Abstract 抽象
Osteoarthritis (OA) is a common joint disease characterized by cartilage degeneration. It can cause severe pain, deformity and even amputation risk. However, existing clinical treatment methods for cartilage repair present certain deficiencies. Meanwhile, the repair effect of cartilage tissue engineering is also unsatisfactory. Cartilage organoids are multicellular aggregates with cartilage-like three-dimensional structure and function. On the one hand, cartilage organoids can be used to explore the pathogenesis of OA by constructing disease models. On the other hand, it can be used as filler for rapid cartilage repair. Extracellular matrix (ECM)-like three-dimensional environment is the key to construct cartilage organoids. Silk fibroin (SF)-based hydrogels not only have ECM-like structure, but also have unique mechanical properties and remarkable biocompatibility. Therefore, SF-based hydrogels are considered as ideal biomaterials for constructing cartilage organoids. In this review, we reviewed the studies of cartilage organoids and SF-based hydrogels. The advantages of SF-based hydrogels in constructing cartilage organoids and the iterative optimization of cartilage organoids through designing hydrogels by using artificial intelligence (AI) calculation are also discussed. This review aims to provide a theoretical basis for the treatment of OA using SF-based biomaterials and cartilage organoids.
骨关节炎 (OA) 是一种以软骨退化为特征的常见关节疾病。它会导致严重的疼痛、畸形,甚至有截肢的风险。然而,现有的临床软骨修复治疗方法存在一定的不足。同时,软骨组织工程的修复效果也不尽如人意。软骨类器官是具有软骨样三维结构和功能的多细胞聚集体。一方面,软骨类器官可用于通过构建疾病模型来探索 OA 的发病机制。另一方面,它可以用作快速软骨修复的填充剂。细胞外基质 (ECM) 样三维环境是构建软骨类器官的关键。基于丝素蛋白 (SF) 的水凝胶不仅具有类似 ECM 的结构,而且还具有独特的机械性能和显著的生物相容性。因此,基于 SF 的水凝胶被认为是构建软骨类器官的理想生物材料。在本综述中,我们回顾了软骨类器官和基于 SF 的水凝胶的研究。还讨论了基于 SF 的水凝胶在构建软骨类器官方面的优势,以及通过使用人工智能 (AI) 计算设计水凝胶来迭代优化软骨类器官。本文旨在为使用 SF 基生物材料和软骨类器官治疗 OA 提供理论依据。
Keywords: Osteoarthritis, Silk fibroin, Hydrogels, Cartilage organoids, Cartilage regeneration.
关键字:骨关节炎、丝素蛋白、水凝胶、软骨类器官、软骨再生。
1. Introduction 1. 引言
Osteoarthritis (OA) is a joint disease characterized by cartilage degeneration 1-5. Pathogenic factors of OA are mainly associated with obesity and age 6. As the global aging and obese population rises, the number of OA patients will increase sharply in the next decade 7, 8. Statistically, approximately 595 million people worldwide are affected by OA. And by 2050, the prevalence is projected to increase by over 200%, resulting in substantial economic burdens on both individuals and society 9. Cartilage degeneration plays a significant role in the progression of OA, as it contributes not only to the disease outcome but also to its worsening 10, 11. Therefore, repairing cartilage is a valuable strategy for the treatment and prevention of OA.
骨关节炎 (OA) 是一种以软骨退化 1 为特征的关节疾病 - 5 。OA 的致病因素主要与肥胖和年龄 6 有关。随着全球老龄化和肥胖人口的增加,未来十年 7 OA 患者的数量将急剧增加。 8 据统计,全球约有 5.95 亿人受到 OA 的影响。到 2050 年,患病率预计将增加 200% 以上,给个人和社会带来沉重的经济负担 9 。软骨变性在 OA 的进展中起着重要作用,因为它不仅会导致疾病结果,还会导致其恶化 10 。 11 因此,修复软骨是治疗和预防 OA 的宝贵策略。
Cartilage has limited self-repair capacity 12-14. As an immune-privileged tissue, it makes engineered cartilage analogs attractive candidates for off-the-shelf grafts in allogeneic transplantation. Organoids are simplified multicellular structures that develop from stem cells or organ progenitors through in vitro 3D culture combined with targeted induction technology, enabling the formation of organ-specific architectures and functions 15-17. Specifically, cartilage organoids are tissues with cartilage structure, function, physiological and pathological characteristics through cultivating and assembling stem cells or chondrocytes 18, 19. This offers a novel approach for cartilage regeneration. Compared to autologous chondrocyte implantation (ACI), cartilage organoid transplantation eliminates the need for a secondary surgery to harvest cells, streamlining the procedure and reducing postoperative complications. Techniques like ACI and matrix-induced autologous chondrocyte implantation (MACI), which rely on 2D chondrocyte expansion, are time-consuming and increase the risk of dedifferentiation into fibrocartilage 20-22. In contrast, cartilage organoids have distinct advantages: they replicate the natural cartilage structure and can be directly implanted to repair defects, ensuring that the regenerated tissue closely resembles native cartilage. Furthermore, as they already mimic cartilage properties, cartilage organoids can seamlessly integrate with native tissue, minimizing the need for further regeneration post-implantation and thus enhancing the overall repair efficiency 23.
软骨的自我修复能力 12 有限 - 14 。作为一种免疫特权组织,它使工程软骨类似物成为同种异体移植中现成移植物的有吸引力的候选者。类器官是从干细胞或器官祖细胞通过体外 3D 培养结合靶向诱导技术发育而来的简化多细胞结构,能够形成器官特异性结构和功能 17 15 。具体来说,软骨类器官是通过培养和组装干细胞或软骨细胞 18 而具有软骨结构、功能、生理和病理特征的组织。 19 这为软骨再生提供了一种新的方法。与自体软骨细胞植入 (ACI) 相比,软骨类器官移植无需二次手术来采集细胞,简化了手术并减少了术后并发症。像 ACI 和基质诱导的自体软骨细胞植入 (MACI) 这样的技术依赖于 2D 软骨细胞扩增,非常耗时,并且增加了去分化为纤维软骨 20 的风险 - 22 。相比之下,软骨类器官具有明显的优势:它们复制天然软骨结构,可以直接植入以修复缺损,确保再生组织与天然软骨非常相似。此外,由于它们已经模拟了软骨的特性,软骨类器官可以与天然组织无缝集成,最大限度地减少植入后进一步再生的需要,从而提高整体修复效率 23 。
Organoid construction methods are broadly divided into scaffold-free self-organization and biomaterial-based co-cultivation. Scaffold-free self-organization allows mesenchymal stem cells (MSCs) to naturally form complex tissue structures. This method is straightforward and has a lower risk of contamination, but it struggles with achieving consistent organoid size and uniformity. On the other hand, biomaterial-assisted co-cultivation uses scaffolds like hydrogels to provide structural support, allowing precise control over the organoid architecture and customization of the environment. This makes it ideal for developing organoids with consistent and predictable properties. Currently, Matrigel is the most commonly used material for organoid construction. However, its undefined composition and batch-to-batch variability result in inconsistent mechanical strength, making it less reliable for precise experimental needs. Additionally, Matrigel lacks the flexibility for customization in specific organoid culture contexts 18, 24, 25. Silk fibroin (SF) hydrogels, as a natural macromolecular material, offer several advantages over Matrigel, including a well-defined structure, controllable mechanical properties, and high customizability. These features make SF hydrogels more suitable for precise tissue engineering applications 26-30. Moreover, SF's excellent processability allows it to be adapted to various processing methods and functional modifications, making it versatile for preparing different types of cartilage tissue engineering materials 31-33. Furthermore, SF hydrogels have excellent printability, enabling their use in 3D bioprinting for advanced biofabrication techniques 34-36. Therefore, SF-based hydrogel has a great prospect for cartilage regeneration and cartilage organoids construction 37.
类器官构建方法大致分为无支架自组织和基于生物材料的共培养。无支架的自组织使间充质干细胞 (MSC) 能够自然形成复杂的组织结构。这种方法简单明了,污染风险较低,但难以实现一致的类器官大小和均匀性。另一方面,生物材料辅助共培养使用水凝胶等支架提供结构支撑,从而可以精确控制类器官结构和环境定制。这使其成为开发具有一致和可预测特性的类器官的理想选择。目前,Matrigel 是类器官构建最常用的材料。然而,其不确定的成分和批次间的可变性导致机械强度不一致,使其在满足精确实验需求方面不太可靠。此外,Matrigel 缺乏在特定类器官培养环境中 18 进行定制的灵活性, 24 、 25 。丝素蛋白 (SF) 水凝胶作为一种天然大分子材料,与 Matrigel 相比具有多项优势,包括结构明确、机械性能可控和高度可定制性。这些特性使 SF 水凝胶更适合于精确的组织工程应用 26 - 30 .此外,SF 出色的加工性能使其能够适应各种加工方法和功能修饰,使其可用于制备不同类型的软骨组织工程材料 31 - 33 。 此外,SF 水凝胶具有出色的可打印性,使其能够用于 3D 生物打印,用于先进的生物制造技术 34 - 36 。因此,SF 基水凝胶在软骨再生和软骨类器官构建 37 方面具有很大的前景。
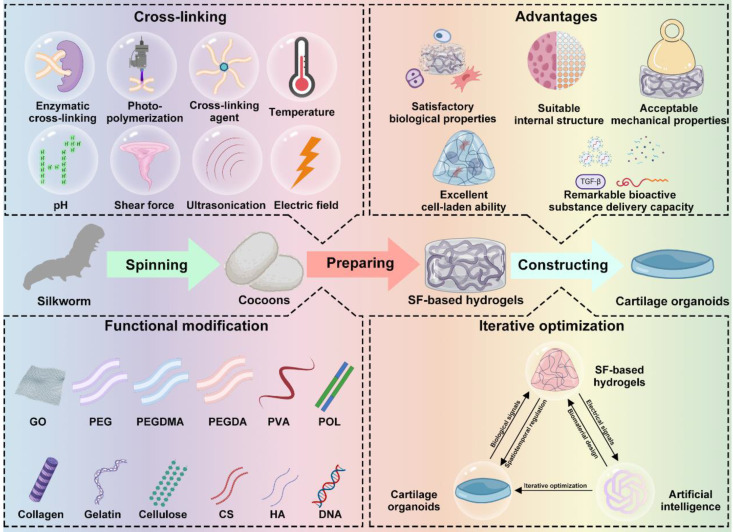
Herein, we summarize recent research on cartilage organoids and SF-based hydrogels, highlighting the advantages of SF-based hydrogels for cartilage organoid construction. The iterative optimization of cartilage organoids through designing hydrogels by using artificial intelligence (AI) calculation is also discussed (Figure 1). We hope that this review can provide a reference for cartilage organoids construction and a promising therapeutic strategy for OA.
在此,我们总结了最近关于软骨类器官和 SF 基水凝胶的研究,强调了 SF 基水凝胶在软骨类器官构建中的优势。还讨论了通过使用人工智能 (AI) 计算设计水凝胶来迭代优化软骨类器官(图 1 )。我们希望本文能为软骨类器官的构建提供参考,并为 OA 的治疗策略提供有前途的治疗策略。
Figure 1. 图 1.
Schematic diagram of SF-based hydrogels for construction of cartilage organoids. Created with BioRender.com.
用于构建软骨类器官的基于 SF 的水凝胶示意图。使用 BioRender.com 创建。
2. Research progress in cartilage organoids
2. 软骨类器官的研究进展
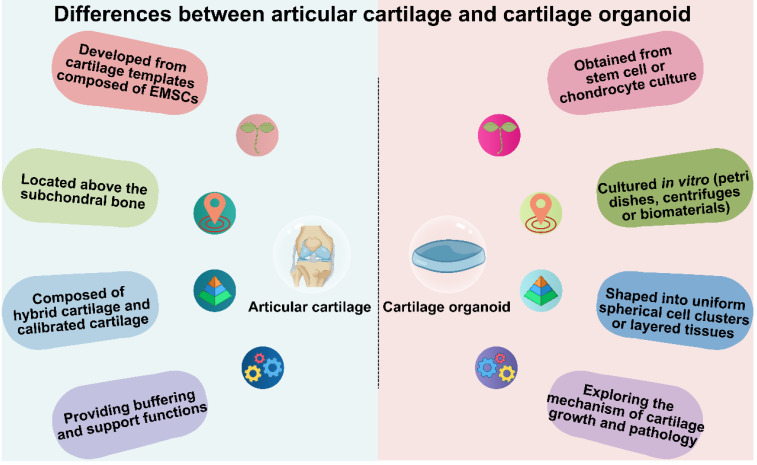
Cartilage is a critical component of the human body, providing essential support for mechanical reinforcement, cushioning, and protection (Figure 2). Cartilage development commences with a cartilaginous template constituted by embryonic mesenchymal stem cells. During this stage, primitive embryonic mesenchymal cells initiate differentiation into chondroblasts 38-40. These chondroblasts proliferate and synthesize collagen fibers and glycosaminoglycans, establishing the extracellular matrix of cartilage. The distribution and orientation of collagen fibers and glycosaminoglycans, along with the degree of chondrocyte calcification, contribute to the multi-layered structure of cartilage. For example, articular cartilage can be broadly categorized into hyaline cartilage and calcified cartilage layers. Cartilage serves various physiological roles, with the most crucial being to provide cushioning and support within joints. It reduces friction between bones and protects them from wear and tear. Moreover, cartilage effectively disperses pressure generated during joint movement, thereby protecting joint tissues. In structures like the nose and ears, the elasticity and flexibility of cartilage allow it to maintain specific shapes 41, 42. Currently, artificially engineered cartilage organoids structures are predominantly composed of uniformly spherical cell clusters or uniformly layered tissues. Research in cartilage organoids mainly focuses on understanding cartilage-related mechanisms, advancing plastic surgery, and promoting cartilage repair (Figure 3 and Figure 4).
软骨是人体的关键组成部分,为机械加固、缓冲和保护提供必要的支撑(图 2 )。软骨发育始于由胚胎间充质干细胞构成的软骨模板。在此阶段,原始胚胎间充质细胞开始分化为成软骨母细胞 38 - 40 。这些软骨母细胞增殖并合成胶原纤维和糖胺聚糖,建立软骨的细胞外基质。胶原纤维和糖胺聚糖的分布和取向,以及软骨细胞钙化的程度,有助于软骨的多层结构。例如,关节软骨大致可分为透明软骨和钙化软骨层。软骨具有多种生理作用,其中最重要的是在关节内提供缓冲和支撑。它减少了骨骼之间的摩擦并保护它们免受磨损。此外,软骨可有效分散关节运动过程中产生的压力,从而保护关节组织。在鼻子和耳朵等结构中,软骨的弹性和柔韧性使其能够保持特定的形状 41 。 42 目前,人工工程软骨类器官结构主要由均匀的球形细胞簇或均匀分层的组织组成。软骨类器官的研究主要集中在了解软骨相关机制、推进整形手术和促进软骨修复(图 3 和图 4 )。
Figure 2. 图 2.
Differences between articular cartilage and cartilage organoids in origin, location, structure, and function. Created with BioRender.com.
关节软骨和软骨类器官在起源、位置、结构和功能上的差异。使用 BioRender.com 创建。
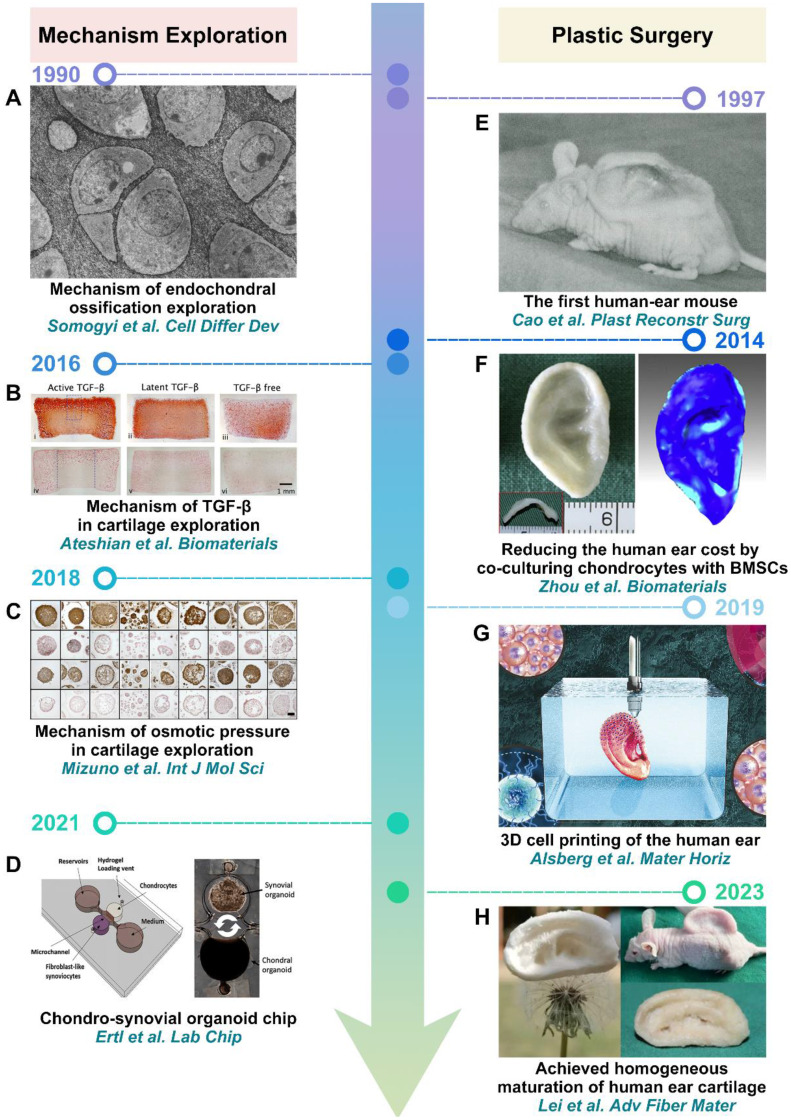
Figure 3. 图 3.
Cartilage organoids in mechanism exploration and plastic surgery: (A) Reproduced with permission from ref 43; Copyright 1990, Elsevier. (B) Reproduced with permission from ref 44; Copyright 2016, Elsevier. (C) Reproduced with permission from ref 45; Copyright 2018, MDPI. (D) Reproduced with permission from ref 46; Copyright 2021, Royal Society of Chemistry. (E) Reproduced with permission from ref 48; Copyright 1997, Wolters Kluwer Health, Inc. (F) Reproduced with permission from ref 49; Copyright 2014, Elsevier. (G) Reproduced with permission from ref 50; Copyright 2019, Royal Society of Chemistry. (H) Reproduced with permission from ref 51; Copyright 2023, Springer Nature.
机制探索和整形手术中的软骨类器官:(A) 经参考文献许可转载 43 ;版权所有 1990,爱思唯尔。(B) 经 ref 许可转载 44 ;版权所有 2016,爱思唯尔。(C) 经 ref 许可转载 45 ;版权所有 2018,MDPI。(D) 经 ref 许可转载 46 ;版权所有 2021,英国皇家化学学会。(E) 经 ref 许可转载 48 ;版权所有 1997, Wolters Kluwer Health, Inc. (F) 经参考文献许可转载 49 ;版权所有 2014,爱思唯尔。(G) 经 ref 许可转载 50 ;版权所有 2019,英国皇家化学学会。(H) 经 ref 许可转载 51 ;版权所有 2023,施普林格自然。
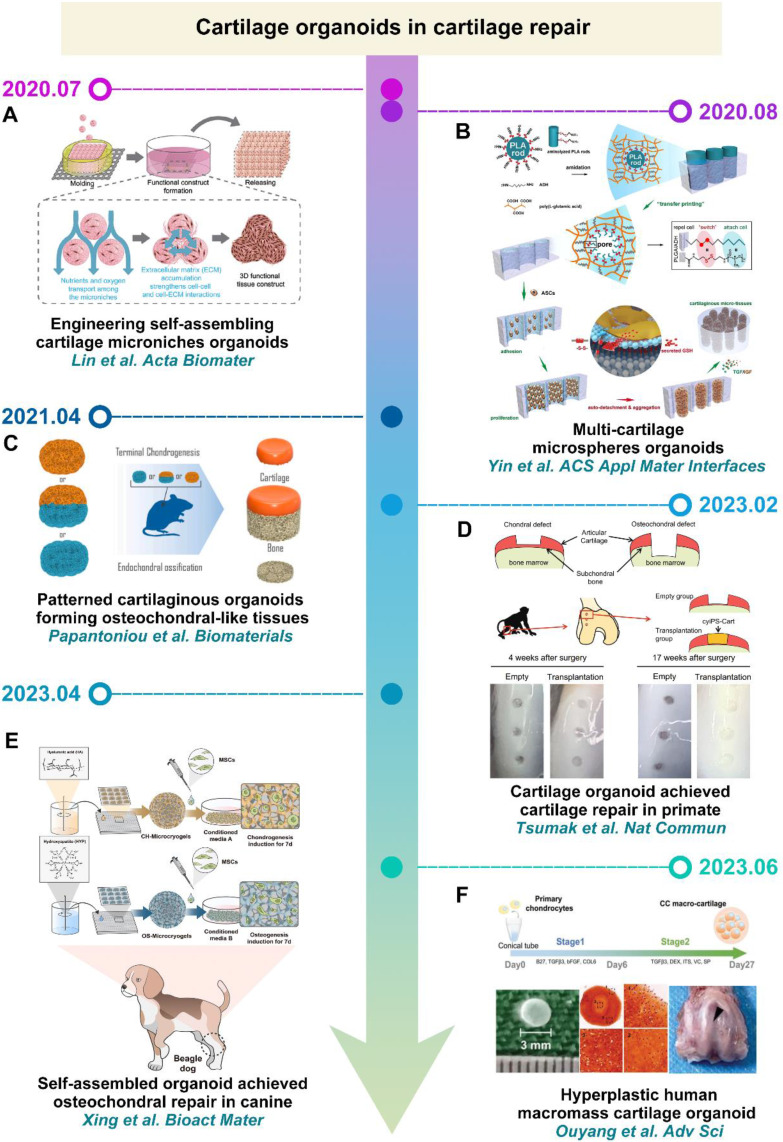
Figure 4. 图 4.
Cartilage organoids in tissue engineering: (A) Reproduced with permission from ref 52; Copyright 2020, Elsevier. (B) Reproduced with permission from ref 53; Copyright 2020, American Chemical Society. (C) Reproduced with permission from ref 54; Copyright 2021, Elsevier. (D) Reproduced with permission from ref 55; Copyright 2023, Springer Nature. (E) Reproduced with permission from ref 56; Copyright 2023, Elsevier. (F) Reproduced with permission from ref 57; Copyright 2023, Wiley-VCH GmbH.
组织工程中的软骨类器官:(A) 经参考文献许可转载 52 ;版权所有 2020,爱思唯尔。(B) 经 ref 许可转载 53 ;版权所有 2020,美国化学会。(C) 经 ref 许可转载 54 ;版权所有 2021,爱思唯尔。(D) 经 ref 许可转载 55 ;版权所有 2023,施普林格自然。(E) 经 ref 许可转载 56 ;版权所有 2023,爱思唯尔。(F) 经 ref 许可转载 57 ;版权所有 2023,Wiley-VCH GmbH。
Cartilage organoids were initially applied to explore cartilage-related mechanisms. In 1990, Somogyi et al. pioneered the in vitro construction of cartilage organoids. By examining their morphology and ECM, they found that osteoblasts promoted mineralization within cartilage, whereas fibroblasts had inhibitory effects (Figure 3A) 43. With the advancement of technology, cartilage organoids have also been utilized to investigate the impact of growth factors (TGF-β) and osmotic pressure on cartilage development (Figure 3B-C) 44, 45. Furthermore, cartilage organoids have also been employed to construct organ-on-a-chip models to study inter-tissue interactions. For example, Ertl et al. constructed the chondro-synovial organoid chip to simulate cross-talk between individual synovial and cartilage organoids. Co-culturing with synovial organoids, it was demonstrated that cartilage organoids induced a heightened degree of cartilage physiology and structure, along with distinct cellular cytokine responses compared to their respective monocultures, underscoring the significance of inter-tissue cross-talk at the organ level in models of arthritic diseases (Figure 3D) 46. In addition to exploring cartilage-related mechanisms, cartilage organoids have also demonstrated remarkable potential in plastic surgery. Notably, one of the most matured applications involves the construction of auricular-shaped cartilage organoids, particularly for reconstructing human ears 47. In 1997, Cao et al. constructed the first human-ear mouse with a polyglycolic acid fiber scaffold (Figure 3E) 48. Subsequently, Zhou et al. advanced the field by constructing human auricular cartilage organoids through co-culturing microtia chondrocytes and bone mesenchymal stem cells (BMSCs) (Figure 3F) 49. This approach not only enhanced the shape stability of human auricular cartilage organoids but also effectively reduced construction costs. In 2019, Alsberg et al. further advanced the field by enhancing the shape resolution of human auricular cartilage organoids using 3D bioprinting technology (Figure 3G) 50. Building on these advances, Lei et al. constructed homogeneous and mature human auricular cartilage organoids using synthetically engineered fiber-reinforced SF super elastic absorbent sponges (Figure 3H) 51.
软骨类器官最初用于探索软骨相关机制。1990 年,Somogyi 等人率先在体外构建软骨类器官。通过检查它们的形态和 ECM,他们发现成骨细胞促进软骨内的矿化,而成纤维细胞具有抑制作用(图 3 A)。 43 随着技术的进步,软骨类器官也被用于研究生长因子 (TGF-β) 和渗透压对软骨发育的影响(图 3 BC), 44 45 。此外,软骨类器官也被用于构建器官芯片模型以研究组织间相互作用。例如,Ertl 等人构建了软骨滑膜类器官芯片,以模拟单个滑膜和软骨类器官之间的串扰。与滑膜类器官共培养,证明软骨类器官诱导了更高程度的软骨生理学和结构,以及与它们各自的单一培养相比,具有不同的细胞因子反应,强调了器官水平组织间串扰在关节炎疾病模型中的重要性(图 3 D) 46 .除了探索软骨相关机制外,软骨类器官在整形手术中也显示出显着的潜力。值得注意的是,最成熟的应用之一涉及耳廓形状软骨类器官的构建,特别是用于重建人耳 47 。1997 年,Cao 等人构建了第一只具有聚乙醇酸纤维支架的人耳小鼠(图 3 E)。 48 随后,周 et al.通过共培养小耳畸形软骨细胞和骨间充质干细胞 (BMSC) 构建人耳软骨类器官,推动了该领域的发展(图 3 F)。 49 这种方法不仅增强了人类耳软骨类器官的形状稳定性,而且有效地降低了施工成本。2019 年,Alsberg 等人通过使用 3D 生物打印技术提高人类耳软骨类器官的形状分辨率,进一步推动了该领域的发展(图 3 G)。 50 在这些进展的基础上,Lei 等人使用合成工程纤维增强 SF 超弹性吸收海绵构建了均质和成熟的人耳软骨类器官(图 3 H)。 51
In recent years, researchers have increasingly directed their focus towards applying cartilage organoids in cartilage repair. For instance, Lin et al. and Yin et al. achieved the stacking of cartilage microsphere organoids using materials with self-assembling properties, enabling the construction of larger-volume cartilage organoids (Figure 4A-B) 52, 53. Papantoniou et al. assembled cartilage microtissues derived from iPSC-derived chondrocytes with callus organoids (COs) sourced from human plasmacytoid dendritic cells to constructed layered osteochondral organoids (Figure 4C) 54. It is worth noting that cartilage organoids constructed by Tsumaki et al. and Xing et al. successfully achieved cartilage repair in primates and canids (Figure 4D-E) 55, 56. In addition, Ouyang et al. constructed macromass cartilage organoids up to 3 mm in diameter by culturing human polydactyly chondrocytes in customized culture, which can be used as implants to facilitate cartilage defect repair (Figure 4F) 57. Based on these studies, it is evident that constructing cartilage organoids requires providing material support for seed cells and directing their chondrogenic differentiation. Consequently, to construct cartilage organoids that closely mimic the natural cartilage structure and function, novel smart biomaterials need to be designed to furnish the chondrogenic microenvironment necessary for seed cells.
