nurko S. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. (2016) 15:S0016-5085(16)00182-7. doi: 10.1053/j.gastro.2016.02.016
31. Petraroli M, Castellone E, Patianna V, Esposito S. Gut microbiota and obesity in adults and children: the state of the art. Front Pediatr. (2021) 9:657020. doi: 10.3389/fped.2021.657020
32. Derrien M, Alvarez AS, de Vos WM. The gut microbiota in the first decade of life. Trend Microbiol. (2019) 27:997–1010. doi: 10.1016/j.tim.2019.08.001
Keywords: polyethylene glycol, Promelaxin microenemas, medical devices based on substances, functional constipation, young children
Citation: Strisciuglio C, Coppola V, Russo M, Tolone C, Marseglia GL, Verrotti A, Caimmi S, Caloisi C, D'Argenio V, Sacchetti L and Staiano A (2021) Promelaxin Microenemas Are Non-inferior to Oral Polyethylene Glycol for the Treatment of Functional Constipation in Young Children: A Randomized Clinical Trial. Front. Pediatr. 9:753938. doi: 10.3389/fped.2021.753938
Received: 05 August 2021; Accepted: 30 September 2021;
Published: 29 October 2021.
Edited by:
Jorge Amil Dias, Centro Hospitalar de São João, PortugalReviewed by:
Moinak Sen Sarma, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), IndiaOsvaldo Borrelli, Great Ormond Street Hospital for Children NHS Foundation Trust, United Kingdom
Mauro Batista De Morais, Federal University of São Paul, Brazil
Copyright © 2021 Strisciuglio, Coppola, Russo, Tolone, Marseglia, Verrotti, Caimmi, Caloisi, D'Argenio, Sacchetti and Staiano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annamaria Staiano, staiano@unina.it
†These authors have contributed equally to this work
‡ORCID: Caterina Strisciuglio orcid.org/0000-0002-9005-6571
Vincenzo Coppola orcid.org/0000-0002-9896-3942
Marina Russo orcid.org/0000-0002-8259-8290
Carlo Tolone orcid.org/0000-0003-0700-5309
Gian Luigi Marseglia orcid.org/0000-0003-366-0159
Alberto Verrotti orcid.org/0000-0001-6323-4187
Silvia Caimmi orcid.org/0000-0003-05158-524X
Claudia Caloisi orcid.org/0000-0003-4437-8040
Valeria D'Argenio orcid.org/0000-0001-9273-3698
Lucia Sacchetti orcid.org/0000-0002-1550-8216
Annamaria Staiano orcid.org/0000-0003-0586-1339