近年来,研究人员越来越多地将注意力转向将软骨类器官应用于软骨修复。例如,Lin 等人和 Yin 等人使用具有自组装特性的材料实现了软骨微球类器官的堆叠,从而能够构建更大容量的软骨类器官(图 4 A-B)。 52 53 Papantoniou 等人将源自 iPSC 衍生的软骨细胞的软骨微组织与源自人浆细胞样树突状细胞的愈伤组织类器官 (CO) 组装成构建的分层骨软骨类器官(图 4 C)。 54 值得注意的是,Tsumaki 等人和 Xing 等人构建的软骨类器官成功地实现了灵长类动物和犬科动物的软骨修复(图 4 D-E)、 55 56 .此外,Ouyang 等人通过在定制培养物中培养人多指软骨细胞构建了直径达 3 mm 的大质量软骨类器官,其可用作植入物以促进软骨缺损修复(图 4 F)。 57 基于这些研究,很明显,构建软骨类器官需要为种子细胞提供物质支持并指导其软骨形成分化。因此,为了构建与天然软骨结构和功能紧密相似的软骨类器官,需要设计新型智能生物材料来提供种子细胞所需的软骨形成微环境。
3. Preparation of silk fibroin-based hydrogel
3. 丝素蛋白基水凝胶的制备
3.1. Characteristics of silk fibroin
3.1. 丝素蛋白的特点
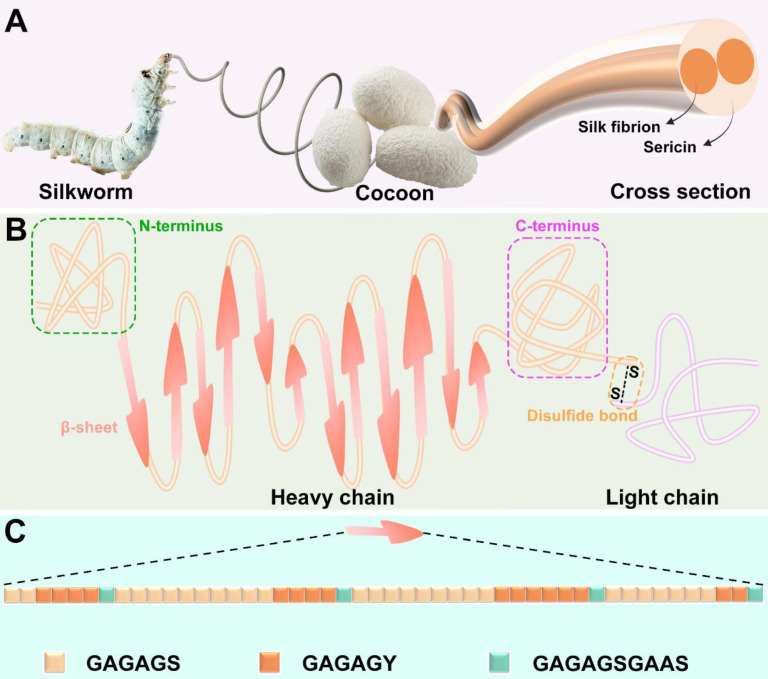
SF is a naturally occurring macromolecular material produced by a range of animals, including silkworms, spiders, scorpions, mites, and flies 58. It is worth noting that SF of different origins has obvious differences in structure and properties. Among them, silkworms-derived SF has been widely studied and applied in the clinic because of its unique mechanical properties and abundant yield 59, 60. Hence, this review only discusses the silkworms derived SF. A single silk filament is composed of two strands of SF, enveloped in sericin (Figure 5A) 61.
SF 是一种天然存在的大分子材料,由一系列动物产生,包括蚕、蜘蛛、蝎子、螨虫和苍蝇 58 。值得注意的是,不同产地的 SF 在结构和性质上存在明显差异。其中,家蚕来源的SF因其独特的力学性能和丰富的产量 59 ,在临床上得到了广泛的研究和应用。 60 因此,本文仅讨论家蚕来源的 SF。单丝由两股 SF 组成,包裹在丝胶蛋白中(图 5 A)。 61
Figure 5. 图 5.
Source and structure of SF: (A) SF can be extracted from cocoon of silkworm. (B) SF molecule is composed of heavy chain and light chain connected by disulfide bond. (C) Crystal structure consists of GAGAGS, GAGAGY and GAGAGSGAAS repetitive sequences. Created with BioRender.com.
SF的来源和结构: (A) SF可以从蚕茧中提取。(B) SF 分子由二硫键连接的重链和轻链组成。(C) 晶体结构由 GAGAGS、GAGAGY 和 GAGAGSGAAS 重复序列组成。使用 BioRender.com 创建。
The molecular structure of SF is very complex, which is composed of disulfide-linked heavy chain and light chain (Figure 5B). The heavy chain includes non-repetitive C-terminal and N-terminal, along with 11 hydrophilic segments composed of 31 amino acid residues and 12 hydrophobic segments. The hydrophobic segments mainly contain Gly-X repeats, where X can be Ala (65%), Ser (23%), or Tyr (9%). Repeated sequences include Gly-Ala-Gly-Ala-Gly-Ser (GAGAGS), Gly-Ala-Gly-Ala-Gly-Tyr (GAGAGY), and Gly-Ala-Gly-Ala-Gly-Ser-Gly-Ala-Ala-Ser (GAGAGSGAAS). These repetitive sequences can form crystalline β-sheet structures through hydrophobic interactions (Figure 5C)
26. Contrary to the heavy chain, the amino acid sequence of the light chain is disordered and tends to form an amorphous structure 62. Recent studies have revealed that SF achieves interfacial self-assembly due to its amphiphilic molecular structure, which promotes the formation of β-sheets at interfaces. This property is particularly useful in hydrogel formation, as the conformation of SF can be modulated by adjusting the water-to-organic phase ratio. These adjustments enable the creation of hydrogels specifically optimized for cartilage regeneration, offering enhanced mechanical properties and bioactivity for improved tissue repair 63.
SF 的分子结构非常复杂,由二硫键连接的重链和轻链组成(图 5 B)。重链包括非重复的 C 端和 N 端,以及由 31 个氨基酸残基和 12 个疏水片段组成的 11 个亲水片段。疏水片段主要包含 Gly-X 重复序列,其中 X 可以是 Ala (65%)、Ser (23%) 或 Tyr (9%)。重复序列包括 Gly-Ala-Gly-Ala-Gly-Ser (GAGAGS)、Gly-Ala-Gly-Ala-Gly-Tyr (GAGAGY) 和 Gly-Ala-Gly-Ala-Gly-Ser-Gly-Ala-Ala-Ser (GAGAGSGAAS)。这些重复序列可以通过疏水相互作用形成结晶β片结构(图 5 C)。 26 与重链相反,轻链的氨基酸序列是无序的,倾向于形成无定形结构 62 。最近的研究表明,SF 由于其两亲性分子结构实现了界面自组装,这促进了界面处β片的形成。这种特性在水凝胶形成中特别有用,因为可以通过调整水与有机相比来调节 SF 的构象。这些调整能够产生专门针对软骨再生优化的水凝胶,从而提供增强的机械性能和生物活性,以改善组织修复 63 。
SF exhibits exceptional physical and chemical properties due to its unique structure and composition. It can adopt four distinct conformations—silk I, silk II, silk III, and an amorphous structure—through intra- and intermolecular interactions. Among these, the amorphous structure and β-sheet-rich silk II conformation endow SF with high mechanical strength and toughness. Furthermore, silk I, silk II, silk III and amorphous structures can be transformed into each other by external effects (temperature, ultrasound, electric field, shear force and pH value) 62. This adaptability makes SF highly suitable for diverse tissue regeneration applications, as its mechanical properties can be finely tuned. As a macromolecular protein material, SF also offers excellent cytocompatibility and biodegradability. It degrades in response to multiple proteases, with its degradation rate primarily controlled by the content of silk II. The degradation products—amino acids and peptides—are non-toxic and can be absorbed by cells, providing essential building blocks for tissue regeneration 76. In addition to these favorable properties, SF's processability allows it to be adapted into various processing methods to meet the complex demands of tissue repair 77. To provide a clearer understanding of SF's properties relative to other biomaterials commonly used in cartilage regeneration, Table 1 presents a quantitative comparison of SF with collagen, alginate, hyaluronic acid (HA), and Matrigel across key parameters, including mechanical properties, degradation rates, and biological performance.
SF 由于其独特的结构和成分而表现出卓越的物理和化学性能。它可以通过分子内和分子间相互作用采用四种不同的构象——丝 I、丝 II、丝 III 和无定形结构。其中,无定形结构和富含 β 片的 silk II 构象赋予 SF 高机械强度和韧性。此外,蚕丝 I、蚕丝 II、蚕丝 III 和非晶结构可以通过外部作用(温度、超声波、电场、剪切力和 pH 值) 62 相互转化。这种适应性使 SF 非常适合各种组织再生应用,因为它的机械性能可以微调。作为一种大分子蛋白质材料,SF 还具有出色的细胞相容性和生物降解性。它响应多种蛋白酶而降解,其降解速率主要受丝 II 的含量控制。降解产物(氨基酸和肽)无毒,可被细胞吸收,为组织再生 76 提供必需的组成部分。除了这些有利的特性外,SF 的可加工性使其能够适应各种加工方法,以满足组织修复 77 的复杂需求。为了更清楚地了解 SF 相对于软骨再生中常用的其他生物材料的特性,下表 1 列出了 SF 与胶原蛋白、藻酸盐、透明质酸 (HA) 和基质胶在关键参数(包括机械性能、降解速率和生物性能)上的定量比较。
Table 1. 表 1.
Comparison of the mechanical properties, degradation, cell viability, and chondrocyte differentiation of SF and other biomaterials used in cartilage tissue engineering.
SF 和其他用于软骨组织工程的生物材料的机械性能、降解、细胞活力和软骨细胞分化的比较。
| Property 财产 | SF | Col 山坳 | Alginate 海藻 酸 | HA | Matrigel 基质胶 |
|---|---|---|---|---|---|
| Young's Modulus (MPa) 杨氏模量 (MPa) | 300-700 MPa | 0.1-10 MPa | 0.01-1.5 MPa | 0.01-0.1 MPa | 0.00004-0.00045 MPa |
| Breaking Elongation (%) 断裂伸长率 (%) | 4%-26% | 10-30% | 10-20% | 5-20% | Not reported 未报告 |
| Toughness (MJ/m3) 韧性 (MJ/m3) |
70-78 MJ/m3 70-78 兆焦耳/立方米 | 1-5 MJ/m³ 1-5 MJ/立方米 | 1-5 MJ/m³ 1-5 MJ/立方米 | 1-2 MJ/m³ 1-2 MJ/立方米 | Not reported 未报告 |
| Degradation Time 退化时间 | Tunable (weeks to months) 可调(数周至数月) |
Days to weeks 天到周 | Days to weeks 天到周 | Days to weeks 天到周 | Within a few days 几天内 |
| Cell Viability (%) 细胞活力 (%) | >90% | >80% | >80% | >85% | >90% |
| Chondrocyte Differentiation 软骨细胞分化 |
Supports viability and promotes collagen type II synthesis 支持活力并促进 II 型胶原蛋白合成 |
Promotes moderate chondrocyte differentiation but limited stable phenotype 促进中等软骨细胞分化,但稳定表型有限 |
Supports chondrocyte viability with moderate differentiation capacity 支持具有中等分化能力的软骨细胞活力 |
Supports viability, requires additional cues for stable phenotype 支持活力,需要额外的线索才能获得稳定的表型 |
Supports growth but does not inherently promote chondrocyte differentiation 支持生长,但本身并不促进软骨细胞分化 |
| References 引用 | 64, 65 | 66, 67 | 68-70 | 71, 72 | 73-75 |
3.2. Cross-linking methods for silk fibroin-based hydrogels preparation
3.2. 丝素蛋白基水凝胶制备的交联方法
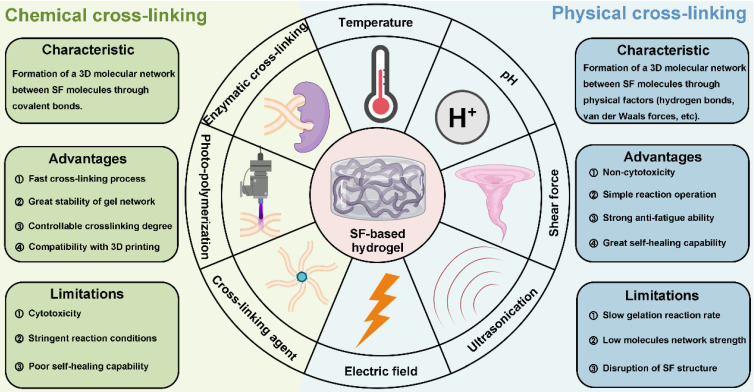
As previously mentioned, SF can be processed using various methods, including hydrogel preparation through cross-linking. Cross-linking methods are generally categorized into chemical and physical approaches 78. Chemical cross-linking promotes the formation of covalent bonds by adding enzymes, cross-linking agents and photo-initiators to accelerate SF gelation. In contrast, physical cross-linking involves the self-assembly of SF into hydrogels by regulating physical parameters such as temperature, pH, shear force, ultrasound, and electric fields, each method offering distinct advantages and limitations (Figure 6, Table 2). The schematic in Figure 7 provides an overview of the mechanisms and preparation techniques employed in these cross-linking strategies for SF hydrogels.
如前所述,SF 可以使用各种方法加工,包括通过交联制备水凝胶。交联方法通常分为化学方法和物理方法 78 。化学交联通过添加酶、交联剂和光引发剂来加速 SF 凝胶化,从而促进共价键的形成。相比之下,物理交联涉及通过调节温度、pH 值、剪切力、超声波和电场等物理参数将 SF 自组装成水凝胶,每种方法都有明显的优点和局限性(图 6 , 表 2 )。图 7 中的示意图概述了 SF 水凝胶的这些交联策略中采用的机制和制备技术。
Figure 6. 图 6.
Cross-linking methods of SF-based hydrogels. Chemical cross-linking: enzymatic cross-linking, photo-polymerization and cross-linking agent. Physical cross-linking: temperature, pH, shear force ultrasonication and electric field. Created with BioRender.com.
SF 基水凝胶的交联方法。化学交联:酶交联、光聚合和交联剂。物理交联:温度、pH、剪切力、超声波和电场。使用 BioRender.com 创建。
Table 2. 表 2.
Cross-linking methods for SF-based hydrogels: advantages and limitations.
SF 基水凝胶的交联方法:优点和局限性。
| Type 类型 | Cross-linking Method 交联方法 | Advantages 优势 | Limitations 局限性 |
|---|---|---|---|
| Chemical cross-linking 化学交联 | Enzymatic cross-linking 酶交联 | High selectivity; biocompatibility; tunable properties 选择性高;生物相容性;可调属性 |
High cost; low reaction velocity; limited scalability 成本高;反应速度低;可扩展性有限 |
| Photo-polymerization 光聚合 | Rapid cross-linking; precise control 快速交联;精确控制 |
Potential cytotoxicity; limited tissue penetration 潜在的细胞毒性;组织渗透受限 |
|
| Cross-linking agents 交联剂 | Cost-effective; enhances mechanical properties 成本效益高;增强机械性能 |
Non-specific reactions; cytotoxicity (for some agents) 非特异性反应;细胞毒性(对于某些药物) |
|
| Physical cross-linking 物理交联 | Temperature 温度 | Non-toxic; simple process 无毒;简单的流程 |
Lacks precision; risk of denaturation at high temperatures 缺乏精确性;高温下变性的风险 |
| pH 酸碱度 | Effective control over gelation 有效控制凝胶化 |
Requires careful pH control; potential impact on cell viability 需要仔细控制 pH 值;对细胞活力的潜在影响 |
|
| Shear force 剪切力 | Creates directional structures; anisotropic properties 创建定向结构;各向异性特性 |
Requires specialized equipment; limited scalability 需要专门的设备;可扩展性有限 |
|
| Ultrasonication 超声检查 | Non-toxic; controllable process; tailored porosity 无毒;过程可控;定制孔隙率 |
Weaker mechanical properties; limited load-bearing capacity 机械性能较弱;承重能力有限 |
|
| Electric field 电场 | Enables gradient structures; useful for tissue engineering 启用渐变结构;适用于组织工程 |
Requires specialized equipment; potential for uneven cross-linking 需要专门的设备;交联不均匀的可能性 |
Figure 7. 图 7.
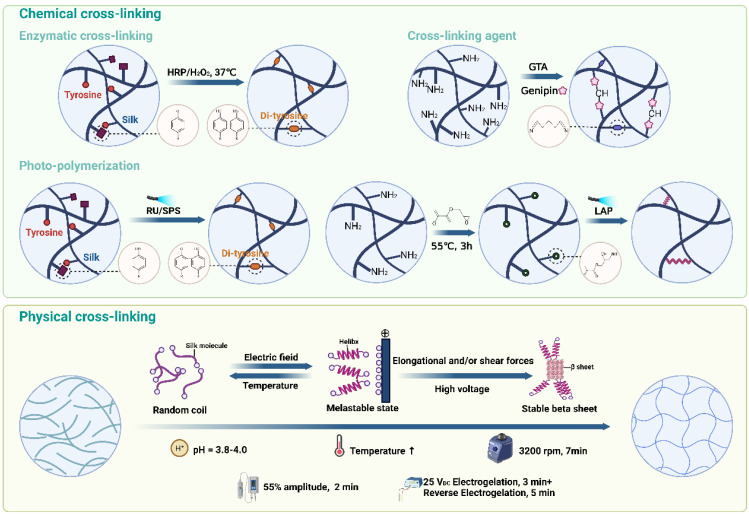
Schematic representation of the mechanisms and preparation methods of different cross-linking approaches for SF-based hydrogels. Created with BioRender.com.
SF 基水凝胶不同交联方法的机理和制备方法的示意图。使用 BioRender.com 创建。
3.2.1. Chemical cross-linking
3.2.1. 化学交联
Enzymatic cross-linking 酶交联
In recent years, enzyme cross-linked hydrogels have attracted wide attention in the biomedicine field. For SF-based hydrogels, enzymes facilitate the formation of intermolecular covalent bonds by activating functional groups within SF. Additionally, enzymatic cross-linking induces the formation of an ECM-like elastic structure by controlling β-sheet formation, resulting in hydrogels with stable structures, controllable mechanical properties, and non-toxic effects on cells 79. This method also supports cell encapsulation due to its cross-linking process being conducted at physiological pH and temperature 80.
近年来,酶交联水凝胶在生物医学领域引起了广泛关注。对于基于 SF 的水凝胶,酶通过激活 SF 内的官能团来促进分子间共价键的形成。此外,酶促交联通过控制 β 片的形成诱导形成类似 ECM 的弹性结构,从而产生结构稳定、机械性能可控且对细胞 79 无毒作用的水凝胶.该方法还支持细胞封装,因为它的交联过程是在生理 pH 值和温度下 80 进行的。
Among the numerous enzymatic cross-linking reactions, horseradish peroxidase (HRP) mediated enzymatic cross-linking reaction is the most commonly used 81, 82. It has the advantages of high selectivity, mild reaction conditions and no toxic components 83, 84. HRP is typically combined with H2O2 to induce SF cross-linking by oxidizing tyrosine residues into o-quinone residues. These o-quinone residues then react with phenol or aniline to form covalent bonds, leading to intermolecular or intramolecular cross-linking 85. For example, Hasturk et al. prepared SF/tyramine-substituted SF (SF-TA) composite hydrogel by using HRP and H2O2
86. The composite hydrogel exhibited adjustable mechanical properties, degradability, and excellent cytocompatibility, making it promising for cartilage defect repair due to its cell encapsulation capability. Li et al. further developed an SF-gelatin (SF-GT) hydrogel with a macroporous structure using HRP/H2O2 in combination with 3D bioprinting 87. SF-GT hydrogel had structural stability, mechanical properties and adjustable degradation rate for cartilage reconstruction. Additionally, SF-GT hydrogel could induce stem cells to synthesize Col II at a higher level and show hyaline cartilage phenotype.
在众多的酶促交联反应中,辣根过氧化物酶 (HRP) 介导的酶促交联反应是最常用的 81 82 。它具有选择性高、反应条件温和、无毒成分 83 等优点。 84 HRP 通常与 H 2 O 2 结合,通过将酪氨酸残基氧化成邻醌残基来诱导 SF 交联。然后,这些邻醌残基与苯酚或苯胺反应形成共价键,导致分子间或分子内交联 85 。例如,Hasturk 等人使用 HRP 和 H2O2 86 制备了 SF/酪胺取代的 SF (SF-TA) 复合水凝胶。复合水凝胶表现出可调节的机械性能、可降解性和出色的细胞相容性,由于其细胞封装能力,使其有望用于软骨缺损修复。Li 等人使用 HRP/H2O2 结合 3D 生物打印 87 进一步开发了一种具有大孔结构的 SF-明胶 (SF-GT) 水凝胶。SF-GT 水凝胶具有结构稳定性、力学性能和可调节的软骨重建降解速率。此外,SF-GT 水凝胶可以诱导干细胞在更高水平上合成 Col II,并显示透明软骨表型。
Photo-polymerization 光聚合
Photo-polymerization is a widely used chemical cross-linking method that utilizes a photo-initiator and light (ultraviolet, visible, or gamma rays) to control the cross-linking process 88. During photo-polymerization, the photo-initiator absorbs light energy and cleaves to produce free radicals, which subsequently react with unsaturated bonds in SF to induce cross-linking. The primary advantage of photo-polymerization is its extremely rapid cross-linking rate 89, 90. For instance, Cui et al. successfully cross-linked SF within 1 minute using tris(2,2-bipyridyl)dichlororuthenium(II) hexahydrate and sodium persulfate as photo-initiators 91. The SF-based hydrogels had stable mechanical properties and supported the long-term culture of human articular chondrocytes and cartilage tissue regeneration. In addition, there is a special photo-polymerization method using high-intensity gamma-ray without adding photo-initiators. This method can completely remove the toxic effects caused by photo-initiators residues 92. For example, Kim et al. prepared chemically cross-linked SF hydrogels by using Co-60 derived gamma-ray (SF C-gel) 93. They found that SF C-gel was biocompatible and could promote the attachment and proliferation of hMSCs.
光聚合是一种广泛使用的化学交联方法,它利用光引发剂和光(紫外线、可见光或伽马射线)来控制交联过程 88 。在光聚合过程中,光引发剂吸收光能并裂解产生自由基,自由基随后与 SF 中的不饱和键反应以诱导交联。光聚合的主要优点是其极快的交联速率 89 。 90 例如,Cui 等人使用三(2,2-联吡啶基)二氯钌 (II) 六水合物和过硫酸钠作为光引发剂,在 1 分钟内成功交联了 SF 91 。基于 SF 的水凝胶具有稳定的机械性能,并支持人关节软骨细胞的长期培养和软骨组织再生。此外,还有一种特殊的光聚合方法,使用高强度伽马射线,无需添加光引发剂。这种方法可以完全去除光引发剂残留物引起的毒性作用 92 。例如,Kim 等人使用 Co-60 衍生的伽马射线 (SF C 凝胶) 93 制备了化学交联的 SF 水凝胶。他们发现 SF C-gel 具有生物相容性,可以促进 hMSC 的附着和增殖。
Cross-linking agents 交联剂
Cross-linking agent molecules can accelerate SF cross-linking by reacting with reactive groups such as -OH, -NH2 and -COOH in SF 94. Compared to enzymes and photo-initiators, cross-linking agents are more cost-effective and can improve the mechanical properties of hydrogels 59. Glutaraldehyde (GTA) is the most widely used cross-linking agent, which can promote SF cross-linking by reacting with the phenolic group of tyrosine. For instance, Srisawasdi et al. prepared polycarbazole/SF (SF/PCZ) hydrogels with glutaraldehyde as cross-linking agent 95. They found that SF/PCZ hydrogel had good dielectric properties and excellent toughness. However, the biotoxicity of GTA limits its applications in tissue engineering and medicine. In contrast, genipin is a promising natural small molecule cross-linking agent due to its excellent biocompatibility. Considering this, Min et al. designed chitosan/SF hydrogels loaded with kartogenin (KGN) and platelet-derived growth factor BB (PDGF-BB) by using genipin as cross-linking agent 96. The hydrogels allowed for the sustained release of KGN and PDGF-BB, supporting the growth of seed chondrocytes and maintaining their phenotype, demonstrating potential in cartilage tissue engineering.
交联剂分子通过与 SF 中的反应性基团如 -OH、-NH2 和 -COOH 反应来加速 SF 交联 94 。与酶和光引发剂相比,交联剂更具成本效益,并且可以改善水凝胶的机械性能 59 。戊二醛 (GTA) 是使用最广泛的交联剂,它可以通过与酪氨酸的酚基反应来促进 SF 交联。例如,Srisawasdi 等人用戊二醛作为交联剂 95 制备了聚咔唑/SF (SF/PCZ) 水凝胶。他们发现 SF/PCZ 水凝胶具有良好的介电性能和优异的韧性。然而,GTA 的生物毒性限制了其在组织工程和医学中的应用。相比之下,genipin 由于其优异的生物相容性,是一种很有前途的天然小分子交联剂。考虑到这一点,Min 等人使用京尼平作为交联剂 96 ,设计了载有 kartogenin (KGN) 和血小板衍生生长因子 BB (PDGF-BB) 的壳聚糖/SF 水凝胶。水凝胶允许 KGN 和 PDGF-BB 的持续释放,支持种子软骨细胞的生长并维持其表型,在软骨组织工程中显示出潜力。
3.2.2. Physical cross-linking
3.2.2. 物理交联
Temperature 温度
Temperature significantly affects the cross-linking of proteins, including SF 97. Increasing the temperature can promote SF cross-linking by enhancing the Brownian motion of SF molecules and increasing the effective collision rate between them. Additionally, elevated temperatures can disrupt the free energy state of SF molecules, exposing internal hydrophobic regions and facilitating the transition from random coil to β-sheet structures, thereby enhancing hydrophobic interactions and accelerating cross-linking 98. For instance, Kim et al. researched the effect of cross-linking temperature for SF hydrogels 99. They found that the cross-linking rate and compressive modulus of SF hydrogels increased with increasing cross-linking temperature within a certain range.
温度显着影响蛋白质的交联,包括 SF 97 。提高温度可以通过增强 SF 分子的布朗运动并提高它们之间的有效碰撞率来促进 SF 交联。此外,高温会破坏 SF 分子的自由能状态,暴露内部疏水区域并促进从无规卷曲过渡到β片结构,从而增强疏水相互作用并加速交联 98 。例如,Kim 等人研究了交联温度对 SF 水凝胶的影响 99 。他们发现,在一定范围内,SF 水凝胶的交联速率和压缩模量随着交联温度的升高而增加。
pH 酸碱度
In addition to temperature, the pH of the SF solution is also a critical factor in SF cross-linking. When the pH of the solution approaches the isoelectric point of SF (pH = 3.8-4.0), the electrostatic repulsion between SF molecules is minimized, making the molecules more prone to aggregation and cross-linking 94. In this condition, the SF molecules are unstable and prone to aggregation and cross-linking. Therefore, adjusting the pH value of the solution is an effective method to induce SF cross-linking. For example, Nagarkar et al. investigated SF cross-linking by changing pH via adding HCl 100. They found that adjusting the solution pH from 8 to 2 could prepare weak SF hydrogels. Additionally, Fini et al. designed and developed SF-based hydrogel though regulating pH via adding citric acid to SF solution 101. They found that the hydrogel had good mechanical properties and excellent cytocompatibility. Additionally, in vivo experiments demonstrated that the hydrogel showed non-inflammatory response after implantation and stimulated cells to produce TGF-β1 to induce tissue regeneration.
除了温度,SF 溶液的 pH 值也是 SF 交联的关键因素。当溶液的 pH 值接近 SF 的等电点(pH = 3.8-4.0)时,SF 分子之间的静电排斥最小,使分子更容易聚集和交联 94 。在这种情况下,SF 分子不稳定,容易聚集和交联。因此,调节溶液的 pH 值是诱导 SF 交联的有效方法。例如,Nagarkar 等人通过添加 HCl 100 来改变 pH 值,从而研究了 SF 交联。他们发现,将溶液 pH 值从 8 调整到 2 可以制备弱 SF 水凝胶。此外,Fini 等人设计并开发了基于 SF 的水凝胶,通过向 SF 溶液 101 中添加柠檬酸来调节 pH 值。他们发现水凝胶具有良好的机械性能和出色的细胞相容性。此外,体内实验表明,水凝胶在植入后表现出非炎症反应,并刺激细胞产生 TGF-β1 以诱导组织再生。
Shear force 剪切力
The shear force cross-linking method typically involves high-speed vortex treatment on SF solution 102. High-speed vortex treatment accelerates β-sheet generation by stretching SF molecules and changing their orientation to promote SF cross-linking 103. Moreover, this method can also be used to prepare SF-based hydrogels with directional structures. For example, Chen et al. fabricated SF/sodium surfactin hydrogels with directional three-dimensional structure by vortex treatment 104. The hydrogel could accelerate 3D directed tissue regeneration due to its excellent anisotropy. Moreover, Kasoju et al. fabricated SF hydrogel with good mechanical properties by combining methanol treatment and vortex treatment 105. These hydrogels demonstrated effective cell encapsulation and controlled drug release capabilities.
剪切力交联法通常涉及对 SF 溶液 102 进行高速涡流处理。高速涡旋处理通过拉伸 SF 分子并改变其方向以促进 SF 交联 103 来加速β片的生成。此外,该方法还可用于制备具有定向结构的 SF 基水凝胶。例如,Chen 等人通过涡旋处理 104 制备了具有定向三维结构的 SF/钠表面活性素水凝胶。由于其优异的各向异性,水凝胶可以加速 3D 定向组织再生。此外,Kasoju 等人通过结合甲醇处理和涡旋处理 105 制备了具有良好机械性能的 SF 水凝胶。这些水凝胶表现出有效的细胞封装和受控的药物释放能力。
Ultrasonication 超声检查
Ultrasonication is a physical cross-linking method commonly used to prepare SF hydrogels. The effect of this method is similar to the anterior silk gland of silkworm, which promotes SF cross-linking through local temperature increase and shear force 106. This method is highly stable and controllable, as it allows for adjustments in output energy and duration. Importantly, it effectively avoids the toxicity issues associated with additives like photo-initiators and cross-linking agents 107. For example, Byram et al. designed and developed SF/xanthan gum hydrogels by ultrasonication 108. Additionally, due to their cartilage ECM-like microstructure, these hydrogels showed potential for applications in cartilage tissue engineering.
超声处理是常用于制备 SF 水凝胶的一种物理交联方法。这种方法的效果类似于蚕的前丝腺,通过局部温度升高和剪切力 106 促进SF交联。这种方法非常稳定和可控,因为它允许调整输出能量和持续时间。重要的是,它有效地避免了与光引发剂和交联剂 107 等添加剂相关的毒性问题。例如,Byram 等人通过超声设计并开发了 SF/黄原胶水凝胶 108 。此外,由于其软骨 ECM 样微观结构,这些水凝胶在软骨组织工程中显示出应用潜力。
Electric field 电场
As we mentioned before, SF is negatively charged in neutral solutions due to its isoelectric point (pH=3.8-4.0). Under an electric field, SF molecules aggregate near the anode, forming micelles that subsequently assemble into hydrogels through physical entanglement of molecular chains 109. For instance, Liu et al. constructed SF electrogels with excellent mechanical properties via a low-voltage electric field 110. They found that the hydrogel had excellent biocompatibility and drug loading capacity. In addition, gradient structure hydrogels which are more suitable for cartilage repair can be prepared by the electric field. Consider this, Xu et al. developed multi-functional beta-sheet rich silk nanofibers (BSNF) hydrogels with adjustable gradient mechanical strength and structure in electric field 111. BSNF hydrogel could regulate BMSCs to differentiate into chondrocytes to promote cartilage repair due to its gradients structure.
正如我们之前提到的,SF 由于其等电点 (pH=3.8-4.0) 而在中性溶液中带负电。在电场下,SF 分子在阳极附近聚集,形成胶束,随后通过分子链 109 的物理纠缠组装成水凝胶。例如,Liu 等人通过低压电场 110 构建了具有优异机械性能的 SF 电凝胶。他们发现水凝胶具有出色的生物相容性和载药能力。此外,可以通过电场制备更适合软骨修复的梯度结构水凝胶。考虑到这一点,Xu 等人开发了多功能富含 β 折叠的蚕丝纳米纤维 (BSNF) 水凝胶,在电场 111 中具有可调节的梯度机械强度和结构。BSNF 水凝胶由于其梯度结构,可以调节 BMSCs 分化为软骨细胞以促进软骨修复。
3.3. Functional modifications
3.3. 功能修改
As we mentioned in 3.1. Characteristics of silk fibroin, SF has excellent biocompatibility, biodegradability and mechanical properties. However, after various physical and chemical treatments, the molecular structure of SF is destroyed, which leads to the unsatisfactory mechanical strength of pure SF hydrogel. In addition, pure SF hydrogel has certain disadvantages, such as insufficient water retention, poor antibacterial properties and unsatisfactory cartilage repair properties 138. In order to improve SF hydrogel, it is an effective strategy to prepare composite SF hydrogel by mixing some functional materials into SF solution 139. These functional materials can be divided into synthetic materials and natural materials (Table 3).
正如我们在 3.1 中提到的。丝素蛋白的特点,SF 具有优异的生物相容性、生物降解性和机械性能。然而,经过各种物理和化学处理后,SF 的分子结构被破坏,导致纯 SF 水凝胶的机械强度不理想。此外,纯SF水凝胶存在一定的缺点,如保水性不足、抗菌性能差、软骨修复性能 138 不理想等。为了改善SF水凝胶,将一些功能材料混合到SF溶液 139 中制备复合SF水凝胶是一种有效的策略。这些功能材料可分为合成材料和天然材料(表 3 )。
Table 3. 表 3.
The functional materials that have been used in combination with SF to prepare composite hydrogels for cartilage repair.
与 SF 联合用于制备用于软骨修复的复合水凝胶的功能材料。
| Type 类型 | Functional materials 功能性材料 | Preparation method 制备方法 | Advantages of composite hydrogels in cartilage repair 复合水凝胶在软骨修复中的优势 |
References 引用 |
|---|---|---|---|---|
| Synthetic 合成 | GO | Photo-polymerization with RuBPY as photo-initiators 以 RuBPY 作为光引发剂的光聚合 |
Excellent mechanical properties 优异的机械性能 |
112, 113 |
| PEG | Ultrasonication 超声检查 | Injectable; rapid gelation; suitable microenvironment 注射;快速凝胶化;适宜的微环境 |
114 | |
| PEGDMA | UV photo-polymerization UV 光聚合 | Adjustable mechanical properties 可调节的机械性能 |
115 | |
| PEGDA | Photo-polymerization with LAP as photo-initiators 以 LAP 为光引发剂的光聚合反应 |
Excellent mechanical properties; bioprinting-compatible 优良的机械性能;兼容 BioPrinting |
116 | |
| poly(N-vinylcaprolactam) 聚(N-乙烯基己内酰胺) | Photo-polymerization with tris(2,2-bipyridyl)dichlororuthenium(II) and ammonium peroxodisulfate as photo-initiators 以三(2,2-联吡啶基)二氯钌 (II) 和过氧化二硫酸铵为光引发剂进行光聚合 |
Enhanced water uptake capacity, elasticity and toughness 增强的吸水能力、弹性和韧性 |
117 | |
| PVA | pH adjustment pH 值调节 | Excellent mechanical properties; high porosity; adjustable swelling ratio 优良的机械性能;高孔隙率;可调节的溶胀率 |
118 | |
| POL | Enzyme cross-linking catalyzed by HRP and H2O2 HRP 和 H2O2 催化的酶交联 |
Thermosensitive; injectable 热 敏;注射 |
119, 120 | |
| MXene | Enzyme cross-linking catalyzed by HRP and H2O2; HRP 和 H2O 2 催化的酶交联; Ultrasound technique 超声技术 |
Metallic conductivity; piezoelectricity; excellent hydrophilicity; diverse surface chemical properties; injectable 金属导电性;压电;优良的亲水性;多种表面化学性质;注射 |
121, 122 | |
| Natural 自然的 | Collagen 胶原 | Ultrasonication 超声检查 | Promotes MSC proliferation; chondrogenic differentiation 促进 MSC 增殖;软骨形成分化 |
123, 124 |
| Gelatin 明胶 | Enzyme cross-linking catalyzed by transglutaminase 转谷氨酰胺酶催化的酶交联 |
Cell attachment, proliferation; excellent mechanical integrity 细胞附着、增殖;出色的机械完整性 |
125, 126 | |
| HPMC | Heating 加热 | Excellent mechanical properties 优异的机械性能 |
127 | |
| CS | Enzyme cross-linking catalyzed by HRP and H2O2 HRP 和 H2O2 催化的酶交联 |
Environmentally sensitive; controlled release of drugs and growth factors 对环境敏感;药物和生长因子的控释 |
128-131 | |
| HA | Enzyme cross-linking catalyzed by HRP and H2O2 HRP 和 H2O2 催化的酶交联 |
MSC recruitment; cell adhesion; cartilage-like mechanical properties MSC 招聘;细胞粘附;类似软骨的机械性能 |
132-135 | |
| GA | Photo-polymerization 光聚合 | Reduced mechanical stress; lower in vivo friction coefficients 减少机械应力;更低的体内摩擦系数 |
136 | |
| DNA | Enzyme cross-linking catalyzed by HRP and H2O2 and complementary base pairing HRP 和 H2O2 催化的酶交联以及互补碱基配对 |
Regulate chondrogenic differentiation of BMSCs 调节 BMSCs 的软骨生成分化 |
137 |
3.3.1. Synthetic material modification
3.3.1. 合成材料改性
Graphene oxide is a kind of functional carbon allotrope. Recently, it has attracted increasing attention in the field of materials due to its excellent mechanical strength, attractive surface volume ratio, high water solubility, easy solution processing and chemical functionality 140. The incorporation of GO into SF solutions can significantly enhance the mechanical properties of SF-based hydrogels. For example, Wang et al. developed nano-hydroxyapatite-GO/SF hydrogels using click chemistry, resulting in enhanced mechanical strength compared to pure SF hydrogels, with a compressive modulus of 95.4 ± 2.0 kPa 112. Furthermore, the addition of GO can improve the toughness of SF hydrogels. Balu et al. fabricated regenerated SF (RSF)/GO nanocomposite hydrogels using RuBPY as a photo-initiator, achieving mechanical properties superior to natural cartilage, with a Young's modulus of 8 MPa and tensile toughness of 2.4 MJ/m3
113.
氧化石墨烯是一种功能性碳同素异形体。近年来,由于其优异的机械强度、有吸引力的表面体积比、高水溶性、易于固溶处理和化学功能 140 ,它在材料领域引起了越来越多的关注。将 GO 掺入 SF 溶液中可以显著提高 SF 基水凝胶的机械性能。例如,Wang 等人使用点击化学开发了纳米羟基磷灰石-GO/SF 水凝胶,与纯 SF 水凝胶相比,机械强度更高,压缩模量为 95.4 ± 2.0 kPa 112 。此外,GO 的添加可以提高 SF 水凝胶的韧性。Balu 等人使用 RuBPY 作为光引发剂制造了再生 SF (RSF)/GO 纳米复合水凝胶,实现了优于天然软骨的机械性能,杨氏模量为 8 MPa,拉伸韧性为 2.4 MJ/m3 113 。
Synthetic polymers are also commonly used to prepare composite hydrogels. Among them, polyethylene glycol (PEG) has received wide attention due to its versatility in molecular weight, topology (linear, branched, star-shaped) 139, 141. Interestingly, the incorporation of PEG can endow the SF composite hydrogel with the injectable property. For example, Zhang et al. prepared injectable BMSC-encapsulated dual-network SF-PEG composite hydrogels via ultrasonication, resulting in hydrogels with a high cross-linking rate, excellent biocompatibility, and strong mechanical strength. Additionally, these dual-network SF-PEG hydrogels promoted cartilage repair by enhancing BMSC chondrogenic differentiation 114. Furthermore, derivatives of PEG can also strengthen the cartilage repair ability of SF hydrogels. For instance, Achachelouei et al. fabricated SF/poly(ethylene glycol) dimethacrylate (PEGDMA) hydrogels with adjustable mechanical properties by photo-polymerization 115. They found that the compression modulus of SF/PEGDMA hydrogel was related to the ratio of SF to PEGDMA. Meanwhile, Bandyopadhyay et al. constructed silk methacrylate (SilMA)-PEG diacrylate hydrogel with excellent mechanical properties and adjustable degradability via photo-polymerization 116. Importantly, this hydrogel was suitable for chondrocytes-laden 3D biological printing to accelerate cartilage repair.
合成聚合物也常用于制备复合水凝胶。其中,聚乙二醇 (PEG) 因其在分子量、拓扑结构(线性、支链、星形) 139 等方面的多功能性而受到广泛关注。 141 有趣的是,PEG 的掺入可以赋予 SF 复合水凝胶可注射的特性。例如,Zhang 等人通过超声处理制备了可注射的 BMSC 封装的双网络 SF-PEG 复合水凝胶,得到的水凝胶具有较高的交联速率、优异的生物相容性和较强的机械强度。此外,这些双网络 SF-PEG 水凝胶通过增强 BMSC 软骨形成分 114 化来促进软骨修复。此外,PEG 的衍生物还可以增强 SF 水凝胶的软骨修复能力。例如,Achachelouei 等人通过光聚合制备了具有可调节机械性能的 SF/聚乙二醇二甲基丙烯酸酯 (PEGDMA) 水凝胶 115 。他们发现 SF/PEGDMA 水凝胶的压缩模量与 SF 与 PEDMA 的比率有关。同时,Bandyopadhyay 等人通过光聚合构建了具有优异机械性能和可调节降解性的甲基丙烯酸丝 (SilMA)-PEG 二丙烯酸酯水凝胶 116 。重要的是,这种水凝胶适用于载有软骨细胞的 3D 生物打印,以加速软骨修复。
Apart from PEG, the performance of SF hydrogels can be enhanced by mixing with poly(N-vinylcaprolactam), poly vinyl alcohol, poloxamer 142. For instance, Whittaker et al. constructed RSF-poly(N-vinylcaprolactam) double network (DN) hydrogel by a rapid one-pot method 117. Compared with SF hydrogels, poly(N-vinylcaprolactam) enhanced elasticity and toughness of hybrid hydrogels. Furthermore, Subramanian et al. developed Mo3Se3-PVA-SF nanowire hydrogel with remarkable mechanical properties by using glutaraldehyde as cross-linking agent 118. They found the composite could stimulate the expression of collagen to accelerate tissue repair. It is worth noting that POL can impart injectability to SF hydrogel 120. For example, Min et al. constructed an injectable alginate-poloxamer (ALG-POL)/SF hydrogel though using HRP and H2O2
119. The injectability of this hydrogel was attributed to its solution-gel transition properties at physiological temperature. In addition, ALG-POL/SF hydrogel accelerated cartilage regeneration by promoting chondrocyte proliferation.
除 PEG 外,SF 水凝胶的性能还可以通过与聚(N-乙烯基己内酰胺)、聚乙烯醇、泊洛沙姆 142 混合来提高。例如,Whittaker 等人通过快速一罐法 117 构建了 RSF-聚(N-乙烯基己内酰胺)双网络 (DN) 水凝胶。与 SF 水凝胶相比,聚 (N-乙烯基己内酰胺) 增强了杂化水凝胶的弹性和韧性。此外,Subramanian 等人通过使用戊二醛作为交联剂 118 开发了具有显着机械性能的 Mo3Se 3-PVA-SF 纳米线水凝胶。他们发现这种复合物可以刺激胶原蛋白的表达以加速组织修复。值得注意的是,POL 可以赋予 SF 水凝胶 120 注射性。例如,Min 等人使用 HRP 和 H2O2 119 构建了一种可注射的藻酸盐-泊洛沙姆 (ALG-POL)/SF 水凝胶。这种水凝胶的可注射性归因于其在生理温度下的溶液-凝胶过渡特性。此外,ALG-POL/SF 水凝胶通过促进软骨细胞增殖来加速软骨再生。
MXenes, as novel 2D nanomaterials composed of transition metal complexes, had been applied in biomedicine due to their metallic conductivity, piezoelectricity, excellent hydrophilicity, and diverse surface chemical properties. Jiang et al. utilized enzyme crosslinking to combine MXene nanosheets with SF, forming a piezoresistive nanocomposite hydrogel that facilitated bone tissue regeneration by restoring the electrical microenvironment 121. Additionally, Yang et al. developed an injectable SF/MXene conductive hydrogel developed through ultrasound techniques, which acted as a stem cell carrier and enabled in vivo electrical stimulation for repairing brain tissue damage 122.
MXenes 作为由过渡金属配合物组成的新型二维纳米材料,由于其金属导电性、压电性、优异的亲水性和多样化的表面化学性质而已应用于生物医学。江等人利用酶交联将MXene纳米片与SF结合,形成压阻纳米复合水凝胶,通过恢复电微环境 121 促进骨组织再生。此外,Yang 等人开发了一种通过超声技术开发的可注射 SF/MXene 导电水凝胶,它充当干细胞载体,能够在体内电刺激以修复脑组织损伤 122 。
3.3.2. Natural material modification
3.3.2. 天然材料改性
The mixing of synthetic materials in SF hydrogels may introduce cytotoxicity. Conversely, the combination of SF with natural materials can avoid the toxicity problems associated with synthetic materials 143. Currently, composite hydrogels have been prepared by combining with GT, collagen, cellulose or HA 144, 145.
合成材料在 SF 水凝胶中的混合可能会引入细胞毒性。相反,SF 与天然材料的结合可以避免与合成材料 143 相关的毒性问题。目前,已经通过与 GT、胶原蛋白、纤维素或 HA 144 、 结合制备了复合水凝胶。 145
Collagen (Col) is the most abundant natural polymer in ECM with remarkable biocompatibility, negligible immunogenicity and strong biological activity. Notably, collagen can enhance cartilage regeneration effects as it promotes the attachment and chondrogenic differentiation of MSCs 123. For example, Zhang et al. developed an injectable BMSC-laden collagen-PEG/SF DN hydrogel via ultrasonication, which exhibited enhanced mechanical strength and cytocompatibility, ultimately accelerating cartilage regeneration 124.
胶原蛋白 (Col) 是 ECM 中最丰富的天然聚合物,具有显著的生物相容性、可忽略不计的免疫原性和强大的生物活性。值得注意的是,胶原蛋白可以增强软骨再生作用,因为它促进 MSC 的附着和软骨形成分化 123 。例如,Zhang 等人通过超声开发了一种可注射的载有 BMSC 的胶原蛋白-PEG/SF DN 水凝胶,它表现出增强的机械强度和细胞相容性,最终加速软骨再生 124 。
GT is a mixture of large polypeptides, denatured from collagen. GT is a promising material in tissue engineering attributed to its high swelling and thermal inversion properties 125. In addition, GT can form an interpenetrating network (IPN) with SF to enhance the compressive moduli of SF-based hydrogel. Park et al. fabricated GT-SF IPN hydrogels through microbial transglutaminase-induced cross-linking, resulting in biodegradable, non-cytotoxic hydrogels with superior mechanical properties compared to individual GT or SF hydrogels. The resulting composite hydrogel also promoted cell adhesion and proliferation 126.
GT 是由胶原蛋白变性的大多肽混合物。GT 是一种很有前途的组织工程材料,因为它具有高溶胀和热反转特性 125 。此外,GT 可以与 SF 形成互穿网络 (IPN),以增强 SF 基水凝胶的压缩模量。Park 等人通过微生物转谷氨酰胺酶诱导的交联制备了 GT-SF IPN 水凝胶,与单个 GT 或 SF 水凝胶相比,产生了可生物降解、无细胞毒性的水凝胶,具有优异的机械性能。所得的复合水凝胶还促进了细胞粘附和增殖 126 。
Cellulose is a polysaccharide biomolecular material available in various sources 146-148. Noticeably, cellulose can be modified in several ways to generate cellulose derivatives 149. These cellulose derivatives strengthen the compressive modulus of SF-based hydrogels by inducing the generation of β-sheets. For instance, Luo et al. fabricated RSF/hydroxypropyl methyl cellulose (HPMC) hydrogel with excellent mechanical properties 127. The maximum compressive and tensile modulus of this hydrogel exceeded 1.0 MPa.
纤维素是一种多糖生物分子材料,有多种来源 146 - 148 。值得注意的是,纤维素可以通过多种方式进行改性以生成纤维素衍生物 149 。这些纤维素衍生物通过诱导 β 片的产生来增强 SF 基水凝胶的压缩模量。例如,Luo 等人制造了具有优异机械性能 127 的 RSF/羟丙基甲基纤维素 (HPMC) 水凝胶。这种水凝胶的最大压缩模量和拉伸模量超过 1.0 MPa。
Chitosan (CS) is a kind of polysaccharide biopolymer, which has an inherent linear structure, excellent biocompatibility and environmental responsiveness. It has been proved that SF hydrogels can be endowed with environmental responsiveness by combining with CS 128. For example, Xu et al. developed pH responsiveness SF/CS hydrogel by chemical cross-linking 129. Meanwhile, Yu et al. prepared CS/SF/amino-functionalized mesoporous silica hydrogel through using genipin as cross-linking agent 130. They found that the excellent injectability of the composite hydrogel was due to its sensitive thermal responsiveness at physiological pH. Additionally, CS-SF composite hydrogels can achieve controlled drug release. Dong et al. prepared a p-hydroxybenzene propanoic acid-modified chitosan (PC)/SF hydrogel using an HRP-mediated enzymatic cross-linking reaction, which sequentially released bioactive molecules to induce MSC homing and chondrogenic differentiation 131.
壳聚糖 (CS) 是一种多糖生物聚合物,具有固有的线性结构、优异的生物相容性和环境响应性。已经证明,SF 水凝胶可以通过与 CS 结合来赋予环境响应性 128 。例如,Xu 等人通过化学交联 129 开发了 pH 响应性 SF/CS 水凝胶。同时,Yu 等人以 genipin 为交联剂 130 制备了 CS/SF/氨基功能化介孔二氧化硅水凝胶。他们发现,复合水凝胶出色的注射性能是由于它在生理 pH 值下敏感的热响应性。此外,CS-SF 复合水凝胶可以实现药物的控释。Dong 等人使用 HRP 介导的酶促交联反应制备了对羟基苯丙酸改性壳聚糖 (PC)/SF 水凝胶,该反应依次释放生物活性分子以诱导 MSC 归巢和软骨形成分 131 化。
HA is a common biodegradable, non-immunogenic and non-inflammatory polysaccharide in the human body. It has been reported that mixing HA can enhance the storage modulus and compressive modulus of SF hydrogels 132, 150. For example, Ziadlou et al. designed and developed HA-TA /SF composite hydrogels via enzymatic cross-linking 133. Compared with pure SF hydrogel, they found this hybrid hydrogel had enhanced storage modulus and drug releasing ability to stimulate cartilage regeneration, remarkable mechanical properties and drug release ability. Moreover, Wang et al. also fabricated an SF-HA composite hydrogel scaffold by combining SF/HA-Tyr hydrogel with SF sponge, finding that this biomaterial accelerated cartilage repair due to its excellent mechanical properties and MSC recruitment ability 134. Fan et al. compounded SF with aldehyde-HA to create a dynamic network that delayed or interrupted the β-sheet-induced hardening of SF chains, producing a hydrogel matrix with mechanical properties similar to biological tissues 135.
HA 是人体内常见的一种可生物降解、非免疫原性和非炎症性的多糖。据报道,混合 HA 可以提高 SF 水凝胶 132 的储能模量和压缩模量。 150 例如,Ziadlou 等人通过酶交联设计并开发了 HA-TA /SF 复合水凝胶 133 。与纯 SF 水凝胶相比,他们发现这种杂化水凝胶具有增强的储能模量和刺激软骨再生的药物释放能力、显著的机械性能和药物释放能力。此外,Wang 等人还通过将 SF/HA-Tyr 水凝胶与 SF 海绵相结合制造了 SF-HA 复合水凝胶支架,发现这种生物材料由于其优异的机械性能和 MSC 募集能力 134 而加速了软骨修复。Fan 等人将 SF 与醛-HA 复合,以创建一个动态网络,延迟或中断 β 片诱导的 SF 链硬化,产生具有类似于生物组织机械性能的水凝胶基质 135 。
Glycyrrhizic acid (GA), a natural compound from licorice roots, effectively inhibits intrinsic inflammation and, when combined with SF, modifies the mechanical properties of materials to reduce mechanical stress and lower friction coefficients in vivo after implantation. Zhang et al. developed a nanocomposite hydrogel for spinal cord repair by creating an interpenetrating polymer network with self-assembled GA as the first network and photo-crosslinked SilMA as the second network 136.
甘草酸 (GA) 是一种来自甘草根的天然化合物,可有效抑制内源性炎症,当与 SF 结合时,可改变材料的机械性能,以减少植入后体内的机械应力和降低摩擦系数。Zhang 等人通过创建一个互穿聚合物网络,以自组装的 GA 作为第一个网络,光交联 SilMA 作为第二个网络 136 ,开发了一种用于脊髓修复的纳米复合水凝胶。
Deoxyribonucleic acid (DNA) is a natural macromolecular material, and hydrogels prepared using DNA exhibit unique programmability, good injectability, controllable mechanical properties, and are adaptable for 3D cell printing 17. It has been demonstrated that DNA hydrogels have significant potential in protecting seed cells, particularly in the field of cartilage repair 151. Recently, our team introduced DNA into SF hydrogels, imparting them with controllable surface rigidity to regulate the chondrogenic differentiation of BMSCs, thereby achieving a breakthrough in cartilage repair 137. Details will be discussed in 4.3. Acceptable mechanical properties.
脱氧核糖核酸 (DNA) 是一种天然的大分子材料,使用 DNA 制备的水凝胶表现出独特的可编程性、良好的注射性、可控的机械性能,适用于3D细胞打印 17 。已经证明 DNA 水凝胶在保护种子细胞方面具有巨大潜力,尤其是在软骨修复 151 领域。最近,我们团队将 DNA 引入 SF 水凝胶中,赋予它们可控的表面刚性,以调节 BMSCs 的软骨生成分化,从而在软骨修复 137 方面取得突破。细节将在 4.3 中讨论。可接受的机械性能。
4. Advantages of silk fibroin-based hydrogels in the construction of cartilage organoids
4. 基于丝素蛋白的水凝胶在构建软骨类器官中的优势
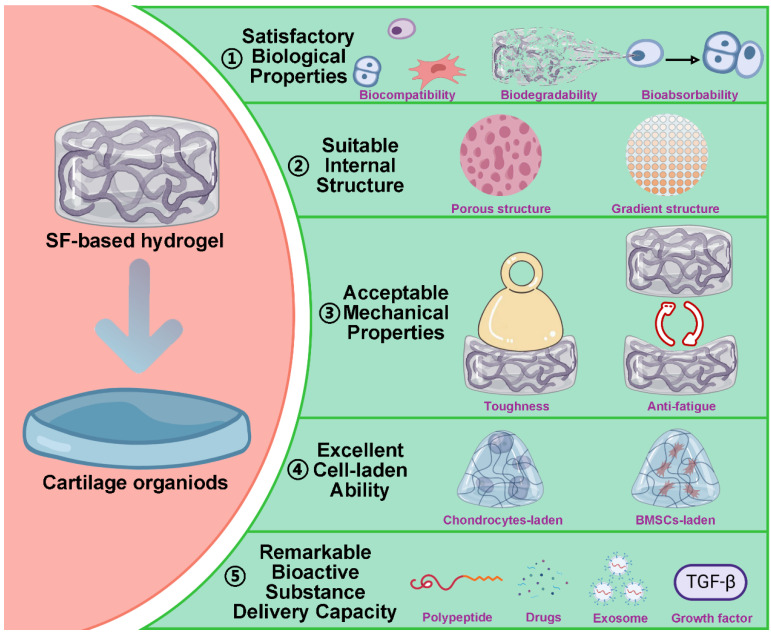
Based on multiple cross-linking and modifications strategy, SF-based hydrogels have great potential in promoting cartilage regeneration 152. Meanwhile, it also demonstrates that SF-based hydrogels have certain advantages in constructing cartilage organoids, including: satisfactory biological properties, suitable internal structure, acceptable mechanical properties, excellent cell-laden ability and remarkable bioactive substance delivery capacity (Figure 8).
基于多种交联和修饰策略,基于 SF 的水凝胶在促进软骨再生 152 方面具有巨大潜力。同时,它还表明基于 SF 的水凝胶在构建软骨类器官方面具有一定的优势,包括:令人满意的生物学特性、合适的内部结构、可接受的机械性能、优异的细胞负载能力和显着的生物活性物质递送能力(图 8 )。
Figure 8. 图 8.
Advantages of SF-based hydrogels in the construction of cartilage organoids: satisfactory biological properties, suitable internal structure, acceptable mechanical properties, excellent cell-laden ability and remarkable bioactive substance delivery capacity. Created with BioRender.com.
SF 基水凝胶在构建软骨类器官中的优势:令人满意的生物学特性、合适的内部结构、可接受的机械特性、优异的载细胞能力和显着的生物活性物质递送能力。使用 BioRender.com 创建。
4.1. Satisfactory biological properties
4.1. 令人满意的生物学特性
An ideal hydrogel for constructing cartilage organoids is inadmissible to inhibit the normal metabolic activity of cells, cause inflammatory responses, or induce apoptosis. For pure SF hydrogels, the problem of immunogenicity can be avoided by degumming completely. For SF-based composite hydrogels, the additional materials should have excellent biocompatibility and biodegradability. In addition, the construction of cartilage organoids is a dynamic and continuous process. Therefore, the SF hydrogel applied to the construction of cartilage organoids should have an appropriate degradation rate 153. During the construction of cartilage organoids, SF hydrogels should be degraded gradually to reserve space for newborn cartilage tissue. Furthermore, all the degradation products of SF hydrogel are non-cytotoxic and can be absorbed by cells to provide a nutritional basis for cartilage regeneration 65.
用于构建软骨类器官的理想水凝胶不能抑制细胞的正常代谢活动、引起炎症反应或诱导细胞凋亡。对于纯 SF 水凝胶,可以通过完全脱胶来避免免疫原性问题。对于 SF 基复合水凝胶,附加材料应具有优异的生物相容性和生物降解性。此外,软骨类器官的构建是一个动态且连续的过程。因此,应用于构建软骨类器官的 SF 水凝胶应具有适当的降解速率 153 。在软骨类器官的构建过程中,SF 水凝胶应逐渐降解,为新生儿软骨组织预留空间。此外,SF 水凝胶的所有降解产物均无细胞毒性,可被细胞吸收,为软骨再生 65 提供营养基础。
4.2. Suitable internal structure
4.2. 合适的内部结构
SF-based hydrogels can mimic the ECM of cartilage due to their hydrophilic three-dimensional porous structure 91. As a substitute for ECM, the porous structure of SF-based hydrogel can provide channels for the diffusion of nutrients into chondrocytes, the migration of chondrocytes and the removal of metabolic waste 87, 154, 155. It is worth noting that the extracellular matrix of cartilage is heterogeneous. From outside to inside, cartilage tissue can be divided into superficial zone, middle zone, deep zone and calcified zone. The composition and content of ECM are different in each zone 156. Therefore, the ideal SF hydrogel for constructing cartilage organoids should have a multi-level or a gradient structure. For instance, Guo et al. fabricated SF/R5 peptide composite hydrogel with gradient structure 157. The hydrogel could regulate the differentiation of BMSCs and accelerate cartilage repair.
基于 SF 的水凝胶由于其亲水性三维多孔结构 91 ,可以模拟软骨的 ECM。作为 ECM 的替代品,SF 基水凝胶的多孔结构可以为营养物质扩散到软骨细胞、软骨细胞迁移和去除代谢废物 87 提供通道。 154 155 值得注意的是,软骨的细胞外基质是异质的。软骨组织由外向内可分为浅表区、中层、深层和钙化区。ECM 的成分和内容在每个区域中 156 都不同。因此,用于构建软骨类器官的理想 SF 水凝胶应具有多级或梯度结构。例如,Guo 等人制备了具有梯度结构 157 的 SF/R5 肽复合水凝胶。水凝胶可以调节 BMSCs 的分化并加速软骨修复。
4.3. Acceptable mechanical properties
4.3. 可接受的机械性能
Cartilage, a smooth, flexible, and durable tissue, plays a supportive and protective role within the skeletal system. Therefore, the construction of cartilage organs requires biomaterials to provide sufficient mechanical support. As we mentioned in 3.3. Functional modification, mixing other materials can improve the mechanical strength of SF-based hydrogel. It is worth noting that the cartilage is in a constant cycle of compression and rebound. Meanwhile, during cartilage regeneration, chondrocytes are more active in a dynamic environment 158, 159. Hence, it is preferable to construct cartilage organoids in a dynamic environment, which requires SF hydrogels with excellent anti-fatigue ability. Consider this, Huang et al. prepared host-guest interactions SF hydrogel (HG-SF hydrogel) with high remarkable anti-fatigue ability by modifying SF with cholesterol or β-cyclodextrin 160. The hydrogel could remain in its original state after 10 times 60% stress compression without any deformation or strength decrease. Interestingly, HG-SF hydrogel could repair itself after stress injury, which showed that it had great potential to construct cartilage organoids in a dynamic environment. Additionally, our recent work found that the introduction of DNA supramolecules effectively aggregated SF molecules, inducing the formation of β-sheet structures in SF and consequently adjusting the surface rigidity of the DNA-SF hydrogels. Simultaneously, we observed that DNA-SF hydrogels with a moderate surface rigidity could induce the secretion of collagen-containing ECM by upregulating the TGF-β and Wnt signaling pathways, thereby promoting the chondrogenic differentiation of BMSCs. In vivo experimental results also demonstrated that DNA-SF hydrogels with moderate surface rigidity could expedite the cartilage repair process 137. Based on this, Su et al. integrated photo-crosslinking with self-assembly technology to develop a novel DNA-SF hydrogel microsphere using a microfluidic system. This microsphere material offers a new option for constructing and sustaining long-term culture of cartilage organoids 161.
软骨是一种光滑、灵活和耐用的组织,在骨骼系统中起着支撑和保护作用。因此,软骨器官的构建需要生物材料提供足够的机械支撑。正如我们在 3.3 中提到的。功能改性,混合其他材料可以提高SF基水凝胶的机械强度。值得注意的是,软骨处于不断的压缩和反弹循环中。同时,在软骨再生过程中,软骨细胞在动态环境中 158 更加活跃。 159 因此,最好在动态环境中构建软骨类器官,这需要具有优异抗疲劳能力的 SF 水凝胶。考虑到这一点,Huang 等人通过用胆固醇或 β-环糊精修饰 SF 制备了具有高显著抗疲劳能力的 SF 水凝胶 (HG-SF 水凝胶 160 )。水凝胶在 10 次 60% 应力压缩后仍能保持其原始状态,没有任何变形或强度降低。有趣的是,HG-SF 水凝胶可以在应力损伤后自我修复,这表明它在动态环境中构建软骨类器官具有巨大潜力。此外,我们最近的工作发现,DNA 超分子的引入有效地聚集了 SF 分子,诱导了 SF 中β片结构的形成,从而调整了 DNA-SF 水凝胶的表面刚度。同时,我们观察到具有中等表面刚性的 DNA-SF 水凝胶可以通过上调 TGF-β 和 Wnt 信号通路诱导含有胶原蛋白的 ECM 的分泌,从而促进 BMSCs 的软骨形成分化。 体内实验结果还表明,具有中等表面刚度的 DNA-SF 水凝胶可以加速软骨修复过程 137 。基于此,Su 等人将光交联与自组装技术相结合,使用微流控系统开发了一种新型 DNA-SF 水凝胶微球。这种微球材料为构建和维持软骨类器官的长期培养提供了新的选择 161 。
4.4. Excellent cell-laden ability
4.4. 出色的载细胞能力
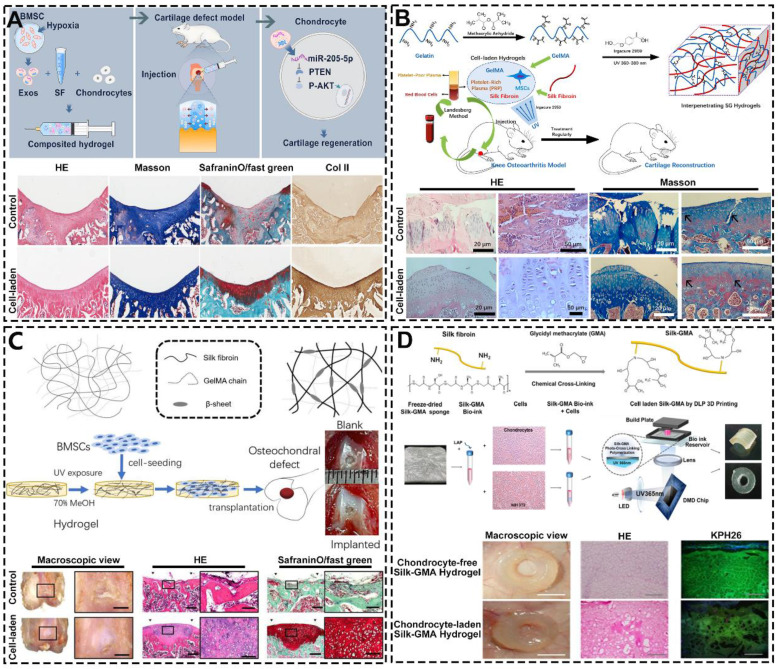
Based on its structure and chemical composition, SF-based hydrogel has excellent cell-laden ability. In the field of constructing cartilage organoids, SF-based hydrogel with excellent cell-laden ability can load chondrocytes and promote their growth and proliferation to achieve cartilage regeneration 162. For instance, Shen et al. prepared injectable SF hydrogel containing articular chondrocytes and hypoxia preconditioned exosomes by ultrasonication (Figure 9A) 163. They found this hydrogel significantly enhances the expression of cartilage marker genes in ACs. Additionally, histological analysis showed that the surface of the cartilage defect area repaired by cell-laden hydrogel was smooth and the regenerated cartilage arranged regularly. However, chondrocyte-laden SF hydrogels are difficult to rapidly construct cartilage organoids due to the poor mitotic activity of chondrocytes. To solve this problem, SF hydrogels can be loaded with stem cells that have the high proliferative capacity and chondrocyte differentiation potential 164, 165. For example, Chen et al. designed and synthesized MSCs-laden GT methacrylate/SF (GelMA/SF) hydrogel with loading platelet-rich plasma (PRP) (Figure 9B) 166. As shown by HE and Masson staining, after the intervention of MSCs-laden GelMA/SF hydrogel, the surface of articular cartilage was smooth, the chondrocytes were arranged neatly. Meanwhile, Zheng et al. constructed BMSCs-laden interpenetrating network GelMA/SF composite hydrogel through UV photo-polymerization (Figure 9C) 167. The macroscopic results, HE and SafraninO/fast green staining showed that osteochondral repair effect of cell-laden group was significantly superior than others group. It is worth noting that the hydrogel can also match 3D biological printing technology to adapt to the regeneration and repair of a variety of cartilage tissues. For instance, Hong et al. fabricated chondrocyte-laden SF-glycidyl-methacrylate (Silk-GMA) hydrogel by using 3D bioprinting technology (Figure 9D) 168. The in vivo experiments showed that the characteristics of the new cartilage tissue (cell morphology, proteoglycan and collagen distribution) of the chondrocyte-laden hydrogel group were closer to the natural cartilage than the chondrocyte-free hydrogel group.
基于其结构和化学成分,SF 基水凝胶具有优异的载细胞能力。在构建软骨类器官领域,具有优异载细胞能力的 SF 基水凝胶可以加载软骨细胞并促进其生长和增殖,以实现软骨再生 162 。例如,Shen 等人通过超声处理制备了含有关节软骨细胞和缺氧预处理外泌体的可注射 SF 水凝胶(图 9 A)。 163 他们发现这种水凝胶显着增强了 AC 中软骨标志基因的表达。此外,组织学分析显示,由载细胞水凝胶修复的软骨缺损区域表面光滑,再生软骨排列规则。然而,由于软骨细胞的有丝分裂活性差,载有软骨细胞的 SF 水凝胶难以快速构建软骨类器官。为了解决这个问题,SF 水凝胶可以加载具有高增殖能力和软骨细胞分化潜力 164 的干细胞。 165 例如,Chen 等人设计并合成了载有 MSC 的 GT 甲基丙烯酸酯/SF (GelMA/SF) 水凝胶和负载富血小板血浆 (PRP)(图 9 B)。 166 HE 和 Masson 染色显示,在载有 MSCs 的 GelMA/SF 水凝胶干预后,关节软骨表面光滑,软骨细胞排列整齐。同时,Zheng 等人通过紫外光聚合构建了载有 BMSC 的互穿网络 GelMA/SF 复合水凝胶(图 9 C)。 167 宏观结果、HE 和番红素O/快速绿染色显示,载细胞组的骨软骨修复效果显著优于其他组。值得注意的是,水凝胶还可以匹配 3D 生物打印技术,以适应多种软骨组织的再生和修复。例如,Hong 等人使用 3D 生物打印技术制造了载有软骨细胞的 SF-甲基丙烯酸缩水甘油酯 (Silk-GMA) 水凝胶(图 9 D)。 168 体内实验表明,与无软骨细胞水凝胶组相比,载软骨细胞水凝胶组的新软骨组织特征 (细胞形态、蛋白多糖和胶原蛋白分布) 更接近天然软骨。
Figure 9. 图 9.
Excellent cell-laden ability of SF-based hydrogels: (A) Injectable chondrocytes-laden SF-based hydrogel promoted cartilage regeneration via the miR-205-5p/PTEN/AKT pathway; Reproduced with permission from ref 163; Copyright 2022, Elsevier. (B) MSCs-laden GelMA/SF hydrogel with the incorporation of PRP for treating osteoarthritis to reconstruct cartilage; Reproduced with permission from ref 166; Copyright 2023, Elsevier. (C) BMSCs-laden GelMA-SF IPN hydrogel for osteochondral defect repair; Reproduced with permission from ref 167; Copyright 2023, MDPI; (D) Digital light processing 3D printed chondrocyte-laden Silk-GMA hydrogel for trachea cartilage tissue engineering; Reproduced with permission from ref 168; Copyright 2019, Elsevier.
基于 SF 的水凝胶的优异载细胞能力: (A) 可注射的载有软骨细胞的 SF 水凝胶通过 miR-205-5p/PTEN/AKT 通路促进软骨再生;经 ref 许可转载 163 ;版权所有 2022,爱思唯尔。(B) 含有 MSCs 的 GelMA/SF 水凝胶,掺入 PRP 用于治疗骨关节炎以重建软骨;经 ref 许可转载 166 ;版权所有 2023,爱思唯尔。(C) 用于骨软骨缺损修复的载 BMSCs GelMA-SF IPN 水凝胶;经 ref 许可转载 167 ;版权所有 2023,MDPI;(D) 用于气管软骨组织工程的数字光处理 3D 打印载软骨细胞 Silk-GMA 水凝胶;经 ref 许可转载 168 ;版权所有 2019,爱思唯尔。
4.5. Remarkable bioactive substance delivery capacity
4.5. 卓越的生物活性物质递送能力
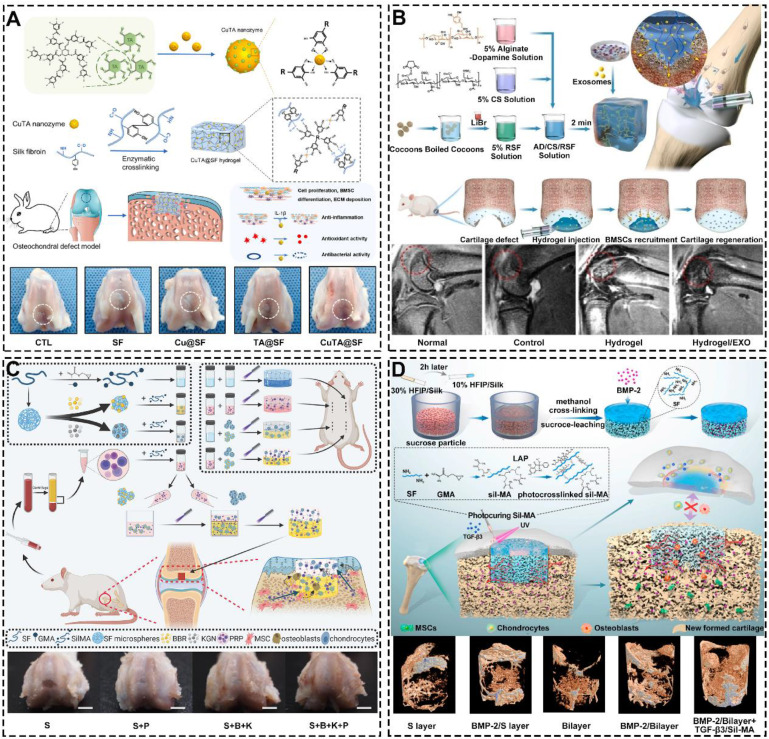
During natural organogenesis (from cells to organs), cells need to undergo multiple differentiations in different directions to form multiple cell populations that reassemble into functionally intact organs 169-172. The construction of cartilage organoids parallels this process, requiring the directed induction of cells. Due to its excellent bioactive substance delivery capability, SF-based hydrogels can regulate cell proliferation, migration, and differentiation by loading polypeptides, drugs, exosomes, or growth factors 173-175. For instance, Cao et al. developed a multifunctional silk-based hydrogel incorporated with metal-organic framework nanozymes (Figure 10A) 176. This hydrogel had the synergistic effects of antioxidation, anti-inflammation and antibacterial, which could regulate the fate of cells and accelerate the regeneration of osteochondral defects. In addition, SF hydrogels loaded with exosomes can mimic the induction of cartilage regeneration by primary cells with no immune rejection 177-179. For example, Zhang et al. fabricated alginate-dopamine/chondroitin sulfate/regenerated SF/exosome (AD/CS/RSF/EXO) hydrogel by enzymatic cross-linking (Figure 10B) 180. The hydrogel had good injectability, mechanical strength and stickiness. Besides, could induce homing and chondrogenic differentiation of BMSCs. Notably, SF hydrogels loaded with growth factors regulates the migration behavior of cells. For instance, Jiang et al. prepared bilayer SilMA hydrogel loaded with Platelet-rich plasma (PRP), SF-KGN microspheres and SF-berberine (BBR) microspheres through stratified photocuring (Figure 10C) 181. PRP enhanced the hydrogel's effect on BMSC migration and pre-differentiation, while the layered incorporation of SF-KGN and SF-BBR microspheres enabled long-term regulation of BMSC differentiation and cartilage regeneration. Meanwhile, Wu et al. constructed silk-GMA (Sil-MA) hydrogel with loading TGF-β3 through UV photo-polymerization (Figure 10D) 182. They found that the hydrogel could promote the migration and chondrogenic differentiation of BMSCs. Importantly, the hydrogel can act as a bridge to induce integration of newborn cartilage and native cartilage. Additionally, Xia et al. utilized click chemistry to coat SF microspheres with heparin disaccharide. They then used heparin disaccharide for selective adsorption of IL-4, while LOX pDNA was preloaded onto the SF microspheres through electrostatic interactions. These functionalized SF microspheres were designed to be injected into the cartilage cavity, where they promote macrophage M2 polarization, with LOX facilitating collagen crosslinking to aid in cartilage repair 183.
在自然器官发生过程中(从细胞到器官),细胞需要在不同方向上经历多次分化,以形成多个细胞群,这些细胞群重新组装成功能完整的器官 169 - 172 。软骨类器官的构建与此过程平行,需要细胞的定向诱导。由于其出色的生物活性物质递送能力,基于 SF 的水凝胶可以通过加载多肽、药物、外泌体或生长因子 173 来调节细胞增殖、迁移和分化 - 175 。例如,Cao 等人开发了一种掺入金属有机框架纳米酶的多功能丝基水凝胶(图 10 A)。 176 这种水凝胶具有抗氧化、抗炎和抗菌的协同作用,可以调节细胞的命运,加速骨软骨缺损的再生。此外,载有外泌体的 SF 水凝胶可以模拟原代细胞对软骨再生的诱导,而没有免疫排斥反应 177 - 179 。例如,Zhang 等人通过酶促交联制备了藻酸盐-多巴胺/硫酸软骨素/再生 SF/外泌体 (AD/CS/RSF/EXO) 水凝胶(图 10 B)。 180 水凝胶具有良好的注射性、机械强度和粘性。此外,可以诱导 BMSCs 的归巢和软骨形成分化。值得注意的是,负载生长因子的 SF 水凝胶调节细胞的迁移行为。例如,江 等人通过分层光固化制备了载有富血小板血浆 (PRP)、SF-KGN 微球和 SF-小檗碱 (BBR) 微球的双层 SilMA 水凝胶(图 10 C)。 181 PRP 增强了水凝胶对 BMSC 迁移和预分化的影响,而 SF-KGN 和 SF-BBR 微球的分层掺入实现了 BMSC 分化和软骨再生的长期调节。同时,Wu 等人通过紫外光聚合构建了负载 TGF-β3 的 silk-GMA (Sil-MA) 水凝胶(图 10 D)。 182 他们发现水凝胶可以促进 BMSC 的迁移和软骨形成分化。重要的是,水凝胶可以作为诱导新生软骨和天然软骨整合的桥梁。此外,Xia 等人利用点击化学用肝素二糖包被 SF 微球。然后,他们使用肝素二糖选择性吸附 IL-4,同时通过静电相互作用将 LOX pDNA 预装到 SF 微球上。这些功能化的 SF 微球被设计为注射到软骨腔中,在那里它们促进巨噬细胞 M2 极化,LOX 促进胶原蛋白交联以帮助软骨修复 183 。
Figure 10. 图 10.
Remarkable bioactive substance delivery capacity of SF-based hydrogels: (A) Silk-based hydrogel incorporated with CuTA nanozymes for enhanced osteochondral defects regeneration via providing a suitable microenvironment; Reproduced with permission from ref 176; Copyright 2022, Elsevier. (B) Injectable SF-based hydrogel with loading exosomes promoted endogenous cell recruitment and cartilage defect regeneration; Reproduced with permission from ref 180; Copyright 2021, Elsevier. (C) SilMA bilayer hydrogel with loading BBR, KGN and PRP stimulated osteochondral defects repair; Reproduced with permission from ref 181; Copyright 2022, Elsevier; (D) Photocurable SF-based hydrogel with loading TGF-β3 accelerated cartilage integration; Reproduced with permission from ref 182; Copyright 2021, Elsevier.
SF 基水凝胶的显着生物活性物质递送能力:(A) 蚕丝基水凝胶与 CuTA 纳米酶结合,通过提供合适的微环境来增强骨软骨缺损再生;经 ref 许可转载 176 ;版权所有 2022,爱思唯尔。(B) 具有负载外泌体的可注射 SF 基水凝胶促进了内源性细胞募集和软骨缺损再生;经 ref 许可转载 180 ;版权所有 2021,爱思唯尔。(C) SilMA 双层水凝胶与负载 BBR、KGN 和 PRP 刺激的骨软骨缺损修复;经 ref 许可转载 181 ;版权所有 2022,爱思唯尔;(D) 具有负载 TGF-β3 加速软骨整合的光固化 SF 基水凝胶;经 ref 许可转载 182 ;版权所有 2021,爱思唯尔。
5. Silk fibroin-based hydrogels in iterative optimization for cartilage organoids
5. 基于丝素蛋白的水凝胶在软骨类器官迭代优化中的应用
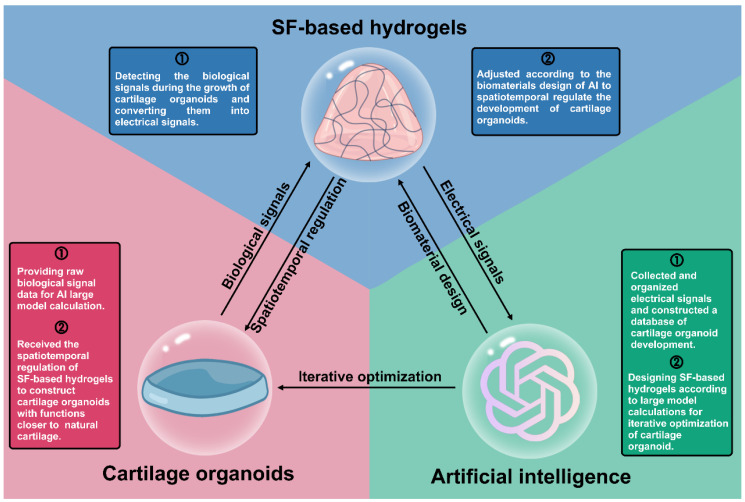
The construction of cartilage organoids is not a one-time process but requires continuous iterative optimization. To meet these needs, SF-based hydrogels must also be continuously upgraded. AI, regarded as the "design master" in life sciences, can utilize large-scale computational models to optimize the design of SF-based hydrogels, thereby enhancing their performance for cartilage organoid construction (Figure 11) 184.
软骨类器官的构建不是一次性的过程,而是需要不断迭代优化。为了满足这些需求,基于 SF 的水凝胶也必须不断升级。人工智能被视为生命科学领域的“设计大师”,可以利用大规模计算模型来优化基于 SF 的水凝胶的设计,从而提高它们在软骨类器官构建方面的性能(图 11 )。 184
Figure 11. 图 11.
Schematic diagram of triangular relationship between cartilage organoids, SF-based hydrogels and artificial intelligence.
软骨类器官、基于 SF 的水凝胶和人工智能之间的三角关系示意图。
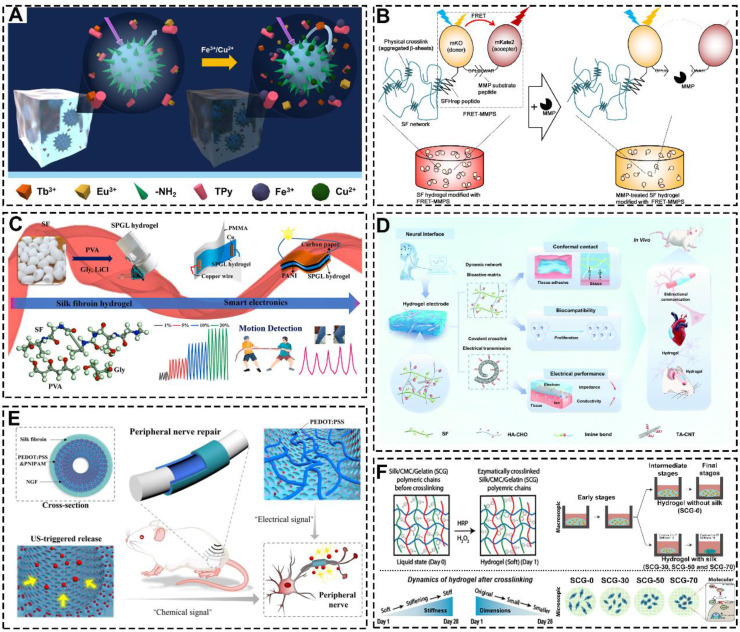
AI for large model computational design requires large amounts of data for model training 185. SF-based hydrogels can be used as biosensors to detect biological signals during the construction of cartilage organoids. For example, Li et al. prepared a highly sensitive responsive hydrogel with SF, pectin, polyvinyl alcohol and lanthanide ions (Figure 12A) 186. This hydrogel could specifically detect Cu2+ and Fe3+ ions. Meanwhile, Yamaoka et al. prepared a Förster/fluorescence resonance energy transfer (FRET)-based SF hydrogel (Figure 12B) 187. This hydrogel could detect the activity of matrix metalloproteinases by FRET signal intensity. Furthermore, SF-based hydrogels can also detect physical signals generated during the construction of cartilage organoids. For example, Feng et al. Prepared mechanical detection hydrogels by combining SF with PVA/glycerol/LiCl (Figure 12C) 188. In addition, Fan et al. constructed SF-HA-CHO hydrogel with dynamic imine crosslinking network (Figure 12D) 188. The hydrogels were biocompatible and could detect and record intercellular electrical signals.
用于大型模型计算设计的 AI 需要大量数据进行模型训练 185 。基于 SF 的水凝胶可用作生物传感器,在软骨类器官构建过程中检测生物信号。例如,Li 等人用 SF、果胶、聚乙烯醇和镧系元素离子制备了一种高灵敏度的响应性水凝胶(图 12 A)。 186 这种水凝胶可以特异性检测 Cu2+ 和 Fe3+ 离子。同时,Yamaoka 等人制备了一种基于 Förster/荧光共振能量转移 (FRET) 的 SF 水凝胶(图 12 B)。 187 这种水凝胶可以通过 FRET 信号强度检测基质金属蛋白酶的活性。此外,基于 SF 的水凝胶还可以检测软骨类器官构建过程中产生的物理信号。例如,Feng 等人。通过将 SF 与 PVA/甘油/LiCl 结合制备机械检测水凝胶(图 12 C)。 188 此外,Fan 等人构建了具有动态亚胺交联网络的 SF-HA-CHO 水凝胶(图 12 D)。 188 水凝胶具有生物相容性,可以检测和记录细胞间电信号。
Figure 12. 图 12.
SF-based hydrogels detect raw biological signal data and provide precision regulation: (A) The SF-based hydrogel detects metal ions. Reproduced with permission from ref 186; Copyright 2020, Elsevier. (B) The SF-based hydrogel detects MMP. Reproduced with permission from ref 187; Copyright 2021, Elsevier. (C) The SF-based hydrogel detects stress stimulus. Reproduced with permission from ref 191; Copyright 2023, Elsevier. (D) The SF-based hydrogel detects biological electrical signals. Reproduced with permission from ref 188; Copyright 2022, Royal Society of Chemistry. (E) The SF-based hydrogel regulated cell behavior through electrical signals. Reproduced with permission from ref 189; Copyright 2023, Wiley-VCH GmbH. (F) The SF-based hydrogels regulate chondrogenic differentiation of cells by contracting and stiffening with time contraction Reproduced with permission from ref 190; Copyright 2022, American Chemical Society.
基于 SF 的水凝胶检测原始生物信号数据并提供精确调节:(A) 基于 SF 的水凝胶检测金属离子。经 ref 许可转载 186 ;版权所有 2020,爱思唯尔。(B) 基于 SF 的水凝胶检测 MMP。经 ref 许可转载 187 ;版权所有 2021,爱思唯尔。(C) 基于 SF 的水凝胶检测压力刺激。经 ref 许可转载 191 ;版权所有 2023,爱思唯尔。(D) 基于 SF 的水凝胶检测生物电信号。经 ref 许可转载 188 ;版权所有 2022,英国皇家化学学会。(E) 基于 SF 的水凝胶通过电信号调节细胞行为。经 ref 许可转载 189 ;版权所有 2023,Wiley-VCH GmbH。(F) 基于 SF 的水凝胶通过随时间收缩和硬化来调节细胞的软骨形成分化 经参考文献许可转载 190 ;版权所有 2022,美国化学会。
AI-driven iterative optimization requires hydrogels with controllable properties, and SF-based hydrogels are well-suited for functional modifications to meet these requirements. For instance, Chai et al. prepared SF-based hydrogel conduits by modifying SF with poly(3,4-ethylenedioxythiophene) and poly(4-styrene sulfonate) (Figure 12E) 189. The hydrogel conduits achieved controlled drug release by modulating electrical signals. Additionally, Katti et al. created ternary network injectable hydrogels using SF, carboxymethyl cellulose, and GT, which showed a time-dependent contraction and stiffening effect that facilitated cartilage regeneration (Figure 12F) 190.
AI 驱动的迭代优化需要具有可控特性的水凝胶,而基于 SF 的水凝胶非常适合进行功能修饰以满足这些要求。例如,Chai 等人通过用聚(3,4-乙烯二氧噻吩)和聚(4-苯乙烯磺酸盐)修饰 SF 制备了基于 SF 的水凝胶导管(图 12 E)。 189 水凝胶导管通过调节电信号实现受控的药物释放。此外,Katti 等人使用 SF、羧甲基纤维素和 GT 创造了三元网络注射水凝胶,它显示出促进软骨再生的时间依赖性收缩和硬化效应(图 12 F)。 190
6. Conclusion and Prospects
6. 结论和展望
As a biomedical material with a long history of applications, SF is considered as an excellent raw material for tissue engineering hydrogels. Here, we reviewed the studies on SF hydrogels on cartilage regeneration in recent years, focusing on the preparation, functionalized modifications and advantages of SF-based hydrogels. Numerous studies demonstrated that SF hydrogels have satisfactory biological properties, suitable internal structure, acceptable mechanical properties, excellent cell-laden ability and remarkable bioactive substance delivery capacity. These features highlight the significant potential of SF hydrogels in cartilage regeneration, repair, and the construction of cartilage organoids.
SF 作为一种具有悠久应用历史的生物医用材料,被认为是组织工程水凝胶的优良原材料。本文综述了近年来SF水凝胶对软骨再生的研究,重点介绍了SF基水凝胶的制备、功能化修饰和优势。大量研究表明,SF 水凝胶具有令人满意的生物学特性、合适的内部结构、可接受的机械性能、优异的载细胞能力和显著的生物活性物质递送能力。这些特征突出了 SF 水凝胶在软骨再生、修复和软骨类器官构建方面的巨大潜力。
Despite promising results in preclinical research, SF-based hydrogels remain largely confined to animal experimentation. Challenges persist in translating these hydrogels into clinical applications and constructing cartilage organoids due to their limited adaptability. One major obstacle lies in the inability of SF hydrogels to respond dynamically to cellular processes post-implantation, as they lack intrinsic "intelligence" or the capacity for real-time adjustment. To address these challenges, recent efforts have focused on creating intelligent SF hydrogels. For instance, Baniassadi et al. designed magnetic field responsive SF hydrogel with excellent mechanical strength and drugs delivery capability 192. The hydrogel achieved controllable drug release by adjusting the magnetic field. In addition, researchers can attempt to combine SF-based hydrogels with programmable DNA to endow SF-based hydrogels with spatio-temporal regulatory capabilities for fine-tuning the construction of cartilage organoids 137, 193.
尽管在临床前研究中取得了可喜的结果,但基于 SF 的水凝胶在很大程度上仍然局限于动物实验。由于适应性有限,将这些水凝胶转化为临床应用和构建软骨类器官的挑战仍然存在。一个主要障碍在于 SF 水凝胶无法动态响应植入后的细胞过程,因为它们缺乏内在的“智能”或实时调整能力。为了应对这些挑战,最近的工作重点是创造智能 SF 水凝胶。例如,Baniassadi 等人设计了具有优异机械强度和药物输送能力 192 的磁场响应 SF 水凝胶。水凝胶通过调节磁场实现了可控的药物释放。此外,研究人员可以尝试将基于 SF 的水凝胶与可编程 DNA 相结合,以赋予基于 SF 的水凝胶时空调节能力,以微调软骨类器官 137 的构建。 193
Looking forward, SF-based hydrogels offer promising avenues for advancing cartilage research and repair. Key future directions include: (1) Developing Disease Models: Cartilage organoids could be utilized to construct disease models, such as OA)or cartilage defect models, to investigate the underlying mechanisms of disease progression and tissue repair more effectively. (2) Drug Screening and Mechanistic Studies: Organoids offer a valuable platform for drug discovery, enabling researchers to screen compounds (loxoprofen sodium, KGN, D-Glucosamine sulfate, etc.) and explore their effects on cartilage regeneration. (3) Overcoming Clinical Translation Barriers: The species gap between animal models and humans remains a significant hurdle in clinical translation. Using cartilage organoids to evaluate biomaterials for regenerative functions and mechanisms can reduce reliance on animal testing and expedite clinical applications. (4) Cartilage Organoids as Implants: Due to their structural and functional similarity to native cartilage, organoids constructed from SF hydrogels could serve as implantable grafts for cartilage repair, potentially bridging the gap between tissue engineering and clinical practice (Figure 13). In conclusion, future efforts to develop smart and programmable SF hydrogels could break through the current bottlenecks in cartilage tissue engineering, offering new tools for modeling disease, discovering therapies, and advancing clinical applications. We believe that SF-based hydrogels are ideal materials for constructing cartilage organoids and can provide new approaches to cartilage regeneration research.
展望未来,基于 SF 的水凝胶为推进软骨研究和修复提供了有前途的途径。未来的主要方向包括:(1) 开发疾病模型:软骨类器官可用于构建疾病模型,例如 OA) 或软骨缺损模型,以更有效地研究疾病进展和组织修复的潜在机制。(2) 药物筛选和机制研究:类器官为药物发现提供了一个有价值的平台,使研究人员能够筛选化合物(洛索洛芬钠、KGN、D-氨基葡萄糖硫酸盐等)并探索它们对软骨再生的影响。(3) 克服临床转化障碍:动物模型和人类之间的物种差距仍然是临床转化中的一个重大障碍。使用软骨类器官评估生物材料的再生功能和机制可以减少对动物试验的依赖并加快临床应用。(4) 作为植入物的软骨类器官:由于它们在结构和功能上与天然软骨相似,由 SF 水凝胶构建的类器官可以作为软骨修复的植入式移植物,有可能弥合组织工程和临床实践之间的差距(图 13 )。总之,未来开发智能和可编程 SF 水凝胶的努力可以突破当前软骨组织工程的瓶颈,为疾病建模、发现疗法和推进临床应用提供新工具。我们相信基于 SF 的水凝胶是构建软骨类器官的理想材料,可以为软骨再生研究提供新的方法。
Figure 13. 图 13.
Development directions of SF-based hydrogels for constructing cartilage organoids: smart SF-based hydrogel and programmable SF-based hydrogel. Application prospects of cartilage organoids: disease modeling, drugs screening, non-animal testing and cartilage defects repairing. Created with BioRender.com.
用于构建软骨类器官的 SF 基水凝胶的发展方向:智能 SF 基水凝胶和可编程 SF 基水凝胶。软骨类器官的应用前景:疾病建模、药物筛选、非动物试验和软骨缺损修复。使用 BioRender.com 创建。
Acknowledgments 确认
This work was supported by the National Key Research and Development Program of China (No. 2022YFB3804300), Integrated Project of Major Research Plan of National Natural Science Foundation of China (92249303), Key Project of the National Natural Science Foundation of China (82230071), National Natural Science Foundation of China (82172098 and 32471395), Shanghai Science and Technology Innovation Action Plan (23141900600), Research Physician Training Program of Shanghai Hospital Development Center (SHDC2023CRT013), and Shanghai Municipal Demonstration Project for Innovative Medical Device Applications (23SHS05700).
这项工作得到了国家重点研发计划(No. 2022YFB3804300)、国家自然科学基金重大研究计划(92249303)、国家自然科学基金(82230071)重点项目、国家自然科学基金(82172098和32471395)、上海市科技创新行动计划(23141900600)的支持。 上海市医院发展中心研究医师培训计划 (SHDC2023CRT013) 和上海市医疗器械创新应用示范项目 (23SHS05700)。
Author contributions 作者贡献
Congyi Shen, Ziyang Zhou, Ruiyang Li and Shike Yang contributed equally to this work. Congyi Shen: Conceptualization, Writing - original draft, Writing - review & editing. Ziyang Zhou: Writing - original draft, Writing - review & editing. Ruiyang Li: Writing - review & editing. Shike Yang: Writing - review & editing. Dongyang Zhou: Writing - review & editing. Fengjin Zhou: Writing - review & editing, Supervision. Zhen Geng: Writing - review & editing, Funding acquisition. Jiacan Su: Funding acquisition, Supervision. All authors read and approved the final manuscript.
沈聪义、周紫阳、李瑞阳和杨世科对这项工作做出了同样的贡献。 沈从义:概念化,写作 - 原稿,写作 - 审查和编辑。周紫阳:写作 - 原稿,写作 - 审查和编辑。李瑞阳:写作 - 审查和编辑。杨世珂:写作 - 审查和编辑。 周东阳:写作 - 审查和编辑。周峰锦:写作 - 审查和编辑,监督。 耿振:写作 - 审查和编辑,资金获取。苏佳灿:资金获取、监督。所有作者都阅读并批准了最终稿件。
Abbreviations 缩写
- OA OA的
osteoarthritis 骨关节炎
- ECM ECM (外表)
extracellular matrix 细胞外基质
- SF SF (旧金山)
silk fibroin 丝素蛋白
- AI 人工智能
artificial intelligence 人工智能
- ACI 国际机场
autologous chondrocyte implantation
自体软骨细胞植入- MACI MACI 公司
mx-induced autologous chondrocyte implantation
MX 诱导的自体软骨细胞植入- MSCs 间充质干细胞
msenchymal stem cells msen充质干细胞
- COs 因为
callus organoids 愈伤组织类器官
- HRP
horseradish peroxidase 辣根过氧化物酶
- TA
tyramine 酪胺
- GT 燃气轮机
gelatin 明胶
- SF C-gel
SF hydrogels by using Co-60 derived gamma-ray
使用 Co-60 衍生的伽马射线制备 SF 水凝胶- GTA 多伦多
glutaraldehyde 戊 二 醛
- SF/PCZ SF/PCZ 系列
polycarbazole/silk fibroin hydrogels
聚咔唑/丝素蛋白水凝胶- KGN KGN型
kartogenin 核晶素
- PDGF-BB PDGF-BB 系列
platelet-derived growth factor BB
血小板衍生生长因子 BB- BSNF BSNF 公司
beta-sheet rich silk nanofibers
富含 β 片的蚕丝纳米纤维- BMSCs BMSC
bone marrow mesenchymal stem cells
骨髓间充质干细胞- RSF
regenerated silk fibroin 再生丝素蛋白
- PEG 楔子
polyethylene glycol 聚乙二醇
- PEGDMA
poly(ethylene glycol) dimethacrylate
聚乙二醇二甲基丙烯酸酯- SilMA 锡尔马
silk methacrylate 甲基丙烯酸丝
- DN DN 系列
double network 双网络
- ALG-POL
alginate-poloxamer 藻酸盐-泊洛沙姆
- Col 山坳
collagen 胶原
- HA 医 管 局
hyaluronic acid 透明质酸
- GA 加语
glycyrrhizic acid 甘草酸
- IPN 国际宣传电话
interpenetrating network 互穿网络
- HPMC 羟丙基甲基纤维素
hydroxypropyl methyl cellulose
羟丙基甲基纤维素- CS CS 系列
chitosan 脱乙酰壳多糖
- PC 个人电脑
propanoic acid-modified chitosan
丙酸改性壳聚糖- DNA 脱氧核糖核酸
deoxyribonucleic acid 脱氧核糖核酸
- HG-SF HG-SF 型
host-guest interactions silk fibroin
主客互动 丝素蛋白- GelMA 凝胶MA
gelatin methacrylate 甲基丙烯酸明胶
- Silk-GMA 丝绸-GMA
SF-glycidyl-methacrylate SF-甲基丙烯酸缩水甘油酯
- AD/CS/RSF/EXO 广告/CS/RSF/EXO
alginate-dopamine/chondroitin sulfate/regenerated silk fibroin/exosome hydrogel
海藻酸盐-多巴胺/硫酸软骨素/再生丝素/外泌体水凝胶- BBR BBR(黑匣子
berberine 黄连素
- FRET 品
förster/fluorescence resonance energy transfer
Förster/荧光共振能量转移
References 引用
-
1.Collins KH, Lenz KL, Pollitt EN, Ferguson D, Hutson I, Springer LE. et al. Adipose tissue is a critical regulator of osteoarthritis. Proc Natl Acad Sci U S A. 2021;118:e2021096118. doi: 10.1073/pnas.2021096118. [DOI] [PMC free article] [PubMed] [Google Scholar]
1.Collins KH, Lenz KL, Pollitt EN, Ferguson D, Hutson I, Springer LE. et al. 脂肪组织是骨关节炎的关键调节因子。美国国家科学院院刊 2021 年;118:e2021096118.doi:10.1073/pnas.2021096118。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
2.Xue X, Liu H, Wang S, Hu Y, Huang B, Li M. et al. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/anti-inflammatory therapy of osteoarthritis. Compos B Eng. 2022;237:109855. [Google Scholar]
2.Xue X, Liu H, Wang S, 胡 Y, Huang B, Li M. et al. 中性粒细胞-红细胞杂化膜包被的空心硫化铜纳米颗粒用于骨关节炎的靶向和光热/抗炎治疗。康波斯工程学士 2022;237:109855。 Google Scholar [ ] -
3.Peng Z, Sun H, Bunpetch V, Koh Y, Wen Y, Wu D. et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268:120555. doi: 10.1016/j.biomaterials.2020.120555. [DOI] [PubMed] [Google Scholar]
3.Peng Z, Sun H, Bunpetch V, Koh Y, 温 Y, Wu D. 等. 关节软骨变性和再生中软骨细胞外基质稳态的调节。生物材料。2021;268:120555. doi: 10.1016/j.biomaterials.2020.120555.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
4.Zheng L, Zhang Z, Sheng P, Mobasheri A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev. 2021;66:101249. doi: 10.1016/j.arr.2020.101249. [DOI] [PubMed] [Google Scholar]
4.郑 L, 张 Z, 盛 P, Mobasheri A.代谢在软骨细胞功能障碍和骨关节炎进展中的作用。老龄化研究修订版 2021;66:101249。doi:10.1016/j.arr.2020.101249.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
5.Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9:20. doi: 10.1038/s41413-021-00147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
5.胡 Y, 陈 X, 王 S, 静 Y, 苏 J. 骨关节炎和疼痛中的软骨下骨微环境。骨骼研究 2021;9:20。doi:10.1038/s41413-021-00147-z。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
6.Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA. et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79:819–28. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
6.Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA. et al. 1990-2017 年全球、区域和国家骨关节炎负担:2017 年全球疾病负担研究的系统分析。Ann Rheum Dis. 2020 年;79:819–28.doi:10.1136/annrheumdis-2019-216515。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
7.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–59. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
7.Hunter DJ,Bierma-Zeinstra S. 骨关节炎。柳叶 刀。2019;393:1745–59.doi: 10.1016/S0140-6736(19)30417-9.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
8.Gao J, Pei H, Lv F, Niu X, You Y, He L. et al. JD-312 - A novel small molecule that facilitates cartilage repair and alleviates osteoarthritis progression. J Orthop Translat. 2024;44:60–71. doi: 10.1016/j.jot.2023.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
8.Gao J, Pei H, Lv F, Niu X, You Y, He L. et al. JD-312 - 一种促进软骨修复和缓解骨关节炎进展的新型小分子。J 骨科翻译。2024;44:60–71.doi:10.1016/j.jot.2023.11.007.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
9.Steinmetz JD, Culbreth GT, Haile LM, Rafferty Q, Lo J, Fukutaki KG. et al. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e508–e22. doi: 10.1016/S2665-9913(23)00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
9.Steinmetz JD, Culbreth GT, Haile LM, Rafferty Q, Lo J, Fukutaki KG. et al. 1990-2020 年全球、区域和国家骨关节炎负担以及到 2050 年的预测:2021 年全球疾病负担研究的系统分析。柳叶刀风湿醇。2023;5:e508–e22。doi:10.1016/S2665-9913(23)00163-7.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
10.van den Bosch MHJ. Osteoarthritis year in review 2020: biology. Osteoarthritis Cartilage. 2021;29:143–50. doi: 10.1016/j.joca.2020.10.006. [DOI] [PubMed] [Google Scholar]
10.范登博世 MHJ.2020 年骨关节炎年度回顾:生物学。骨关节炎软骨。2021;29:143–50.doi:10.1016/j.joca.2020.10.006.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
11.Liang M, Wang K, Wei X, Gong X, Tang H, Xue H. et al. Replenishing decoy extracellular vesicles inhibits phenotype remodeling of tissue-resident cells in inflammation-driven arthritis. Cell Rep Med. 2023;4:101228. doi: 10.1016/j.xcrm.2023.101228. [DOI] [PMC free article] [PubMed] [Google Scholar]
11.Liang M, Wang K, Wei X, Gong X, Tang H, Xue H. et al. 补充诱饵细胞外囊泡抑制炎症驱动性关节炎中组织驻留细胞的表型重塑。细胞代表医学 2023;4:101228。doi:10.1016/j.xcrm.2023.101228。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
12.Onnerfjord P, Khabut A, Reinholt FP, Svensson O, Heinegard D. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J Biol Chem. 2012;287:18913–24. doi: 10.1074/jbc.M111.298968. [DOI] [PMC free article] [PubMed] [Google Scholar]
12.Onnerfjord P, Khabut A, Reinholt FP, Svensson O, Heinegard D. 八种软骨组织的定量蛋白质组学分析揭示了亚组之间的特征差异和相似性。J Biol Chem. 2012 年;287:18913–24.doi:10.1074/jbc。M111.298968. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar -
13.Vilela CA, Correia C, Oliveira JM, Sousa RA, Espregueira-Mendes J, Reis RL. Cartilage Repair Using Hydrogels: A Critical Review of in Vivo Experimental Designs. ACS Biomater Sci Eng. 2015;1:726–39. doi: 10.1021/acsbiomaterials.5b00245. [DOI] [PubMed] [Google Scholar]
13.Vilela CA, Correia C, Oliveira JM, Sousa RA, Espregueira-Mendes J, Reis RL.使用水凝胶修复软骨:体内实验设计的批判性回顾。ACS Biomater Sci Eng. 2015 年;1:726–39.doi:10.1021/acsbiomaterials.5b00245。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
14.Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040. doi: 10.1038/boneres.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
14.Zhang W, Ouyang H, Dass CR, Xu J. 骨关节炎药物和再生疗法的当前研究。骨骼研究 2016;4:15040。doi:10.1038/boneres.2015.40。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
15.Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6:402–20. doi: 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
15.Hofer M, Lutolf MP.工程类器官。Nat Rev Mater.2021;6:402–20.doi:10.1038/s41578-021-00279-y.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
16.Drakhlis L, Biswanath S, Farr CM, Lupanow V, Teske J, Ritzenhoff K. et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat Biotechnol. 2021;39:737–46. doi: 10.1038/s41587-021-00815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
16.Drakhlis L, Biswanath S, Farr CM, Lupanow V, Teske J, Ritzenhoff K. et al. 人类心脏形成类器官概括了早期心脏和前肠发育。国家生物技术.2021;39:737–46.doi:10.1038/s41587-021-00815-9。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
17.Ma Z, Wu Y, Li G, Liu J, Geng Z, Su J. Extracellular vesicles-loaded DNA hydrogels: A promising candidate for cartilage organoids engineering. Chem Eng J. 2023;477:147146. [Google Scholar]
17.马 Z, 吴 Y, 李 G, 刘 J, 耿 Z, 苏 J. 细胞外囊泡负载的 DNA 水凝胶:软骨类器官工程的有前途的候选者。化学工程杂志 2023;477:147146。 Google Scholar [ ] -
18.Shen C, Li Z, Li G, Wang G, Geng Z, Su J. DNA-based hydrogels: Ideal biomaterials for cartilage organoids. Fundam Res. 2024.
18.Shen C, Li Z, Li G, Wang G, Geng Z, Su J. 基于 DNA 的水凝胶:软骨类器官的理想生物材料。Fundam Res. 2024 年。 -
19.Crispim JF, Ito K. De novo neo-hyaline-cartilage from bovine organoids in viscoelastic hydrogels. Acta Biomater. 2021;128:236–49. doi: 10.1016/j.actbio.2021.04.008. [DOI] [PubMed] [Google Scholar]
19.Crispim JF, Ito K. 粘弹性水凝胶中来自牛类器官的从头新透明软骨。生物学报。2021;128:236–49.doi:10.1016/j.actbio.2021.04.008。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
20.Singh YP, Moses JC, Bhardwaj N, Mandal BB. Injectable hydrogels: a new paradigm for osteochondral tissue engineering. J Mater Chem B. 2018;6:5499–529. doi: 10.1039/c8tb01430b. [DOI] [PubMed] [Google Scholar]
20.辛格 YP, 摩西 JC, 巴德瓦吉 N, 曼达尔 BB.可注射水凝胶:骨软骨组织工程的新范式。J Mater Chem B. 2018 年;6:5499–529.doi:10.1039/c8tb01430b。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
21.Galperin A, Oldinski RA, Florczyk SJ, Bryers JD, Zhang M, Ratner BD. Integrated bi-layered scaffold for osteochondral tissue engineering. Adv Healthc Mater. 2013;2:872–83. doi: 10.1002/adhm.201200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
21.Galperin A, Oldinski RA, Florczyk SJ, Bryers JD, Zhang M, Ratner BD. 用于骨软骨组织工程的集成双层支架。Adv Healthc Mater.2013;2:872–83.doi:10.1002/adhm.201200345。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
22.Zhao X, Hu DA, Wu D, He F, Wang H, Huang L. et al. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front Bioeng Biotechnol. 2021;9:603444. doi: 10.3389/fbioe.2021.603444. [DOI] [PMC free article] [PubMed] [Google Scholar]
22.赵晓军, 胡达, 吴丹, 何峰, 王 H, 黄玲等. 生物相容性支架材料在干细胞软骨组织工程中的应用.Front Bioeng 生物技术公司。2021;9:603444.doi:10.3389/fbioe.2021.603444。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
23.Chen S, Chen X, Geng Z, Su J. The horizon of bone organoid: A perspective on construction and application. Bioact Mater. 2022;18:15–25. doi: 10.1016/j.bioactmat.2022.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
23.陈 S, 陈 X, 耿 Z, 苏 J.骨类器官的视野:构建和应用的观点。生物行为材料。2022;18:15–25.doi:10.1016/j.bioactmat.2022.01.048。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
24.Gai T, Zhang Y, Li G, Zhou F, He C, Wang X. et al. Engineered hydrogel microspheres for spheroids and organoids construction. Chem Eng J. 2024;498:155131. [Google Scholar]
24.Gai T, Zhang Y, Li G, 周 F, He C, Wang X. et al. 用于球状体和类器官构建的工程水凝胶微球.化学工程杂志 2024;498:155131。 Google Scholar [ ] -
25.Kaur S, Kaur I, Rawal P, Tripathi DM, Vasudevan A. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021;504:58–66. doi: 10.1016/j.canlet.2021.01.025. [DOI] [PubMed] [Google Scholar]
25.Kaur S, Kaur I, Rawal P, Tripathi DM, Vasudevan A. 用于类器官培养的非基质胶支架。癌症Lett. 2021;504:58–66.doi:10.1016/j.canlet.2021.01.025。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
26.Holland C, Numata K, Rnjak-Kovacina J, Seib FP. The Biomedical Use of Silk: Past, Present, Future. Adv Healthc Mater. 2019;8:e1800465. doi: 10.1002/adhm.201800465. [DOI] [PubMed] [Google Scholar]
26.Holland C, Numata K, Rnjak-Kovacina J, Seib FP.丝绸的生物医学用途:过去、现在、未来。Adv Healthc Mater.2019;8:e1800465.doi:10.1002/adhm.201800465。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
27.Johari N, Khodaei A, Samadikuchaksaraei A, Reis RL, Kundu SC, Moroni L. Ancient fibrous biomaterials from silkworm protein fibroin and spider silk blends: Biomechanical patterns. Acta Biomater. 2022;153:38–67. doi: 10.1016/j.actbio.2022.09.030. [DOI] [PubMed] [Google Scholar]
27.Johari N, Khodaei A, Samadikuchaksaraei A, Reis RL, Kundu SC, Moroni L. 来自蚕蛋白丝素蛋白和蜘蛛丝混合物的古老纤维生物材料:生物力学模式。生物学报。2022;153:38–67.doi:10.1016/j.actbio.2022.09.030。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
28.Ju Y-X, Hu R-Z, Wang P-Y, Shen Y-S, Shuang F-F, Wang J. et al. Strong Silk Fibroin/PVA/Chitosan Hydrogels with High Water Content Inspired by Straw Rammed Earth Brick Structures. ACS Sustain Chem Eng. 2022;10:13070–80. [Google Scholar]
28.Ju Y-X, 胡 R-Z, 王P-Y, 沈Y-S, 双F-F, Wang J. et al. 受稻草夯土砖结构启发的高含水量强丝素蛋白/PVA/壳聚糖水凝胶.ACS Sustain Chem Eng. 2022 年;10:13070-80。 Google Scholar [ ] -
29.Shuang F-F, Zong C-M, Wang C-C, Hu R-Z, Shen Y-S, Ju Y-X. et al. Chlorogenic acid and cellulose nanocrystal-assisted crosslinking preparation of a silk-based film to extend the shelf life of strawberries. LWT-Food Sci. Technol. 2022;172:114218. [Google Scholar]
29.Shuang F-F, Zong C-M, Wang C-C, 胡 R-Z, Shen Y-S, Ju Y-X. et al. 绿原酸和纤维素纳米晶辅助交联制备蚕丝基薄膜以延长草莓的保质期.LWT-食品科学技术。2022;172:114218。 Google Scholar [ ] -
30.Laomeephol C, Ferreira H, Kanokpanont S, Luckanagul JA, Neves NM, Damrongsakkul S. Osteogenic differentiation of encapsulated cells in dexamethasone-loaded phospholipid-induced silk fibroin hydrogels. Biomater Transl. 2022;3:213–20. doi: 10.12336/biomatertransl.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
30.Laomeephol C, Ferreira H, Kanokpanont S, Luckanagul JA, Neves NM, Damrongsakkul S. 载有地塞米松的磷脂诱导的丝素蛋白水凝胶中包膜细胞的成骨分化。Biomater Transl. 2022;3:213–20.doi: 10.12336/biomatertransl.2022.03.005.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
31.Ribeiro VP, Pina S, Oliveira JM, Reis RL. Silk Fibroin-Based Hydrogels and Scaffolds for Osteochondral Repair and Regeneration. Adv Exp Med Biol. 2018;1058:305–25. doi: 10.1007/978-3-319-76711-6_14. [DOI] [PubMed] [Google Scholar]
31.Ribeiro 副总裁,Pina S,Oliveira JM,Reis RL。用于骨软骨修复和再生的基于丝素蛋白的水凝胶和支架。Adv Exp Med Biol. 2018;1058:305–25.doi: 10.1007/978-3-319-76711-6_14.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
32.Qiu W, Liu XY. Recent Progress of Applying Mesoscopic Functionalization Engineering Principles to Spin Advanced Regenerated Silk Fibroin Fibers. Adv Fiber Mater. 2022;4:390–403. [Google Scholar]
32.邱文,刘晓。应用介观功能化工程原理纺丝高级再生丝素蛋白纤维的最新进展。高级纤维材料。2022;4:390–403。 Google Scholar [ ] -
33.Liu H, Sun Z, Guo C. Chemical Modification of Silk Proteins: Current Status and Future Prospects. Adv Fiber Mater. 2022;4:705–19. [Google Scholar]
33.Liu H, Sun Z, Guo C. 蚕丝蛋白的化学修饰:现状和未来展望。高级纤维材料。2022;4:705-19。 Google Scholar [ ] -
34.Costa JB, Silva-Correia J, Ribeiro VP, Morais AD, Oliveira JM, Reis RL. Engineering patient-specific bioprinted constructs for treatment of degenerated intervertebral disc. Mater Today Commun. 2019;19:506–12. [Google Scholar]
34.Costa JB, Silva-Correia J, Ribeiro VP, Morais AD, Oliveira JM, Reis RL.设计患者特异性生物打印结构用于治疗退化的椎间盘。今日通讯。2019;19:506-12。 Google Scholar [ ] -
35.Bhar B, Das E, Manikumar K, Mandal BB. 3D Bioprinted Human Skin Model Recapitulating Native-Like Tissue Maturation and Immunocompetence as an Advanced Platform for Skin Sensitization Assessment. Adv Healthc Mater. 2024;13:2303312. doi: 10.1002/adhm.202303312. [DOI] [PubMed] [Google Scholar]
35.Bhar B, Das E, Manikumar K, Mandal BB. 3D生物打印的人体皮肤模型概括了天然样组织的成熟和免疫能力,作为皮肤致敏评估的先进平台。Adv Healthc Mater.2024;13:2303312.doi:10.1002/adhm.202303312。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
36.Li XL, Sheng SH, Li GF, Hu Y, Zhou FJ, Geng Z. et al. Research Progress in Hydrogels for Cartilage Organoids. Adv Healthc Mater. 2024;13:2400431. doi: 10.1002/adhm.202400431. [DOI] [PubMed] [Google Scholar]
36.Li XL, Sheng SH, Li GF, 胡 Y, 周 FJ, 耿志等. 软骨类器官水凝胶的研究进展.Adv Healthc Mater.2024;13:2400431.doi:10.1002/adhm.202400431。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
37.Wang HY, Zhang YQ. Processing silk hydrogel and its applications in biomedical materials. Biotechnol Prog. 2015;31:630–40. doi: 10.1002/btpr.2058. [DOI] [PubMed] [Google Scholar]
37.Wang HY, 张 YQ.蚕丝水凝胶加工及其在生物医用材料中的应用。生物技术进展 2015;31:630–40.doi:10.1002/btpr.2058。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
38.Myatt JP, Schilling N, Thorpe SK. Distribution patterns of fibre types in the triceps surae muscle group of chimpanzees and orangutans. J Anat. 2011;218:402–12. doi: 10.1111/j.1469-7580.2010.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
38.Myatt JP, Schilling N, Thorpe SK. 黑猩猩和猩猩小腿三头肌中纤维类型的分布模式。J Anat. 2011;218:402–12.doi: 10.1111/j.1469-7580.2010.01338.x.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
39.Taheri S, Ghazali HS, Ghazali ZS, Bhattacharyya A, Noh I. Correction: Progress in biomechanical stimuli on the cell-encapsulated hydrogels for cartilage tissue regeneration. Biomater Res. 2023;27:74. doi: 10.1186/s40824-023-00358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
39.Taheri S, Ghazali HS, Ghazali ZS, Bhattacharyya A, Noh I. 更正:细胞包膜水凝胶上用于软骨组织再生的生物力学刺激的进展。生物材料研究 2023;27:74。doi:10.1186/s40824-023-00358-x.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
40.Taheri S, Ghazali HS, Ghazali ZS, Bhattacharyya A, Noh I. Progress in biomechanical stimuli on the cell-encapsulated hydrogels for cartilage tissue regeneration. Biomater Res. 2023;27:22. doi: 10.1186/s40824-023-00358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
40.Taheri S, Ghazali HS, Ghazali ZS, Bhattacharyya A, Noh I. 细胞包封水凝胶上用于软骨组织再生的生物力学刺激的进展。生物材料研究 2023;27:22。doi:10.1186/s40824-023-00358-x.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
41.Li MM, Yu B, Wang SC, Zhou FJ, Cui J, Su JC. Microenvironment-responsive nanocarriers for targeted bone disease therapy. Nano Today. 2023;50:101838. [Google Scholar]
41.李MM, 于斌, 王SC, 周FJ, 崔J, 苏锦成.用于靶向骨病治疗的微环境响应纳米载体。今天的纳米。2023;50:101838。 Google Scholar [ ] -
42.Wei W, Dai H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact Mater. 2021;6:4830–55. doi: 10.1016/j.bioactmat.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
42.Wei W, Dai H. 关节软骨和骨软骨组织工程技术:最近的进展和挑战。生物行为材料。2021;6:4830–55.doi: 10.1016/j.bioactmat.2021.05.011.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
43.Zimmermann. B, Wachtel. HC, Somogyi. H. Endochondral mineralization in cartilage organoid culture. Cell Differ Dev. 1990;31:11–22. doi: 10.1016/0922-3371(90)90086-c. [DOI] [PubMed] [Google Scholar]
43.齐默尔曼。B, 瓦赫特尔.HC,Somogyi。H. 软骨类器官培养物中的软骨内矿化。Cell Differ Dev. 1990;31:11–22.doi: 10.1016/0922-3371(90)90086-c.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
44.Albro MB, Nims RJ, Durney KM, Cigan AD, Shim JJ, Vunjak-Novakovic G. et al. Heterogeneous engineered cartilage growth results from gradients of media-supplemented active TGF-beta and is ameliorated by the alternative supplementation of latent TGF-beta. Biomaterials. 2016;77:173–85. doi: 10.1016/j.biomaterials.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
44.Albro MB, Nims RJ, Durney KM, Cigan AD, Shim JJ, Vunjak-Novakovic G. et al. 异质工程软骨生长由补充培养基的活性 TGF-β 的梯度引起,并通过替代补充潜在的 TGF-β 得到改善。生物材料。2016;77:173–85.doi: 10.1016/j.biomaterials.2015.10.018.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
45.Takada E, Mizuno S. Reproduction of Characteristics of Extracellular Matrices in Specific Longitudinal Depth Zone Cartilage within Spherical Organoids in Response to Changes in Osmotic Pressure. Int J Mol Sci. 2018;19:1507. doi: 10.3390/ijms19051507. [DOI] [PMC free article] [PubMed] [Google Scholar]
45.Takada E, Mizuno S. 响应渗透压变化,球形类器官内特定纵向深度区软骨中细胞外基质特征的再现。国际分子科学杂志 2018;19:1507。doi:10.3390/ijms19051507。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
46.Rothbauer M, Byrne RA, Schobesberger S, Olmos Calvo I, Fischer A, Reihs EI. et al. Establishment of a human three-dimensional chip-based chondro-synovial coculture joint model for reciprocal cross talk studies in arthritis research. Lab Chip. 2021;21:4128–43. doi: 10.1039/d1lc00130b. [DOI] [PubMed] [Google Scholar]
46.Rothbauer M, Byrne RA, Schobesberger S, Olmos Calvo I, Fischer A, Reihs EI. et al. 建立基于人体三维芯片的软骨-滑膜共培养联合模型,用于关节炎研究中互惠串扰研究。实验室芯片。2021;21:4128–43.doi:10.1039/d1lc00130b。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
47.Wei J, Baptista-Hon DT, Wang Z, Li G, Herrler T, Dai C. et al. Bioengineered human tissue regeneration and repair using endogenous stem cells. Cell Rep Med. 2023;4:101156. doi: 10.1016/j.xcrm.2023.101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
47.Wei J, Baptista-Hon DT, Wang Z, Li G, Herrler T, Dai C. et al. 使用内源性干细胞进行生物工程人体组织再生和修复。细胞代表医学 2023;4:101156. doi: 10.1016/j.xcrm.2023.101156.[ DOI ] [ PMC free article ] [ PubMed ] Google Scholar [ ] [ ] [ ] -
48.Cao. Y, Vacanti. JP, Paige. KT, Upton. J, Vacanti. CA. Transplantation of Chondrocytes Utilizing a Polymer-Cell Construct to Produce Tissue-Engineered Cartilage in the Shape of a Human Ear. Plast. Reconstr. Surg. 1997;100:297–302. doi: 10.1097/00006534-199708000-00001. [DOI] [PubMed] [Google Scholar]
48.曹.Y,瓦坎蒂。JP,佩奇。KT,厄普顿。J,瓦坎蒂。利用聚合物细胞构建体移植软骨细胞,以产生人耳形状的组织工程软骨。塑料。重新勘察。外科杂志 1997;100:297–302.doi: 10.1097/00006534-199708000-00001.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
49.Zhang L, He A, Yin Z, Yu Z, Luo X, Liu W. et al. Regeneration of human-ear-shaped cartilage by co-culturing human microtia chondrocytes with BMSCs. Biomaterials. 2014;35:4878–87. doi: 10.1016/j.biomaterials.2014.02.043. [DOI] [PubMed] [Google Scholar]
49.Zhang L, He A, Yin Z, Yu Z, Luo X, Liu W. et al. 通过共培养人小耳症软骨细胞与 BMSC 再生人耳形软骨。生物材料。2014 年;35:4878–87.doi: 10.1016/j.biomaterials.2014.02.043.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
50.Jeon O, Lee YB, Jeong H, Lee SJ, Wells D, Alsberg E. Individual cell-only bioink and photocurable supporting medium for 3D printing and generation of engineered tissues with complex geometries. Mater Horiz. 2019;6:1625–31. doi: 10.1039/c9mh00375d. [DOI] [PMC free article] [PubMed] [Google Scholar]
50.Jeon O, Lee YB, Jeong H, Lee SJ, Wells D, Alsberg E. 用于 3D 打印和生成具有复杂几何形状的工程组织的单个细胞生物墨水和光固化支持介质。马特·霍里兹。2019;6:1625–31.doi:10.1039/c9MH00375D。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
51.Wang Q, Ran X, Wang J, Wang S, Zhang P, Gao E. et al. Elastic Fiber-Reinforced Silk Fibroin Scaffold with A Double-Crosslinking Network for Human Ear-Shaped Cartilage Regeneration. Adv Fiber Mater. 2023;5:1008–1024. [Google Scholar]
51.Wang Q, Ran X, Wang J, Wang S, Zhang P, Gao E. et al. 用于人耳形软骨再生的具有双交联网络的弹性纤维增强丝素蛋白支架。高级纤维材料。2023;5:1008–1024。 Google Scholar [ ] -
52.Xing D, Liu W, Li JJ, Liu L, Guo A, Wang B. et al. Engineering 3D functional tissue constructs using self-assembling cell-laden microniches. Acta Biomater. 2020;114:170–82. doi: 10.1016/j.actbio.2020.07.058. [DOI] [PubMed] [Google Scholar]
52.Xing D, Liu W, Li JJ, Liu L, Guo A, Wang B. et al. 使用自组装载细胞微生态位设计 3D 功能组织构建。生物学报。2020;114:170–82.doi:10.1016/j.actbio.2020.07.058。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
53.Xiahou Z, She Y, Zhang J, Qin Y, Li G, Zhang L. et al. Designer Hydrogel with Intelligently Switchable Stem-Cell Contact for Incubating Cartilaginous Microtissues. ACS Appl Mater Interfaces. 2020;12:40163–75. doi: 10.1021/acsami.0c13426. [DOI] [PubMed] [Google Scholar]
53.Xiahou Z, She Y, Zhang J, Qin Y, Li G, Zhang L. et al. 设计用于孵化软骨微组织的具有智能可切换干细胞接触的水凝胶。ACS 应用程序接口。2020;12:40163–75.doi:10.1021/acsami.0c13426。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
54.Hall GN, Tam WL, Andrikopoulos KS, Casas-Fraile L, Voyiatzis GA, Geris L. et al. Patterned, organoid-based cartilaginous implants exhibit zone specific functionality forming osteochondral-like tissues in vivo. Biomaterials. 2021;273:120820. doi: 10.1016/j.biomaterials.2021.120820. [DOI] [PubMed] [Google Scholar]
54.Hall GN, Tam WL, Andrikopoulos KS, Casas-Fraile L, Voyiatzis GA, Geris L. et al. 图案化的、基于类器官的软骨植入物表现出在体内形成骨软骨样组织的区域特异性功能。生物材料。2021;273:120820.doi:10.1016/j.biomaterials.2021.120820.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
55.Abe K, Yamashita A, Morioka M, Horike N, Takei Y, Koyamatsu S. et al. Engraftment of allogeneic iPS cell-derived cartilage organoid in a primate model of articular cartilage defect. Nat Commun. 2023;14:804. doi: 10.1038/s41467-023-36408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
55.Abe K, Yamashita A, Morioka M, Horike N, Takei Y, Koyamatsu S. et al. 同种异体 iPS 细胞衍生的软骨类器官在关节软骨缺损灵长类动物模型中的植入。Nat Commun.2023;14:804.doi:10.1038/s41467-023-36408-0.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
56.Yang Z, Wang B, Liu W, Li X, Liang K, Fan Z. et al. In situ self-assembled organoid for osteochondral tissue regeneration with dual functional units. Bioact Mater. 2023;27:200–15. doi: 10.1016/j.bioactmat.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
56.Yang Z, Wang B, Liu W, Li X, Liang K, Fan Z. et al.用于具有双功能单元的骨软骨组织再生的原位自组装类器官。生物行为材料。2023;27:200–15.doi:10.1016/j.bioactmat.2023.04.002。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
57.Wen Y, Chen Y, Wu W, Zhang H, Peng Z, Yao X. et al. Hyperplastic Human Macromass Cartilage for Joint Regeneration. Adv Sci (Weinh) 2023;10:2301833. doi: 10.1002/advs.202301833. [DOI] [PMC free article] [PubMed] [Google Scholar]
57.温 Y, 陈 Y, 吴伟, 张 H, 彭 Z, 姚 X. 等. 用于关节再生的增生性人类大块软骨.高级科学 (Weinh) 2023;10:2301833。doi: 10.1002/advs.202301833.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
58.Farokhi M, Aleemardani M, Solouk A, Mirzadeh H, Teuschl AH, Redl H. Crosslinking strategies for silk fibroin hydrogels: promising biomedical materials. Biomed Mater. 2021;16:022004. doi: 10.1088/1748-605X/abb615. [DOI] [PubMed] [Google Scholar]
58.Farokhi M, Aleemardani M, Solouk A, Mirzadeh H, Teuschl AH, Redl H. 丝素蛋白水凝胶的交联策略:有前途的生物医学材料。生物医学材料。2021;16:022004.doi:10.1088/1748-605X/abb615。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
59.Zhou Z, Cui J, Wu S, Geng Z, Su J. Silk fibroin-based biomaterials for cartilage/osteochondral repair. Theranostics. 2022;12:5103–24. doi: 10.7150/thno.74548. [DOI] [PMC free article] [PubMed] [Google Scholar]
59.周 Z, 崔 J, 吴 S, 耿 Z, 苏 J. 用于软骨/骨软骨修复的丝素蛋白基生物材料.治疗诊断学。2022;12:5103–24.doi:10.7150/THNO.74548。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
60.Schneider KH, Goldberg BJ, Hasturk O, Mu X, Dotzlhofer M, Eder G. et al. Silk fibroin, gelatin, and human placenta extracellular matrix-based composite hydrogels for 3D bioprinting and soft tissue engineering. Biomater Res. 2023;27:117. doi: 10.1186/s40824-023-00431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
60.Schneider KH, Goldberg BJ, Hasturk O, Mu X, Dotzlhofer M, Eder G. et al. 用于 3D 生物打印和软组织工程的丝素蛋白、明胶和基于人胎盘细胞外基质的复合水凝胶。生物材料研究 2023;27:117。doi:10.1186/s40824-023-00431-5。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
61.Kapoor S, Kundu SC. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016;31:17–32. doi: 10.1016/j.actbio.2015.11.034. [DOI] [PubMed] [Google Scholar]
61.Kapoor S, Kundu SC. 基于丝蛋白的水凝胶:用于生物医学应用的有前途的先进材料。生物学报。2016;31:17–32.doi:10.1016/j.actbio.2015.11.034。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
62.Liu J, Ge X, Liu L, Xu W, Shao R. Challenges and opportunities of silk protein hydrogels in biomedical applications. Mater Adv. 2022;3:2291–308. [Google Scholar]
62.Liu J, Ge X, Liu L, Xu W, Shao R. 蚕丝蛋白水凝胶在生物医学应用中的挑战和机遇.材料广告 2022;3:2291-308。 Google Scholar [ ] -
63.Wang ZX, Yin XY, Zhuang CY, Wu K, Wang HR, Shao ZZ. et al. Injectable Regenerated Silk Fibroin Micro/Nanosphere with Enhanced Permeability and Stability for Osteoarthritis Therapy. Small. 2024;20:e2405049. doi: 10.1002/smll.202405049. [DOI] [PubMed] [Google Scholar]
63.Wang ZX, Yin XY, Zhuang CY, Wu K, Wang HR, Shao ZZ. et al. 用于骨关节炎治疗的可注射再生丝素蛋白微/纳米球,具有增强的渗透性和稳定性。小。2024;20:e2405049.doi:10.1002/smll.202405049。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
64.Floren M, Migliaresi C, Motta A. Processing Techniques and Applications of Silk Hydrogels in Bioengineering. J Funct Biomater. 2016;7:7030026. doi: 10.3390/jfb7030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
64.Floren M, Migliaresi C, Motta A. 蚕丝水凝胶的加工技术和在生物工程中的应用。J Funct 生物材料。2016;7:7030026.doi:10.3390/jfb7030026。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
65.Sun W, Gregory DA, Tomeh MA, Zhao X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int J Mol Sci. 2021;22:1499. doi: 10.3390/ijms22031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
65.Sun W, Gregory DA, Tomeh MA, Zhao X. 蚕丝素蛋白作为组织工程的功能性生物材料。国际分子科学杂志 2021;22:1499。doi:10.3390/ijms22031499。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
66.Lin KL, Zhang DW, Macedo MH, Cui WG, Sarmento B, Shen GF. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv Funct Mater. 2019;29:1804943. [Google Scholar]
66.Lin KL, Zhang DW, Macedo MH, Cui WG, Sarmento B, Shen GF.用于再生生物医学的先进胶原蛋白基生物材料。Adv Funct Mater.2019;29:1804943。 Google Scholar [ ] -
67.Dong LP, Liu QL, Gao YL, Jia HX, Dai WL, Guo LK. et al. The effect of collagen hydrogels on chondrocyte behaviors through restricting the contraction of cell/hydrogel constructs. Regen Biomater. 2021;8:rbab030. doi: 10.1093/rb/rbab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
67.董 LP, 刘 QL, 高 YL, 贾 HX, 戴 WL, 郭 LK. 等胶原水凝胶通过限制细胞/水凝胶构建体的收缩对软骨细胞行为的影响。再生生物材料。2021;8:rbab030.doi:10.1093/rb/rbab030。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
68.Sahoo DR, Biswal T. Alginate and its application to tissue engineering. SN Appl Sci. 2021;3:30. [Google Scholar]
68.Sahoo DR, Biswal T. 海藻酸盐及其在组织工程中的应用。SN 应用科学 2021;3:30。 Google Scholar [ ] -
69.Venkatesan J, Bhatnagar I, Manivasagan P, Kang KH, Kim SK. Alginate composites for bone tissue engineering: A review. Int J Biol Macromol. 2015;72:269–81. doi: 10.1016/j.ijbiomac.2014.07.008. [DOI] [PubMed] [Google Scholar]
69.Venkatesan J, Bhatnagar I, Manivasagan P, Kang KH, Kim SK. 用于骨组织工程的海藻酸盐复合材料:综述。国际生物学杂志 Macromol.2015;72:269–81.doi:10.1016/j.ijbiomac.2014.07.008.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
70.Smith AM, Senior JJ. Alginate Hydrogels with Tuneable Properties. Adv Biochem Eng Biotechnol. 2021;178:37–61. doi: 10.1007/10_2020_161. [DOI] [PubMed] [Google Scholar]
70.Smith AM, Senior JJ. 具有可调特性的藻酸盐水凝胶。Adv Biochem Eng 生物技术。2021;178:37–61.doi:10.1007/10_2020_161。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
71.Hu YP, Liu NN, Chen K, Liu MX, Wang F, Liu P. et al. Resilient and Self-Healing Hyaluronic Acid/Chitosan Hydrogel With Ion Conductivity, Low Water Loss, and Freeze-Tolerance for Flexible and Wearable Strain Sensor. Front Bioeng Biotechnol. 2022;10:837750. doi: 10.3389/fbioe.2022.837750. [DOI] [PMC free article] [PubMed] [Google Scholar]
71.胡 YP, Liu NN, Chen K, Liu MX, Wang F, Liu P. et al. 弹性和自修复透明质酸/壳聚糖水凝胶,具有离子导电性、低水分损失和耐冻性,适用于柔性和可穿戴应变传感器。Front Bioeng 生物技术公司。2022;10:837750.doi:10.3389/fbioe.2022.837750。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
72.Tavsanli B, Okay O. Mechanically strong hyaluronic acid hydrogels with an interpenetrating network structure. Eur Polym J. 2017;94:185–95. [Google Scholar]
72.Tavsanli B, Okay O. 具有互穿网络结构的机械强度透明质酸水凝胶。Eur Polym J. 2017 年;94:185-95。 Google Scholar [ ] -
73.Borries M, Barooji YF, Yennek S, Grapin-Botton A, Berg-Sorensen K, Oddershede LB. Quantification of Visco-Elastic Properties of a Matrigel for Organoid Development as a Function of Polymer Concentration. Front Phys. 2020;8:579168. [Google Scholar]
73.Borries M, Barooji YF, Yennek S, Grapin-Botton A, Berg-Sorensen K, Oddershede LB. 用于类器官发育的基质胶的粘弹性特性与聚合物浓度的函数关系的定量。前物理学 2020;8:579168。 Google Scholar [ ] -
74.Méhes E, Biri-Kovács B, Isai DG, Gulyás M, Nyitray L, Czirók A. Matrigel patterning reflects multicellular contractility. PLoS Comput Biol. 2019;15:e1007431. doi: 10.1371/journal.pcbi.1007431. [DOI] [PMC free article] [PubMed] [Google Scholar]
74.Bees E, Biri-Kovács B, Isai DG, Gulyás M, Nyitray L, Czirók A. 基质胶图案反映了多细胞收缩性。PLoS 计算生物学。2019;15:E1007431。doi:10.1371/journal.pcbi.1007431。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ ] [ Google Scholar ] -
75.Passaniti A, Kleinman HK, Martin GR. Matrigel: history/background, uses, and future applications. J Cell Commun Signal. 2022;16:621–6. doi: 10.1007/s12079-021-00643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
75.Passaniti A, Kleinman HK, Martin GR. Matrigel:历史/背景、用途和未来应用。J Cell Commun 信号。2022;16:621–6.doi:10.1007/s12079-021-00643-1。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
76.Wongpinyochit T, Johnston BF, Seib FP. Degradation Behavior of Silk Nanoparticles-Enzyme Responsiveness. ACS Biomater Sci Eng. 2018;4:942–51. doi: 10.1021/acsbiomaterials.7b01021. [DOI] [PubMed] [Google Scholar]
76.Wongpinyochit T, Johnston BF, Seib FP. 蚕丝纳米颗粒的降解行为 - 酶反应性。ACS Biomater Sci Eng. 2018 年;4:942–51.doi:10.1021/acsbiomaterials.7b01021。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
77.Shuang F F, Wang C C, Zhu W J, Chen T, Yao X H, Zhang D Y. et al. Preparation of a robust silk fibroin scaffold with a reinforced concrete structure constructed with silk nanofibers as the skeleton based on a CaCl2-formic acid solution and freeze-drying method. Polym Test. 2022;111:107599. [Google Scholar]
77.Shuang F F, Wang C C, Zhu W J, Chen T, Yao X H, Zhang D Y. et al. 基于 CaCl2-甲酸溶液和冷冻干燥方法制备具有钢筋混凝土结构的坚固丝素蛋白支架,该支架以蚕丝纳米纤维为骨架。Polym 测试。2022;111:107599。 Google Scholar [ ] -
78.Yang C, Li S, Huang X, Chen X, Shan H, Chen X. et al. Silk Fibroin Hydrogels Could Be Therapeutic Biomaterials for Neurological Diseases. Oxid Med Cell Longev. 2022;2022:2076680. doi: 10.1155/2022/2076680. [DOI] [PMC free article] [PubMed] [Google Scholar]
78.Yang C, Li S, Huang X, Chen X, Shan H, Chen X. et al. 丝素蛋白水凝胶可作为神经系统疾病的治疗性生物材料。氧化医学细胞隆耶夫。2022;2022:2076680.doi:10.1155/2022/2076680.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
79.Teixeira LS, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials. 2012;33:1281–90. doi: 10.1016/j.biomaterials.2011.10.067. [DOI] [PubMed] [Google Scholar]
79.Teixeira LS, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M. 酶催化的交联水凝胶:组织工程的新兴策略。生物材料。2012;33:1281–90.doi: 10.1016/j.biomaterials.2011.10.067.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
80.Chen F, Yu S, Liu B, Ni Y, Yu C, Su Y. et al. An Injectable Enzymatically Crosslinked Carboxymethylated Pullulan/Chondroitin Sulfate Hydrogel for Cartilage Tissue Engineering. Sci Rep. 2016;6:20014. doi: 10.1038/srep20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
80.Chen F, Yu S, Liu B, Ni Y, Yu C, Su Y. 等.一种用于软骨组织工程的可注射酶交联羧甲基化支链淀粉/硫酸软骨素水凝胶。科学代表 2016;6:20014。doi:10.1038/srep20014。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
81.Chen W, Fu M, Zhu X, Liu Q. Protein recognition by polydopamine-based molecularly imprinted hollow spheres. Biosens Bioelectron. 2019;142:111492. doi: 10.1016/j.bios.2019.111492. [DOI] [PubMed] [Google Scholar]
81.Chen W, Fu M, Zhu X, Liu Q. 基于聚多巴胺的分子印记空心球体的蛋白质识别。Biosens 生物电子。2019;142:111492.doi:10.1016/j.bios.2019.111492.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
82.Chen W, Fu M, Zhu X, Liu Q. A close-packed imprinted colloidal array for naked-eye detection of glycoproteins under physiological pH. Biosens Bioelectron. 2019;142:111499. doi: 10.1016/j.bios.2019.111499. [DOI] [PubMed] [Google Scholar]
82.陈文, 付明, 朱晓, 刘群.一种紧密排列的印迹胶体阵列,用于在生理 pH 值下肉眼检测糖蛋白。Biosens 生物电子。2019;142:111499.doi:10.1016/j.bios.2019.111499。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
83.Tabatabai AP, Partlow BP, Raia NR, Kaplan DL, Blair DL. Silk Molecular Weight Influences the Kinetics of Enzymatically Cross-linked Silk Hydrogel Formation. Langmuir. 2018;34:15383–7. doi: 10.1021/acs.langmuir.8b02950. [DOI] [PubMed] [Google Scholar]
83.Tabatabai AP, Partlow BP, Raia NR, Kaplan DL, Blair DL.蚕丝分子量影响酶交联蚕丝水凝胶形成的动力学。朗缪尔。2018;34:15383–7.doi:10.1021/acs.langmuir.8b02950。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
84.Chen W, Zhang L, Sun Y, Yu H, Fu M. pH-Responsive molecularly imprinted hollow spheres for selective separation of ribavirin from water samples. Chem Eng J. 2022;450:138064. [Google Scholar]
84.Chen W, Zhang L, Sun Y, Yu H, Fu M. 用于从水样中选择性分离利巴韦林的 pH 响应分子印记空心球体。化学工程杂志 2022;450:138064。 Google Scholar [ ] -
85.Partlow BP, Hanna CW, Rnjak-Kovacina J, Moreau JE, Applegate MB, Burke KA. et al. Highly tunable elastomeric silk biomaterials. Adv Funct Mater. 2014;24:4615–24. doi: 10.1002/adfm.201400526. [DOI] [PMC free article] [PubMed] [Google Scholar]
85.Partlow BP, Hanna CW, Rnjak-Kovacina J, Moreau JE, Applegate MB, Burke KA. et al. 高度可调的弹性丝生物材料。Adv Funct Mater.2014;24:4615–24.doi:10.1002/adfm.201400526。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
86.Hasturk O, Jordan KE, Choi J, Kaplan DL. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials. 2020;232:119720. doi: 10.1016/j.biomaterials.2019.119720. [DOI] [PMC free article] [PubMed] [Google Scholar]
86.Hasturk O, Jordan KE, Choi J, Kaplan DL.酶交联丝和丝-明胶水凝胶,具有可调的凝胶动力学、机械性能和生物活性,适用于细胞培养和封装。生物材料。2020;232:119720.doi:10.1016/j.biomaterials.2019.119720.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
87.Li Q, Xu S, Feng Q, Dai Q, Yao L, Zhang Y. et al. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact Mater. 2021;6:3396–410. doi: 10.1016/j.bioactmat.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
87.Li Q, Xu S, Feng Q, Dai Q, Yao L, Zhang Y. et al. 3D 具有不同多孔结构和细胞接种策略的印刷丝-明胶水凝胶支架用于软骨再生。生物行为材料。2021;6:3396–410.doi:10.1016/j.bioactmat.2021.03.013.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
88.Wang L, Zhang YF, Xia YJ, Xu CZ, Meng KJ, Lian J. et al. Photocross-linked silk fibroin/hyaluronic acid hydrogel loaded with hDPSC for pulp regeneration. Int J Biol Macromol. 2022;215:155–68. doi: 10.1016/j.ijbiomac.2022.06.087. [DOI] [PubMed] [Google Scholar]
88.Wang L, Zhang YF, Xia YJ, Xu CZ, Meng KJ, Lian J. et al. 负载 hDPSC 的光交联丝素/透明质酸水凝胶用于牙髓再生。国际生物学杂志 Macromol.2022;215:155–68.doi:10.1016/j.ijbiomac.2022.06.087.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
89.Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C. et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater. 2014;26:85–123. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
89.Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C. et al. 25周年纪念文章:水凝胶在再生医学中的合理设计和应用。高级材料。2014;26:85–123.doi: 10.1002/adma.201303233.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
90.Piluso S, Flores Gomez D, Dokter I, Moreira Texeira L, Li Y, Leijten J. et al. Rapid and cytocompatible cell-laden silk hydrogel formation via riboflavin-mediated crosslinking. J Mater Chem B. 2020;8:9566–75. doi: 10.1039/d0tb01731k. [DOI] [PubMed] [Google Scholar]
90.Piluso S, Flores Gomez D, Dokter I, Moreira Texeira L, Li Y, Leijten J. et al. 通过核黄素介导的交联快速且具有细胞相容性的载细胞丝水凝胶形成。J Mater Chem B. 2020 年;8:9566–75.doi:10.1039/d0tb01731k。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
91.Cui X, Soliman BG, Alcala-Orozco CR, Li J, Vis MAM, Santos M. et al. Rapid Photocrosslinking of Silk Hydrogels with High Cell Density and Enhanced Shape Fidelity. Adv Healthc Mater. 2020;9:e1901667. doi: 10.1002/adhm.201901667. [DOI] [PubMed] [Google Scholar]
91.Cui X, Soliman BG, Alcala-Orozco CR, Li J, Vis MAM, Santos M. et al. 具有高细胞密度和增强形状保真度的丝绸水凝胶的快速光交联。Adv Healthc Mater.2020;9:e1901667.doi:10.1002/adhm.201901667.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
92.Kim MH, Kim BS, Lee J, Cho D, Kwon OH, Park WH. Silk fibroin/hydroxyapatite composite hydrogel induced by gamma-ray irradiation for bone tissue engineering. Biomater Res. 2017;21:12. doi: 10.1186/s40824-017-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
92.Kim MH, Kim BS, Lee J, Cho D, Kwon OH, Park WH. 伽马射线照射诱导的丝素蛋白/羟基磷灰石复合水凝胶用于骨组织工程。生物材料研究 2017;21:12。doi:10.1186/s40824-017-0098-2。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
93.Kim MH, Park WH. Chemically cross-linked silk fibroin hydrogel with enhanced elastic properties, biodegradability, and biocompatibility. Int J Nanomedicine. 2016;11:2967–78. doi: 10.2147/IJN.S106467. [DOI] [PMC free article] [PubMed] [Google Scholar]
93.Kim MH, Park WH. 化学交联丝素蛋白水凝胶,具有增强的弹性、生物降解性和生物相容性。国际纳米医学杂志。2016;11:2967–78.doi: 10.2147/日本.S106467。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
94.Zheng H, Zuo B. Functional silk fibroin hydrogels: preparation, properties and applications. J Mater Chem B. 2021;9:1238–58. doi: 10.1039/d0tb02099k. [DOI] [PubMed] [Google Scholar]
94.Zheng H, Zuo B. 功能性丝素蛋白水凝胶:制备、性质和应用。J Mater Chem B. 2021 年;9:1238–58.doi:10.1039/d0tb02099k。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
95.Srisawasdi T, Petcharoen K, Sirivat A, Jamieson AM. Electromechanical response of silk fibroin hydrogel and conductive polycarbazole/silk fibroin hydrogel composites as actuator material. Mater Sci Eng C Mater Biol Appl. 2015;56:1–8. doi: 10.1016/j.msec.2015.06.005. [DOI] [PubMed] [Google Scholar]
95.Srisawasdi T, Petcharoen K, Sirivat A, Jamieson AM.丝素蛋白水凝胶和导电聚咔唑/丝素蛋白水凝胶复合材料作为致动器材料的机电响应。Mater Sci Eng C Mater Biol Appl. 2015;56:1–8.doi:10.1016/j.msec.2015.06.005。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
96.Min Q, Tian D, Zhang Y, Wang C, Wan Y, Wu J. Strong and Elastic Chitosan/Silk Fibroin Hydrogels Incorporated with Growth-Factor-Loaded Microspheres for Cartilage Tissue Engineering. Biomimetics (Basel) 2022;7:41. doi: 10.3390/biomimetics7020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
96.Min Q, Tian D, Zhang Y, Wang C, Wan Y, Wu J. 强壮而有弹性的壳聚糖/丝素蛋白水凝胶与负载生长因子的微球相结合,用于软骨组织工程。仿生学(巴塞尔)2022;7:41。doi:10.3390/biomimetics7020041。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
97.Wang W, Nema S, Teagarden D. Protein aggregation-pathways and influencing factors. Int J Pharm. 2010;390:89–99. doi: 10.1016/j.ijpharm.2010.02.025. [DOI] [PubMed] [Google Scholar]
97.Wang W, Nema S, Teagarden D. 蛋白质聚集途径和影响因素。国际药学杂志 2010;390:89–99.doi:10.1016/j.ijpharm.2010.02.025。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
98.Nagarkar S, Nicolai T, Chassenieux C, Lele A. Structure and gelation mechanism of silk hydrogels. Phys Chem Chem Phys. 2010;12:3834–44. doi: 10.1039/b916319k. [DOI] [PubMed] [Google Scholar]
98.Nagarkar S, Nicolai T, Chassenieux C, Lele A. 蚕丝水凝胶的结构和凝胶化机制。Phys Chem Chem Phys. 2010 年;12:3834–44.doi:10.1039/b916319k。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
99.Kim U-J, Park J, Li C, Jin H-J, Valluzzi R, Kaplan DL. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5:786–92. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
99.Kim U-J, Park J, Li C, Jin H-J, Valluzzi R, Kaplan DL.蚕丝水凝胶的结构和性能。生物大分子。2004;5:786–92.doi:10.1021/bm0345460。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
100.Nagarkar S, Patil A, Lele A, Bhat S, Bellare J, Mashelkar RA. Some Mechanistic Insights into the Gelation of Regenerated Silk Fibroin Sol. Ind Eng Chem Res. 2009;48:8014–23. [Google Scholar]
100.Nagarkar S, Patil A, Lele A, Bhat S, Bellare J, Mashelkar RA.对再生丝素蛋白凝胶化的一些机制见解溶胶。Ind Eng Chem Res. 2009;48:8014-23。 Google Scholar [ ] -
101.Fini M, Motta A, Torricelli P, Giavaresi G, Nicoli Aldini N, Tschon M. et al. The healing of confined critical size cancellous defects in the presence of silk fibroin hydrogel. Biomaterials. 2005;26:3527–36. doi: 10.1016/j.biomaterials.2004.09.040. [DOI] [PubMed] [Google Scholar]
101.Fini M, Motta A, Torricelli P, Giavaresi G, Nicoli Aldini N, Tschon M. et al.在丝素蛋白水凝胶存在下局限临界大小的松质性缺损的愈合。生物材料。2005;26:3527–36.doi:10.1016/j.biomaterials.2004.09.040。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
102.Kojic N, Pritchard EM, Tao H, Brenckle MA, Mondia JP, Panilaitis B. et al. Focal Infection Treatment using Laser-Mediated Heating of Injectable Silk Hydrogels with Gold Nanoparticles. Adv Funct Mater. 2012;22:3793–8. doi: 10.1002/adfm.201200382. [DOI] [PMC free article] [PubMed] [Google Scholar]
102.Kojic N, Pritchard EM, Tao H, Brenckle MA, Mondia JP, Panilaitis B. et al. 使用激光介导的金纳米颗粒注射蚕丝水凝胶的局部感染治疗。Adv Funct Mater.2012;22:3793–8.doi:10.1002/adfm.201200382。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
103.Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE. et al. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials. 2012;33:6691–7. doi: 10.1016/j.biomaterials.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
103.Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE. et al. 与蚕丝水凝胶中的 ECM 蛋白和间充质基质细胞共封装的胰岛的增强功能。生物材料。2012;33:6691–7.doi:10.1016/j.biomaterials.2012.06.015.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
104.Chen D, Yin Z, Wu F, Fu H, Kundu SC, Lu S. Orientational behaviors of silk fibroin hydrogels. J Appl Polym Sci. 2017;134:45050. [Google Scholar]
104.Chen D, Yin Z, Wu F, Fu H, Kundu SC, Lu S. 丝素蛋白水凝胶的取向行为。应用多利姆科学杂志 2017;134:45050。 Google Scholar [ ] -
105.Kasoju N, Hawkins N, Pop-Georgievski O, Kubies D, Vollrath F. Silk fibroin gelation via non-solvent induced phase separation. Biomater Sci. 2016;4:460–73. doi: 10.1039/c5bm00471c. [DOI] [PubMed] [Google Scholar]
105.Kasoju N, Hawkins N, Pop-Georgievski O, Kubies D, Vollrath F. 通过非溶剂诱导相分离的丝素蛋白凝胶化。Biomater Sci. 2016;4:460–73.doi:10.1039/c5bm00471c.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
106.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–64. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
106.Wang X, Kluge JA, Leisk GG, Kaplan DL.用于细胞包埋的丝素蛋白超声诱导凝胶化。生物材料。2008;29:1054–64.doi: 10.1016/j.biomaterials.2007.11.003.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
107.Yuan T, Li Z, Zhang Y, Shen K, Zhang X, Xie R. et al. Injectable Ultrasonication-Induced Silk Fibroin Hydrogel for Cartilage Repair and Regeneration. Tissue Eng Part A. 2021;27:1213–24. doi: 10.1089/ten.TEA.2020.0323. [DOI] [PubMed] [Google Scholar]
107.Yuan T, Li Z, Zhang Y, Shen K, Zhang X, Xie R. et al. 用于软骨修复和再生的可注射超声诱导的丝素蛋白水凝胶。纸巾工程 A 部分 2021;27:1213–24.doi:10.1089/十。茶.2020.0323. [ DOI ] [ PubMed ] [ Google Scholar ] -
108.Byram PK, Sunka KC, Barik A, Kaushal M, Dhara S, Chakravorty N. Biomimetic silk fibroin and xanthan gum blended hydrogels for connective tissue regeneration. Int J Biol Macromol. 2020;165:874–82. doi: 10.1016/j.ijbiomac.2020.09.231. [DOI] [PubMed] [Google Scholar]
108.Byram PK, Sunka KC, Barik A, Kaushal M, Dhara S, Chakravorty N. 用于结缔组织再生的仿生丝素蛋白和黄原胶混合水凝胶。国际生物学杂志 Macromol.2020;165:874–82.doi:10.1016/j.ijbiomac.2020.09.231。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
109.Leisk GG, Lo TJ, Yucel T, Lu Q, Kaplan DL. Electrogelation for protein adhesives. Adv Mater. 2010;22:711–5. doi: 10.1002/adma.200902643. [DOI] [PubMed] [Google Scholar]
109.Leisk GG, Lo TJ, Yucel T, Lu Q, Kaplan DL.用于蛋白质粘合剂的电凝胶化。高级材料。2010;22:711–5.doi:10.1002/adma.200902643。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
110.Liu H, Ming J, Guo X, Huang X, Zuo B, Ning X. Low voltage electric field governs fibrous silk electrogels. Mater Des. 2021;199:109401. [Google Scholar]
110.Liu H, Ming J, Guo X, Huang X, Zuo B, Ning X. 低压电场控制纤维丝电凝胶。材料描述2021;199:109401。 Google Scholar [ ] -
111.Xu G, Ding Z, Lu Q, Zhang X, Zhou X, Xiao L. et al. Electric field-driven building blocks for introducing multiple gradients to hydrogels. Protein Cell. 2020;11:267–85. doi: 10.1007/s13238-020-00692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
111.Xu G, Ding Z, Lu Q, Zhang X, 周 X, Xiao L. et al. 用于向水凝胶引入多个梯度的电场驱动构建块.蛋白质细胞。2020;11:267–85.doi:10.1007/s13238-020-00692-z。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
112.Wang B, Yuan S, Xin W, Chen Y, Fu Q, Li L. et al. Synergic adhesive chemistry-based fabrication of BMP-2 immobilized silk fibroin hydrogel functionalized with hybrid nanomaterial to augment osteogenic differentiation of rBMSCs for bone defect repair. Int J Biol Macromol. 2021;192:407–16. doi: 10.1016/j.ijbiomac.2021.09.036. [DOI] [PubMed] [Google Scholar]
112.Wang B, Yuan S, Xin W, Chen Y, Fu Q, Li L. et al. 用杂化纳米材料功能化的 BMP-2 固定化丝素蛋白水凝胶的协同胶粘剂化学制备,以增强 rBMSCs 的成骨分化,用于骨缺损修复。国际生物学杂志 Macromol.2021;192:407–16.doi:10.1016/j.ijbiomac.2021.09.036.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
113.Balu R, Reeder S, Knott R, Mata J, de Campo L, Dutta NK. et al. Tough Photocrosslinked Silk Fibroin/Graphene Oxide Nanocomposite Hydrogels. Langmuir. 2018;34:9238–51. doi: 10.1021/acs.langmuir.8b01141. [DOI] [PubMed] [Google Scholar]
113.Balu R, Reeder S, Knott R, Mata J, de Campo L, Dutta NK. et al. 坚韧的光交联丝素蛋白/氧化石墨烯纳米复合水凝胶。朗缪尔。2018;34:9238–51.doi:10.1021/acs.langmuir.8b01141。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
114.Zhang Y, Cao Y, Zhang L, Zhao H, Ni T, Liu Y. et al. Fabrication of an injectable BMSC-laden double network hydrogel based on silk fibroin/PEG for cartilage repair. J Mater Chem B. 2020;8:5845–8. doi: 10.1039/d0tb01017k. [DOI] [PubMed] [Google Scholar]
114.Zhang Y, Cao Y, Zhang L, Zhao H, Ni T, Liu Y. et al. 基于丝素蛋白/PEG 的可注射载有 BMSC 的双网络水凝胶的制备用于软骨修复。J Mater Chem B. 2020 年;8:5845–8.doi:10.1039/d0tb01017k.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
115.Fathi-Achachelouei M, Keskin D, Bat E, Vrana NE, Tezcaner A. Dual growth factor delivery using PLGA nanoparticles in silk fibroin/PEGDMA hydrogels for articular cartilage tissue engineering. J Biomed Mater Res B Appl Biomater. 2020;108:2041–62. doi: 10.1002/jbm.b.34544. [DOI] [PubMed] [Google Scholar]
115.Fathi-Achachelouei M, Keskin D, Bat E, Vrana NE, Tezcaner A. 在丝素蛋白/PEGDMA 水凝胶中使用 PLGA 纳米颗粒进行双生长因子递送,用于关节软骨组织工程。J Biomed Mater Res B Appl 生物材料。2020;108:2041–62.doi:10.1002/jbm.b.34544。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
116.Bandyopadhyay A, Mandal BB, Bhardwaj N. 3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering. J Biomed Mater Res A. 2022;110:884–98. doi: 10.1002/jbm.a.37336. [DOI] [PubMed] [Google Scholar]
116.Bandyopadhyay A、Mandal BB、Bhardwaj N. 3D用于软骨组织工程的光交联甲基丙烯酸丝 (SilMA)-聚乙二醇二丙烯酸酯 (PEGDA) 生物墨水的生物打印。J Biomed Mater Res A. 2022 年;110:884–98.doi:10.1002/jbm.a.37336。[ DOI ] [ PubMed ] [ ] ] Google Scholar -
117.Whittaker JL, Dutta NK, Zannettino A, Choudhury NR. Engineering DN hydrogels from regenerated silk fibroin and poly(N-vinylcaprolactam) J Mater Chem B. 2016;4:5519–33. doi: 10.1039/c6tb01055e. [DOI] [PubMed] [Google Scholar]
117.Whittaker JL, Dutta NK, Zannettino A, Choudhury NR.来自再生丝素蛋白和聚(N-乙烯基己内酰胺)的工程 DN 水凝胶 J Mater Chem B. 2016;4:5519–33.doi:10.1039/c6tb01055e.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
118.Allur Subramanian S, Oh S, Mariadoss AVA, Chae S, Dhandapani S, Parasuraman PS. et al. Tunable mechanical properties of Mo3Se3-poly vinyl alcohol-based/silk fibroin-based nanowire ensure the regeneration mechanism in tenocytes derived from human bone marrow stem cells. Int J Biol Macromol. 2022;210:196–207. doi: 10.1016/j.ijbiomac.2022.04.211. [DOI] [PubMed] [Google Scholar]
118.Allur Subramanian S, Oh S, Mariadoss AVA, Chae S, Dhandapani S, Parasuraman PS. et al. Mo3Se3-聚乙烯醇基/丝素蛋白基纳米线的可调机械性能确保了源自人骨髓干细胞的肌腱细胞的再生机制。国际生物学杂志 Macromol.2022;210:196–207.doi:10.1016/j.ijbiomac.2022.04.211。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
119.Min Q, Liu J, Zhang Y, Yang B, Wan Y, Wu J. Dual Network Hydrogels Incorporated with Bone Morphogenic Protein-7-Loaded Hyaluronic Acid Complex Nanoparticles for Inducing Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells. Pharmaceutics. 2020;12:613. doi: 10.3390/pharmaceutics12070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
119.Min Q, Liu J, Zhang Y, Yang B, Wan Y, Wu J. 掺入载有骨形态生成蛋白-7 的透明质酸复合物纳米颗粒的双网络水凝胶诱导滑膜来源的间充质干细胞的软骨形成分化。制药学。2020;12:613.doi: 10.3390/pharmaceutics12070613.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
120.Liu J, Fang Q, Lin H, Yu X, Zheng H, Wan Y. Alginate-poloxamer/silk fibroin hydrogels with covalently and physically cross-linked networks for cartilage tissue engineering. Carbohydr Polym. 2020;247:116593. doi: 10.1016/j.carbpol.2020.116593. [DOI] [PubMed] [Google Scholar]
120.Liu J, Fang Q, Lin H, Yu X, Zheng H, Wan Y. 藻酸盐-泊洛沙姆/丝素蛋白水凝胶,具有共价和物理交联网络,用于软骨组织工程。碳水化合物 Polym.2020;247:116593.doi:10.1016/j.carbpol.2020.116593.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
121.Hu ZC, Lu JQ, Zhang TW, Liang HF, Yuan H, Su DH. et al. Piezoresistive MXene/Silk fibroin nanocomposite hydrogel for accelerating bone regeneration by Re-establishing electrical microenvironment. Bioact Mater. 2023;22:1–17. doi: 10.1016/j.bioactmat.2022.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
121.胡 ZC, 卢建军, 张TW, 梁 HF, 袁华, 苏 DH. 等. 压阻式 MXene/Silk 丝素蛋白纳米复合水凝胶通过重建电微环境加速骨再生。生物行为材料。2023;22:1–17.doi:10.1016/j.bioactmat.2022.08.025。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
122.Yang ZZ, You YX, Liu XY, Wan Q, Xu ZP, Shuai YJ. et al. Injectable Bombyx mori (B. mori) silk fibroin/MXene conductive hydrogel for electrically stimulating neural stem cells into neurons for treating brain damage. J Nanobiotechnology. 2024;22:111. doi: 10.1186/s12951-024-02359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
122.Yang ZZ, You YX, Liu XY, Wan Q, Xu ZP, Shuai YJ. et al. 可注射的家蚕丝素/MXene 导电水凝胶,用于电刺激神经干细胞进入神经元,用于治疗脑损伤。J 纳米生物技术。2024;22:111.doi:10.1186/s12951-024-02359-x.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
123.Long S, Huang D, Ma Z, Shi S, Xiao Y, Zhang X. A sonication-induced silk-collagen hydrogel for functional cartilage regeneration. J Mater Chem B. 2022;10:5045–57. doi: 10.1039/d2tb00564f. [DOI] [PubMed] [Google Scholar]
123.龙S, 黄 D, 马 Z, 石 S, 肖 Y, 张 X.一种超声处理诱导的丝胶原水凝胶,用于功能性软骨再生。J Mater Chem B. 2022 年;10:5045–57. doi: 10.1039/d2tb00564f.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
124.Zhang Y, Liu M, Pei R. An in situ gelling BMSC-laden collagen/silk fibroin double network hydrogel for cartilage regeneration. Mater Adv. 2021;2:4733–42. [Google Scholar]
124.张 Y, 刘 M, 裴 R.一种用于软骨再生的原位胶凝含有 BMSC 的胶原蛋白/丝素蛋白双网络水凝胶。材料广告 2021;2:4733-42。 Google Scholar [ ] -
125.Trucco D, Sharma A, Manferdini C, Gabusi E, Petretta M, Desando G. et al. Modeling and Fabrication of Silk Fibroin-Gelatin-Based Constructs Using Extrusion-Based Three-Dimensional Bioprinting. ACS Biomater Sci Eng. 2021;7:3306–20. doi: 10.1021/acsbiomaterials.1c00410. [DOI] [PubMed] [Google Scholar]
125.Trucco D, Sharma A, Manferdini C, Gabusi E, Petretta M, Desando G. et al. 使用基于挤出的三维生物打印对基于丝素蛋白-明胶的结构进行建模和制造。ACS Biomater Sci Eng. 2021 年;7:3306–20.doi:10.1021/acsbiomaterials.1c00410。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
126.Park S, Edwards S, Hou S, Boudreau R, Yee R, Jeong KJ. A multi-interpenetrating network (IPN) hydrogel with gelatin and silk fibroin. Biomater Sci. 2019;7:1276–80. doi: 10.1039/c8bm01532e. [DOI] [PMC free article] [PubMed] [Google Scholar]
126.Park S, Edwards S, Hou S, Boudreau R, Yee R, Jeong KJ.一种含有明胶和丝素蛋白的多互穿网络 (IPN) 水凝胶。生物材料科学 2019;7:1276–80.doi: 10.1039/c8bm01532e.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
127.Luo K, Yang Y, Shao Z. Physically Crosslinked Biocompatible Silk-Fibroin-Based Hydrogels with High Mechanical Performance. Adv Funct Mater. 2016;26:872–80. [Google Scholar]
127.Luo K, Yang Y, Shao Z. 具有高机械性能的物理交联生物相容性丝素蛋白基水凝胶。Adv Funct Mater.2016;26:872-80。 Google Scholar [ ] -
128.Mirahmadi F, Tafazzoli-Shadpour M, Shokrgozar MA, Bonakdar S. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2013;33:4786–94. doi: 10.1016/j.msec.2013.07.043. [DOI] [PubMed] [Google Scholar]
128.Mirahmadi F, Tafazzoli-Shadpour M, Shokrgozar MA, Bonakdar S. 通过丝纤维增强热敏壳聚糖水凝胶的机械性能,用于软骨组织工程。Mater Sci Eng C Mater Biol Appl. 2013;33:4786–94.doi: 10.1016/j.msec.2013.07.043.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
129.Xu Z, Tang E, Zhao H. An Environmentally Sensitive Silk Fibroin/Chitosan Hydrogel and Its Drug Release Behaviors. Polymers (Basel) 2019;11:1980. doi: 10.3390/polym11121980. [DOI] [PMC free article] [PubMed] [Google Scholar]
129.徐 Z, 唐 E, 赵 H.一种环境敏感的丝素蛋白/壳聚糖水凝胶及其药物释放行为。聚合物(巴塞尔)2019;11:1980。doi:10.3390/polym11121980。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
130.Yu Y, Yu X, Tian D, Yu A, Wan Y. Thermo-responsive chitosan/silk fibroin/amino-functionalized mesoporous silica hydrogels with strong and elastic characteristics for bone tissue engineering. Int J Biol Macromol. 2021;182:1746–58. doi: 10.1016/j.ijbiomac.2021.05.166. [DOI] [PubMed] [Google Scholar]
130.Yu Y, Yu X, Tian D, Yu A, Wan Y. 热响应壳聚糖/丝素蛋白/氨基功能化介孔二氧化硅水凝胶,具有用于骨组织工程的强度和弹性特性。国际生物学杂志 Macromol.2021;182:1746–58.doi:10.1016/j.ijbiomac.2021.05.166.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
131.Dong Y, Liu Y, Chen Y, Sun X, Zhang L, Zhang Z. et al. Spatiotemporal regulation of endogenous MSCs using a functional injectable hydrogel system for cartilage regeneration. NPG Asia Mater. 2021;13:71. [Google Scholar]
131.Dong Y, Liu Y, Chen Y, Sun X, Zhang L, Zhang Z. et al. 使用功能性可注射水凝胶系统进行软骨再生的内源性 MSC 的时空调控。NPG 亚洲材料。2021;13:71。 Google Scholar [ ] -
132.Weitkamp JT, Woltje M, Nusspickel B, Schmidt FN, Aibibu D, Bayer A. et al. Silk Fiber-Reinforced Hyaluronic Acid-Based Hydrogel for Cartilage Tissue Engineering. Int J Mol Sci. 2021;22:3635. doi: 10.3390/ijms22073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
132.Weitkamp JT, Woltje M, Nusspickel B, Schmidt FN, Aibibu D, Bayer A. et al. 用于软骨组织工程的丝纤维增强透明质酸基水凝胶。国际分子科学杂志 2021;22:3635. doi: 10.3390/ijms22073635.[ DOI ] [ PMC free article PubMed ] [ ] Google Scholar [ ] [ ] [ ] -
133.Ziadlou R, Rotman S, Teuschl A, Salzer E, Barbero A, Martin I. et al. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater Sci Eng C Mater Biol Appl. 2021;120:111701. doi: 10.1016/j.msec.2020.111701. [DOI] [PubMed] [Google Scholar]
133.Ziadlou R, Rotman S, Teuschl A, Salzer E, Barbero A, Martin I. et al. 透明质酸-酪胺/丝素蛋白复合水凝胶的优化,用于软骨组织工程和抗炎和合成代谢药物的递送。材料科学工程 C 材料生物学应用2021;120:111701。doi:10.1016/j.msec.2020.111701.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
134.Wang X, Song X, Li T, Chen J, Cheng G, Yang L. et al. Aptamer-Functionalized Bioscaffold Enhances Cartilage Repair by Improving Stem Cell Recruitment in Osteochondral Defects of Rabbit Knees. Am J Sports Med. 2019;47:2316–26. doi: 10.1177/0363546519856355. [DOI] [PubMed] [Google Scholar]
134.Wang X, Song X, Li T, Chen J, Cheng G, Yang L. et al. 适配体功能化生物支架通过改善兔膝骨软骨缺损中的干细胞募集来增强软骨修复。美国运动医学杂志 2019;47:2316–26.doi: 10.1177/0363546519856355.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
135.Ding J, Chen ZH, Liu XY, Tian Y, Jiang J, Qiao Z. et al. A mechanically adaptive hydrogel neural interface based on silk fibroin for high-efficiency neural activity recording. Mater Horiz. 2022;9:2215–25. doi: 10.1039/d2mh00533f. [DOI] [PubMed] [Google Scholar]
135.丁 J, 陈 志, 刘 XY , 田 Y, 江 J, 乔 Z 等.一种基于丝素蛋白的机械自适应水凝胶神经接口,用于高效神经活动记录。马特·霍里兹。2022;9:2215–25.doi:10.1039/d2MH00533F。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
136.Zhang BC, Wang WS, Gao P, Li X, Chen LL, Lin ZF. et al. Injectable, Electroconductive, Free Radical Scavenging Silk Fibroin/Black Phosphorus/Glycyrrhizic Acid Nanocomposite Hydrogel for Enhancing Spinal Cord Repair. Adv Healthc Mater. 2024;13:2304300. doi: 10.1002/adhm.202304300. [DOI] [PubMed] [Google Scholar]
136.Zhang BC, Wang WS, Gao P, Li X, Chen LL, Lin ZF. et al. 注射、导电、自由基清除丝素蛋白/黑磷/甘草酸纳米复合水凝胶用于增强脊髓修复。Adv Healthc Mater.2024;13:2304300.doi:10.1002/adhm.202304300.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
137.Zhou Z, Song P, Wu Y, Wang M, Shen C, Ma Z. et al. Dual-network DNA-Silk fibroin hydrogels with controllable surface rigidity for regulating chondrogenic differentiation. Mater Horiz. 2024;11:1465–1483. doi: 10.1039/d3mh01581e. [DOI] [PubMed] [Google Scholar]
137.周 Z, 宋 P, 吴宇, 王 M, 沈 C, 马 Z. 等. 具有可控表面刚性的双网络 DNA-丝素蛋白水凝胶,用于调节软骨形成分化。马特·霍里兹。2024;11:1465–1483.doi: 10.1039/d3MH01581E.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
138.Zhao Y, Zhu ZS, Guan J, Wu SJ. Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels. Acta Biomater. 2021;125:57–71. doi: 10.1016/j.actbio.2021.02.018. [DOI] [PubMed] [Google Scholar]
138.赵 Y, 朱 ZS, 关 J, 吴 SJ.丝素蛋白基高强度水凝胶的加工、机械性能和生物应用。生物学报。2021;125:57–71.doi:10.1016/j.actbio.2021.02.018。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
139.Kim JS, Choi J, Ki CS, Lee KH. 3D Silk Fiber Construct Embedded Dual-Layer PEG Hydrogel for Articular Cartilage Repair - In vitro Assessment. Front Bioeng Biotechnol. 2021;9:653509. doi: 10.3389/fbioe.2021.653509. [DOI] [PMC free article] [PubMed] [Google Scholar]
139.Kim JS, Choi J, Ki CS, Lee KH. 3D 用于关节软骨修复的蚕丝纤维构建嵌入双层 PEG 水凝胶 - 体外评估。Front Bioeng 生物技术公司。2021;9:653509.doi:10.3389/fbioe.2021.653509。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
140.Kim SH, Hong H, Ajiteru O, Sultan MT, Lee YJ, Lee JS. et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat Protoc. 2021;16:5484–532. doi: 10.1038/s41596-021-00622-1. [DOI] [PubMed] [Google Scholar]
140.Kim SH、Hong H、Ajiteru O、Sultan MT、Lee YJ、Lee JS. 等al. 3D用于组织工程的生物打印丝素蛋白水凝胶。Nat Protoc.2021;16:5484–532.doi:10.1038/s41596-021-00622-1。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
141.Xue X, Zhang H, Liu H, Wang S, Li J, Zhou Q. et al. Rational Design of Multifunctional CuS Nanoparticle-PEG Composite Soft Hydrogel-Coated 3D Hard Polycaprolactone Scaffolds for Efficient Bone Regeneration. Adv Funct Mater. 2022;32:2202470. [Google Scholar]
141.薛晓、张海、刘海、王S、李杰、周Q.等. 多功能CuS纳米颗粒-PEG复合软水凝胶涂层3D硬质聚己内酯支架的合理设计,用于高效骨再生。Adv Funct Mater.2022;32:2202470。 Google Scholar [ ] -
142.Lee JM, Sultan MT, Kim SH, Kumar V, Yeon YK, Lee OJ. et al. Artificial Auricular Cartilage Using Silk Fibroin and Polyvinyl Alcohol Hydrogel. Int J Mol Sci. 2017;18:1707. doi: 10.3390/ijms18081707. [DOI] [PMC free article] [PubMed] [Google Scholar]
142.Lee JM, Sultan MT, Kim SH, Kumar V, Yeon YK, Lee OJ. et al. 使用丝素蛋白和聚乙烯醇水凝胶的人工耳软骨。国际分子科学杂志 2017;18:1707。doi:10.3390/ijms18081707。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
143.Wang K, Ma Q, Zhang Y-M, Han G-T, Qu C-X, Wang S-D. Preparation of bacterial cellulose/silk fibroin double-network hydrogel with high mechanical strength and biocompatibility for artificial cartilage. Cellulose. 2019;27:1845–52. [Google Scholar]
143.王 K, 马 Q, 张 Y-M, 韩 G-T, 瞿 C-X, 王 S-D.制备具有高机械强度和生物相容性的细菌纤维素/丝素蛋白双网络水凝胶,用于人工软骨。纤维素。2019;27:1845-52。 Google Scholar [ ] -
144.Adali T, Kalkan R, Karimizarandi L. The chondrocyte cell proliferation of a chitosan/silk fibroin/egg shell membrane hydrogels. Int J Biol Macromol. 2019;124:541–7. doi: 10.1016/j.ijbiomac.2018.11.226. [DOI] [PubMed] [Google Scholar]
144.阿达利 T, 卡尔坎 R, 卡里米扎兰迪 L.壳聚糖/丝素蛋白/蛋壳膜水凝胶的软骨细胞细胞增殖。You J Biol Macromol.2019;124:541–7.doi:10.1016/j.ijbiomac.2018.11.226.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
145.Dong T, Mi R, Wu M, Zhong N, Zhao X, Chen X. et al. The regenerated silk fibroin hydrogel with designed architecture bioprinted by its microhydrogel. J Mater Chem B. 2019;7:4328–37. [Google Scholar]
145.董 T, 米 R, 吴 M, 钟 N, 赵 X, 陈 X. 等。具有设计结构的再生丝素蛋白水凝胶由其微水凝胶生物打印。J Mater Chem B. 2019 年;7:4328-37。 Google Scholar [ ] -
146.Dorishetty P, Balu R, Athukoralalage SS, Greaves TL, Mata J, de Campo L. et al. Tunable Biomimetic Hydrogels from Silk Fibroin and Nanocellulose. ACS Sustain Chem Eng. 2020;8:2375–89. [Google Scholar]
146.Dorishetty P, Balu R, Athukoralalage SS, Greaves TL, Mata J, de Campo L. et al. 来自丝素蛋白和纳米纤维素的可调仿生水凝胶.ACS Sustain Chem Eng. 2020 年;8:2375-89。 Google Scholar [ ] -
147.Hu RZ, Zhang ZF, Yu BQ, Wang J, Yao XH, Chen T. et al. Natural phenolics and flavonoids modified the hierarchical cellular cellulose sponges for efficient water disinfection. Carbohydr Polym. 2022;296:119962. doi: 10.1016/j.carbpol.2022.119962. [DOI] [PubMed] [Google Scholar]
147.胡 RZ, 张 ZF, 于BQ, 王 J, 姚 XH, 陈 T. 等. 天然酚类化合物和类黄酮改性了分层细胞纤维素海绵,用于高效水消毒。碳水化合物 Polym.2022;296:119962.doi:10.1016/j.carbpol.2022.119962.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
148.Zhang DY, Yang JX, Liu EJ, Hu RZ, Yao XH, Chen T. et al. Soft and elastic silver nanoparticle-cellulose sponge as fresh-keeping packaging to protect strawberries from physical damage and microbial invasion. Int J Biol Macromol. 2022;211:470–80. doi: 10.1016/j.ijbiomac.2022.05.092. [DOI] [PubMed] [Google Scholar]
148.张迪, 杨建军, 刘EJ, 胡 RZ, 姚晓华, 陈T. et al. 柔软有弹性的银纳米颗粒纤维素海绵作为保鲜包装,保护草莓免受物理损伤和微生物入侵。国际生物学杂志 Macromol.2022;211:470–80.doi:10.1016/j.ijbiomac.2022.05.092.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
149.Hu R-Z, Zhang X-Q, Yao X-H, Yang J-X, Liu E-J, Chen T. et al. A bio-inspired dynamic filter with unidirectional channels based on cross-linked cellulose loading palladium nanoparticles for catalytic reduction of 4-nitrophenol. Ind Crops Prod. 2022;179:114690. [Google Scholar]
149.胡 R-Z, 张晓泉, 姚晓华, 杨俊杰, 刘奕杰, 陈T. 等.一种基于交联纤维素负载钯纳米颗粒的生物启发式动态过滤器,具有单向通道,用于催化还原 4-硝基苯酚。工业作物产品 2022;179:114690。 Google Scholar [ ] -
150.Gan D, Jiang Y, Hu Y, Wang X, Wang Q, Wang K. et al. Mussel-inspired extracellular matrix-mimicking hydrogel scaffold with high cell affinity and immunomodulation ability for growth factor-free cartilage regeneration. J Orthop Translat. 2022;33:120–31. doi: 10.1016/j.jot.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
150.甘 D, 江 Y, 胡 Y, 王 X, 王 Q, 王 K. 等. 受贻贝启发的细胞外基质模拟水凝胶支架,具有高细胞亲和力和免疫调节能力,可实现无生长因子的软骨再生。J 骨科翻译。2022;33:120–31.doi:10.1016/j.jot.2022.02.006.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
151.Yuan T, Shao Y, Zhou X, Liu Q, Zhu Z, Zhou B. et al. Highly Permeable DNA Supramolecular Hydrogel Promotes Neurogenesis and Functional Recovery after Completely Transected Spinal Cord Injury. Adv Mater. 2021;33:e2102428. doi: 10.1002/adma.202102428. [DOI] [PubMed] [Google Scholar]
151.袁T, 邵勇, 周X, 刘群, 朱志, 周B. 等. 高渗透性DNA超分子水凝胶促进完全横断脊髓损伤后的神经发生和功能恢复。高级材料。2021;33:e2102428.doi:10.1002/adma.202102428。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
152.Mao ZN, Bi XW, Wu CA, Zheng YF, Shu X, Wu SJ. et al. A Cell-Free Silk Fibroin Biomaterial Strategy Promotes In Situ Cartilage Regeneration Via Programmed Releases of Bioactive Molecules. Adv Healthc Mater. 2022;12:2201588. doi: 10.1002/adhm.202201588. [DOI] [PubMed] [Google Scholar]
152.毛振军, 毕旭, 吴 CA, 郑玉峰, 舒 X, 吴SJ. 等.一种无细胞丝素蛋白生物材料策略通过生物活性分子的程序性释放促进原位软骨再生。Adv Healthc Mater.2022;12:2201588.doi:10.1002/adhm.202201588.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
153.Cao Y, Wang BC. Biodegradation of Silk Biomaterials. Int J Mol Sci. 2009;10:1514–24. doi: 10.3390/ijms10041514. [DOI] [PMC free article] [PubMed] [Google Scholar]
153.曹 Y, 王 BC.真丝生物材料的生物降解。国际分子科学杂志 2009;10:1514–24.doi:10.3390/ijms10041514。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
154.Haung SM, Lin YT, Liu SM, Chen JC, Chen WC. In Vitro Evaluation of a Composite Gelatin-Hyaluronic Acid-Alginate Porous Scaffold with Different Pore Distributions for Cartilage Regeneration. Gels. 2021;7:165. doi: 10.3390/gels7040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
154.Haung SM, Lin YT, Liu SM, Chen JC, Chen WC.具有不同孔隙分布的复合明胶-透明质酸-海藻酸盐多孔支架的体外评估,用于软骨再生。凝 胶。2021;7:165.doi:10.3390/gels7040165。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
155.Singh YP, Bhardwaj N, Mandal BB. Potential of Agarose/Silk Fibroin Blended Hydrogel for in Vitro Cartilage Tissue Engineering. ACS Appl Mater Interfaces. 2016;8:21236–49. doi: 10.1021/acsami.6b08285. [DOI] [PubMed] [Google Scholar]
155.Singh YP, Bhardwaj N, Mandal BB.琼脂糖/丝素蛋白混合水凝胶在体外软骨组织工程中的潜力。ACS 应用程序接口。2016;8:21236–49.doi:10.1021/acsami.6b08285。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
156.Izadifar Z, Chen X, Kulyk W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J Funct Biomater. 2012;3:799–838. doi: 10.3390/jfb3040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
156.Izadifar Z, Chen X, Kulyk W. 用于关节软骨修复的工程支架的战略设计和制造。J Funct 生物材料。2012;3:799–838.doi:10.3390/jfb3040799。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
157.Guo J, Li C, Ling S, Huang W, Chen Y, Kaplan DL. Multiscale design and synthesis of biomimetic gradient protein/biosilica composites for interfacial tissue engineering. Biomaterials. 2017;145:44–55. doi: 10.1016/j.biomaterials.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
157.郭军, 李 C, 凌 S, 黄 W, 陈 Y, 卡普兰 DL.用于界面组织工程的仿生梯度蛋白/生物二氧化硅复合材料的多尺度设计和合成。生物材料。2017;145:44–55.doi:10.1016/j.biomaterials.2017.08.025.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
158.Sawatjui N, Limpaiboon T, Schrobback K, Klein T. Biomimetic scaffolds and dynamic compression enhance the properties of chondrocyte- and MSC-based tissue-engineered cartilage. J Tissue Eng Regen Med. 2018;12:1220–9. doi: 10.1002/term.2653. [DOI] [PubMed] [Google Scholar]
158.Sawatjui N, Limpaiboon T, Schrobback K, Klein T. 仿生支架和动态压缩增强了基于软骨细胞和 MSC 的组织工程软骨的特性。J Tissue Eng Regen Med. 2018 年;12:1220–9.doi:10.1002/term.2653。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
159.Jess R, Ling T, Xiong Y, Wright CJ, Zhao F. Mechanical environment for in vitro cartilage tissue engineering assisted by in silico models. Biomater Transl. 2023;4:18–26. doi: 10.12336/biomatertransl.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
159.Jess R, Ling T, Xiong Y, Wright CJ, Zhao F. 计算机模型辅助体外软骨组织工程的机械环境。Biomater Transl. 2023;4:18–26.doi: 10.12336/biomatertransl.2023.01.004.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
160.Huang X, Zhang M, Ming J, Ning X, Bai S. High-Strength and High-Toughness Silk Fibroin Hydrogels: A Strategy Using Dynamic Host-Guest Interactions. ACS Appl Bio Mater. 2020;3:7103–12. doi: 10.1021/acsabm.0c00933. [DOI] [PubMed] [Google Scholar]
160.Huang X, Zhang M, Ming J, Ning X, Bai S. 高强度和高韧性蚕丝素蛋白水凝胶:一种使用动态主客互动的策略。ACS 应用生物材料。2020;3:7103–12.doi:10.1021/acsabm.0c00933。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
161.Shen C, Wang J, Li G, Hao S, Wu Y, Song P. et al. Boosting cartilage repair with silk fibroin-DNA hydrogel-based cartilage organoid precursor. Bioact Mater. 2024;35:429–44. doi: 10.1016/j.bioactmat.2024.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
161.Shen C, Wang J, Li G, Hao S, Wu Y, Song P. et al. 用丝素蛋白-DNA 水凝胶基软骨类器官前体促进软骨修复。生物行为材料。2024;35:429–44.doi:10.1016/j.bioactmat.2024.02.016。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
162.Xu B, Ye J, Yuan FZ, Zhang JY, Chen YR, Fan BS. et al. Advances of Stem Cell-Laden Hydrogels With Biomimetic Microenvironment for Osteochondral Repair. Front Bioeng Biotechnol. 2020;8:247. doi: 10.3389/fbioe.2020.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
162.Xu B, Ye J, Yuan FZ, Zhang JY, Chen YR, Fan BS. et al. 具有仿生微环境的载干细胞水凝胶在骨软骨修复中的研究进展.Front Bioeng 生物技术公司。2020;8:247.doi:10.3389/fbioe.2020.00247。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
163.Shen K, Duan A, Cheng J, Yuan T, Zhou J, Song H. et al. Exosomes derived from hypoxia preconditioned mesenchymal stem cells laden in a silk hydrogel promote cartilage regeneration via the miR-205-5p/PTEN/AKT pathway. Acta Biomater. 2022;143:173–88. doi: 10.1016/j.actbio.2022.02.026. [DOI] [PubMed] [Google Scholar]
163.Shen K, Duan A, Cheng J, Yuan T, 周 J, Song H. et al. 源自载于丝水凝胶中的缺氧预处理间充质干细胞的外泌体通过 miR-205-5p/PTEN/AKT 途径促进软骨再生。生物学报。2022;143:173–88.doi:10.1016/j.actbio.2022.02.026。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
164.Ni T, Liu M, Zhang Y, Cao Y, Pei R. 3D Bioprinting of Bone Marrow Mesenchymal Stem Cell-Laden Silk Fibroin Double Network Scaffolds for Cartilage Tissue Repair. Bioconjug Chem. 2020;31:1938–47. doi: 10.1021/acs.bioconjchem.0c00298. [DOI] [PubMed] [Google Scholar]
164.Ni T, Liu M, Zhang Y, Cao Y, Pei R. 3D 用于软骨组织修复的骨髓间充质干细胞丝素蛋白双网络支架的生物打印。生物共轭化学 2020;31:1938–47.doi:10.1021/acs.bioconjchem.0c00298。[ DOI ] [ PubMed ] [ ] ] Google Scholar -
165.Calabrese R, Raia N, Huang W, Ghezzi CE, Simon M, Staii C. et al. Silk-ionomer and silk-tropoelastin hydrogels as charged three-dimensional culture platforms for the regulation of hMSC response. J Tissue Eng Regen Med. 2017;11:2549–64. doi: 10.1002/term.2152. [DOI] [PubMed] [Google Scholar]
165.Calabrese R, Raia N, Huang W, Ghezzi CE, Simon M, Staii C. et al. 蚕丝-离聚物和蚕丝-原弹性蛋白水凝胶作为调节 hMSC 反应的带电三维培养平台。J Tissue Eng Regen Med. 2017 年;11:2549–64.doi:10.1002/term.2152。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
166.Chen D, Chang P, Ding P, Liu S, Rao Q, Okoro OV. et al. MSCs-laden silk Fibroin/GelMA hydrogels with incorporation of platelet-rich plasma for chondrogenic construct. Heliyon. 2023;9:e14349. doi: 10.1016/j.heliyon.2023.e14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
166.Chen D, Chang P, Ding P, Liu S, Rao Q, Okoro OV. et al. 载有 MSCs 的丝素蛋白/GelMA 水凝胶,掺入富含血小板的血浆用于软骨形成构建体。赫利永。2023;9:e14349.doi:10.1016/j.heliyon.2023.e14349。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
167.Zheng K, Zheng X, Yu M, He Y, Wu D. BMSCs-Seeded Interpenetrating Network GelMA/SF Composite Hydrogel for Articular Cartilage Repair. J Funct Biomater. 2023;14:39. doi: 10.3390/jfb14010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
167.Zheng K, Zheng X, Yu M, He Y, Wu D. 用于关节软骨修复的 BMSCs 种子穿穿网络 GelMA/SF 复合水凝胶。J Funct 生物材料。2023;14:39.doi:10.3390/jfb14010039。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
168.Hong H, Seo YB, Kim DY, Lee JS, Lee YJ, Lee H. et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679. doi: 10.1016/j.biomaterials.2019.119679. [DOI] [PubMed] [Google Scholar]
168.Hong H, Seo YB, Kim DY, Lee JS, Lee YJ, Lee H. et al. 用于软骨组织工程的数字光加工 3D 打印丝素蛋白水凝胶。生物材料。2020;232:119679.doi:10.1016/j.biomaterials.2019.119679。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
169.Zorn AM, Wells JM. Vertebrate Endoderm Development and Organ Formation. Annu Rev Cell Dev Bi. 2009;25:221–51. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
169.佐恩 AM,威尔斯 JM。脊椎动物内胚层发育和器官形成。Annu Rev Cell Dev Bi.2009;25:221–51.doi:10.1146/annurev.cellbio.042308.113344。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
170.Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact Mater. 2021;14:169–81. doi: 10.1016/j.bioactmat.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
170.Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J. 细菌细胞外囊泡作为药物递送的生物活性纳米载体:进展和前景。生物行为材料。2021;14:169–81.doi:10.1016/j.bioactmat.2021.12.006.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
171.Meng FY, Xue X, Yin ZF, Gao F, Wang XH, Geng Z. Research Progress of Exosomes in Bone Diseases: Mechanism, Diagnosis and Therapy. Front Bioeng Biotech. 2022;10:866627. doi: 10.3389/fbioe.2022.866627. [DOI] [PMC free article] [PubMed] [Google Scholar]
171.Meng FY, Xue X, Yin ZF, Gao F, Wang XH, Geng Z. 外泌体在骨病中的研究进展:机制、诊断和治疗.前 Bioeng Biotech。2022;10:866627.doi:10.3389/fbioe.2022.866627.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
172.Meng FY, Yin ZF, Ren XX, Geng Z, Su JC. Construction of Local Drug Delivery System on Titanium-Based Implants to Improve Osseointegration. Pharmaceutics. 2022;14:1069. doi: 10.3390/pharmaceutics14051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
172.孟飞, 尹ZF, 任XX, 耿Z, 苏JC.在钛基植入物上构建局部药物递送系统以改善骨结合。制药学。2022;14:1069.doi: 10.3390/pharmaceutics14051069.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
173.Gao XJ, Dai QY, Yao LT, Dong H, Li QT, Cao XD. A medical adhesive used in a wet environment by blending tannic acid and silk fibroin. Biomater Sci-Uk. 2020;8:2694–701. doi: 10.1039/d0bm00322k. [DOI] [PubMed] [Google Scholar]
173.高晓军, 戴QY, 姚LT, 董浩, 李QT, 曹欣。一种医用胶粘剂,在潮湿环境中通过混合单宁酸和丝素蛋白而使用。Biomater Sci-Uk.2020;8:2694–701.doi:10.1039/d0bm00322k。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
174.Liu H, Li M, Zhang T, Liu X, Zhang H, Geng Z. et al. Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem Eng J. 2022;450:138309. [Google Scholar]
174.Liu H, Li M, Zhang T, Liu X, Zhang H, Geng Z. et al. 用于骨质疏松症治疗的工程细菌细胞外囊泡。化学工程杂志 2022;450:138309。 Google Scholar [ ] -
175.Zhang W, Zhang Y, Li X, Cao Z, Mo Q, Sheng R. et al. Multifunctional polyphenol-based silk hydrogel alleviates oxidative stress and enhances endogenous regeneration of osteochondral defects. Mater Today Bio. 2022;14:100251. doi: 10.1016/j.mtbio.2022.100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
175.Zhang W, Zhang Y, Li X, Cao Z, Mo Q, Sheng R. et al. 多功能多酚基蚕丝水凝胶减轻氧化应激并增强骨软骨缺损的内源性再生。今日材料生物。2022;14:100251。doi:10.1016/j.mtbio.2022.100251。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
176.Cao ZC, Wang HM, Chen JL, Zhang YA, Mo QY, Zhang P. et al. Silk-based hydrogel incorporated with metal-organic framework nanozymes for enhanced osteochondral regeneration. Bioact Mater. 2023;20:221–42. doi: 10.1016/j.bioactmat.2022.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
176.Cao ZC, Wang HM, Chen JL, Zhang YA, Mo QY, Zhang P. et al. 蚕丝基水凝胶与金属有机框架纳米酶结合以增强骨软骨再生。生物行为材料。2023;20:221–42.doi:10.1016/j.bioactmat.2022.05.025。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
177.Chen PF, Zheng L, Wang YY, Tao M, Xie Z, Xia C. et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9:2439–59. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
177.Chen PF, Zheng L, Wang YY, Tao M, Xie Z, Xia C. et al. 用于骨软骨缺损再生的径向细胞外基质/间充质干细胞外泌体生物墨水的桌面立体光固化 3D 打印。治疗诊断学。2019;9:2439–59.doi:10.7150/THNO.31017。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
178.Liu H, Zhang H, Han Y, Hu Y, Geng Z, Su J. Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy. Theranostics. 2022;12:6576–94. doi: 10.7150/thno.78034. [DOI] [PMC free article] [PubMed] [Google Scholar]
178.Liu H, Zhang H, Han Y, 胡 Y, 耿 Z, Su J. 基于细菌细胞外囊泡的骨和软组织肿瘤治疗策略。治疗诊断学。2022;12:6576–94.doi:10.7150/THNO.78034。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
179.Hu Y, Li X, Zhang Q, Gu Z, Luo Y, Guo J. et al. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021;6:2905–13. doi: 10.1016/j.bioactmat.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
179.胡 Y, 李 X, 张 Q, 顾 Z, 罗 Y, 郭 J. 等. 外泌体引导的骨靶向递送 Antagomir-188 作为骨质流失的合成代谢疗法。生物行为材料。2021;6:2905–13.doi: 10.1016/j.bioactmat.2021.02.014.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
180.Zhang FX, Liu P, Ding W, Meng QB, Su DH, Zhang QC. et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278:121169. doi: 10.1016/j.biomaterials.2021.121169. [DOI] [PubMed] [Google Scholar]
180.Zhang FX, Liu P, Ding W, Meng QB, Su DH, Zhang QC. et al. 注射贻贝启发的高粘附性水凝胶与外泌体,用于内源性细胞募集和软骨缺损再生。生物材料。2021;278:121169.doi: 10.1016/j.biomaterials.2021.121169.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
181.Jiang W, Xiang X, Song M, Shen J, Shi Z, Huang W. et al. An all-silk-derived bilayer hydrogel for osteochondral tissue engineering. Mater Today Bio. 2022;17:100485. doi: 10.1016/j.mtbio.2022.100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
181.江 W, 向 X, 宋 M, 沈 J, 石 Z, 黄 W. 等.一种用于骨软骨组织工程的全丝衍生双层水凝胶。今日材料生物。2022;17:100485。doi: 10.1016/j.mtbio.2022.100485.[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
182.Wu X, Zhou M, Jiang F, Yin S, Lin S, Yang G. et al. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact Mater. 2021;6:3976–86. doi: 10.1016/j.bioactmat.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
182.Wu X, 周 M, 江 F, 尹 S, 林 S, 杨 G. 等. 基于光固化丝水凝胶修复骨软骨缺损的整体双层支架周围的边缘密封。生物行为材料。2021;6:3976–86.doi:10.1016/j.bioactmat.2021.04.005。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
183.He SJ, Fu XJ, Wang L, Xue YY, Zhou L, Qiao SG. et al. Self-Assemble Silk Fibroin Microcapsules for Cartilage Regeneration through Gene Delivery and Immune Regulation. Small. 2023;19:2302799. doi: 10.1002/smll.202302799. [DOI] [PubMed] [Google Scholar]
183.He SJ, Fu XJ, Wang L, Xue YY, 周 L, Qiao SG. et al. 通过基因递送和免疫调节自组装丝素蛋白微胶囊用于软骨再生。小。2023;19:2302799.doi:10.1002/smll.202302799。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
184.Chang L, Perez A. Ranking Peptide Binders by Affinity with AlphaFold. Angew Chem Int Ed Engl. 2023;62:e202213362. doi: 10.1002/anie.202213362. [DOI] [PubMed] [Google Scholar]
184.Chang L, Perez A. 根据 AlphaFold 的亲和力对肽结合物进行排名。Angew Chem Int Ed Engl. 2023;62:e202213362.doi:10.1002/anie.202213362.[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
185.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O. et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
185.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O. et al. 使用 AlphaFold 进行高精度蛋白质结构预测。自然界。2021;596:583–9.doi:10.1038/s41586-021-03819-2。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
186.Tang KY, Jiang L, Yeo JCC, Owh C, Ye E, Loh XJ. et al. Engineering luminescent pectin-based hydrogel for highly efficient multiple sensing. Int J Biol Macromol. 2021;166:869–75. doi: 10.1016/j.ijbiomac.2020.10.243. [DOI] [PubMed] [Google Scholar]
186.Tang KY, 江 L, Yeo JCC, Owh C, Ye E, Loh XJ. et al. 用于高效多传感的工程发光果胶基水凝胶.国际生物学杂志 Macromol.2021;166:869–75.doi:10.1016/j.ijbiomac.2020.10.243。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
187.Kambe Y, Yamaoka T. Initial immune response to a FRET-based MMP sensor-immobilized silk fibroin hydrogel in vivo. Acta Biomater. 2021;130:199–210. doi: 10.1016/j.actbio.2021.05.030. [DOI] [PubMed] [Google Scholar]
187.Kambe Y, Yamaoka T. 体内对基于 FRET 的 MMP 传感器固定化丝素蛋白水凝胶的初始免疫反应。生物学报。2021;130:199–210.doi:10.1016/j.actbio.2021.05.030。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
188.Ding J, Chen Z, Liu X, Tian Y, Jiang J, Qiao Z. et al. A mechanically adaptive hydrogel neural interface based on silk fibroin for high-efficiency neural activity recording. Mater Horiz. 2022;9:2215–25. doi: 10.1039/d2mh00533f. [DOI] [PubMed] [Google Scholar]
188.丁军, 陈志强, 刘旭, 田勇, 江军, 乔志强等.一种基于丝素蛋白的机械自适应水凝胶神经接口,用于高效神经活动记录。马特·霍里兹。2022;9:2215–25.doi:10.1039/d2MH00533F。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
189.Zhang H, Wang H, Wen B, Lu L, Zhao Y, Chai R. Ultrasound-Responsive Composited Conductive Silk Conduits for Peripheral Nerve Regeneration. Small Struct. 2023;4:2300045. [Google Scholar]
189.Zhang H, Wang H, 温 B, Lu L, Zhao Y, Chai R. 用于周围神经再生的超声响应复合导电丝导管.小结构体。2023;4:2300045。 Google Scholar [ ] -
190.Mahajan A, Singh A, Datta D, Katti DS. Bioinspired Injectable Hydrogels Dynamically Stiffen and Contract to Promote Mechanosensing-Mediated Chondrogenic Commitment of Stem Cells. ACS Appl Mater Interfaces. 2022;14:7531–50. doi: 10.1021/acsami.1c11840. [DOI] [PubMed] [Google Scholar]
190.Mahajan A, Singh A, Datta D, Katti DS.仿生注射水凝胶动态硬化和收缩,以促进机械感应介导的干细胞软骨形成定型。ACS 应用程序接口。2022;14:7531–50.doi:10.1021/acsami.1c11840。[ DOI ] [ PubMed ] [ ] [ Google Scholar ] -
191.Tao XY, Zhu KH, Chen HM, Ye SF, Cui PX, Dou LY. et al. Recyclable, anti-freezing and anti-drying silk fibroin-based hydrogels for ultrasensitive strain sensors and all-hydrogel-state super-capacitors. Mater Today Chem. 2023;32:101624. [Google Scholar]
191.Tao XY, Zhu KH, Chen HM, Ye SF, Cui PX, Dou LY. et al. 用于超敏感应变传感器和全水凝胶状态超级电容器的可回收、防冻和防干丝素蛋白基水凝胶.今日材料化学 2023;32:101624。 Google Scholar [ ] -
192.Haghighattalab M, Kajbafzadeh A, Baghani M, Gharehnazifam Z, Jobani BM, Baniassadi M. Silk Fibroin Hydrogel Reinforced With Magnetic Nanoparticles as an Intelligent Drug Delivery System for Sustained Drug Release. Front Bioeng Biotechnol. 2022;10:891166. doi: 10.3389/fbioe.2022.891166. [DOI] [PMC free article] [PubMed] [Google Scholar]
192.Haghighattalab M, Kajbafzadeh A, Baghani M, Gharehnazifam Z, Jobani BM, Baniassadi M. 用磁性纳米颗粒增强的丝素蛋白水凝胶作为智能药物输送系统,用于持续药物释放。Front Bioeng 生物技术公司。2022;10:891166.doi:10.3389/fbioe.2022.891166。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ] -
193.Sun S, Liu H, Hu Y, Wang Y, Zhao M, Yuan Y. et al. Selection and identification of a novel ssDNA aptamer targeting human skeletal muscle. Bioact Mater. 2023;20:166–78. doi: 10.1016/j.bioactmat.2022.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
193.Sun S, Liu H, 胡 Y, Wang Y, Zhao M, Yuan Y. et al. 靶向人骨骼肌的新型 ssDNA 适配体的选择和鉴定.生物行为材料。2023;20:166–78.doi:10.1016/j.bioactmat.2022.05.016。[ DOI ] [ PMC free article ] [ PubMed ] [ ] [ ] [ Google Scholar ]