Abstract 抽象的
Acute liver injury (ALI) is an acute inflammatory liver disease with a high mortality rate. Alternatively, activated macrophages (AAMs) have been linked to the inflammation and recovery of ALI. However, the mechanism underlying AAM death in ALI has not been studied sufficiently. We used liensinine (Lie) as a drug of choice after screening a library of small-molecule monomers with 1488 compounds from traditional Chinese remedies. In ALI, we evaluated the potential therapeutic effects and underlying mechanisms of action of the drug in ALI and found that it effectively inhibited RSL3-induced ferroptosis in AAM. Lie significantly reduced lipid peroxidation in RSL3-generated AAM. It also improved the survival rate of LPS/D-GalN-treated mice, reduced serum transaminase activity, suppressed inflammatory factor production, and may have lowered AAM ferroptosis in ALI. Lie also inhibited ferritinophagy and blocked Fe2+ synthesis. Following combined treatment with RSL3 and Lie, super-resolution microscopy revealed a close correlation between ferritin and LC3-positive vesicles in the AAM. The co-localization of ferritin and LC3 with LAMP1 was significantly reduced. These findings suggest that Lie may ameliorate ALI by inhibiting ferritinophagy and enhancing AMM resistance to ferroptosis by inhibiting autophagosome-lysosome fusion. Therefore, Lie may be used as a potential therapeutic agent for patients with ALI.
急性肝损伤(ALI)是一种死亡率很高的急性炎症性肝病。另外,活化的巨噬细胞 (AAM) 与 ALI 的炎症和恢复有关。然而,ALI 中 AAM 死亡的机制尚未得到充分研究。在筛选了含有 1488 种中药化合物的小分子单体库后,我们选择了莲心碱 (Lie) 作为首选药物。在 ALI 中,我们评估了该药物在 ALI 中的潜在治疗效果和潜在作用机制,发现它能有效抑制 AAM 中 RSL3 诱导的铁死亡。 Lie 显着降低了 RSL3 生成的 AAM 中的脂质过氧化。它还提高了 LPS/D-GalN 治疗小鼠的存活率,降低血清转氨酶活性,抑制炎症因子的产生,并可能降低 ALI 中的 AAM 铁死亡。 Lie 还抑制铁蛋白自噬并阻断 Fe 2+合成。 RSL3 和 Lie 联合治疗后,超分辨率显微镜揭示了 AAM 中铁蛋白和 LC3 阳性囊泡之间的密切相关性。铁蛋白和 LC3 与 LAMP1 的共定位显着减少。这些发现表明,Lie 可能通过抑制铁蛋白自噬来改善 ALI,并通过抑制自噬体-溶酶体融合来增强 AMM 对铁死亡的抵抗力。因此,Lie可作为ALI患者的潜在治疗剂。
Similar content being viewed by others
其他人正在查看类似内容
Introduction 介绍
Acute liver injury (ALI), also known as acute liver failure, is caused by various factors, including drug stimulation [1], viral infection [2], alcohol consumption [3], and chemical toxins [4]. It is associated with severe impairment or liver function loss. Further, ALI is characterized by its rapid onset, severe disease, and several other complications [5]. Unfortunately, no specific drugs or treatment approaches for ALI are available [6], and artificial liver support treatment cannot completely restore liver function due to large-scale liver cell necrosis [7]. Therefore, the sole viable treatment option is liver transplantation. However, due to the lack of suitable donor tissue, the associated life-long immunosuppression, high costs, and technical limitations, it is not an ideal treatment option [8]. Therefore, developing safe drugs with effective mechanisms of action and enhanced therapeutic outcomes for patients with ALI is crucial.
急性肝损伤(ALI)又称急性肝功能衰竭,是由多种因素引起的,包括药物刺激[ 1 ]、病毒感染[ 2 ]、饮酒[ 3 ]和化学毒素[ 4 ]。它与严重损伤或肝功能丧失有关。此外,ALI 的特点是发病迅速、病情严重和其他一些并发症 [ 5 ]。遗憾的是,目前尚无针对ALI的特效药物或治疗方法[ 6 ],且人工肝支持治疗因肝细胞大面积坏死而无法完全恢复肝功能[ 7 ]。因此,唯一可行的治疗选择是肝移植。然而,由于缺乏合适的供体组织、相关的终生免疫抑制、高成本和技术限制,它并不是一种理想的治疗选择[ 8 ]。因此,开发具有有效作用机制并增强 ALI 患者治疗效果的安全药物至关重要。
Liver inflammation plays a significant role in the etiology of ALI [9]. Macrophages have innate immune and paracrine functions; hence, an imbalance in their function can lead to ALI-related liver inflammation progression or resolution [10,11,12]. Based on their activity, macrophages are widely categorized into two phenotypes: classical macrophages (M1) and alternatively activated macrophages (AAM) [13]. Intrahepatic macrophages in ALI are mainly M1 macrophages, which synthesize and secrete various inflammatory factors, such as interleukin (IL)-1β and IL-6, accelerating liver inflammation progression [14,15,16]. AAM macrophages play a hepatoprotective role in ALI [11, 17, 18], such as protecting the liver in acute and chronic liver failure by inhibiting the necroptosis-S100A9-necroinflammatory axis [19]. AAM macrophages reduced hepatocyte necrosis and neutrophil infiltration while reducing liver inflammation in an APAP-ALI experimental model [20]. Therefore, the balance between M1 macrophages and AAM may be an effective therapeutic target for ALI. ALI causes significant damage and death of intrahepatic cells, including hepatocytes and macrophages [21]. It is unknown whether M1 macrophages and AAM have different mortality patterns, which could affect their balance. Oxidative stress results in several cell death patterns [22,23,24,25]. Owing to the variable expression of antioxidant-related proteins, including inducible nitric oxide synthase (iNOS), M1 macrophages and AAM have differing antioxidative defense capabilities [26]. Therefore, we hypothesized that M1 macrophages and AAM would be differentially sensitive to one type of oxidative stress-related cell death, potentially resulting in an imbalance between both phenotypes and exacerbating liver inflammation progression in ALI. Elucidating the potential differential death mechanisms of M1 macrophages and AAM could lead to a novel strategy for developing effective drugs for treating ALI.
肝脏炎症在 ALI 的病因学中起着重要作用[ 9 ]。巨噬细胞具有先天免疫和旁分泌功能;因此,它们的功能失衡可能导致 ALI 相关的肝脏炎症进展或消退 [10,11,12 ] 。根据其活性,巨噬细胞被广泛分为两种表型:经典巨噬细胞(M1)和替代激活巨噬细胞(AAM)[ 13 ]。 ALI中肝内巨噬细胞主要为M1型巨噬细胞,其合成并分泌多种炎症因子,如白细胞介素(IL)-1β和IL-6,加速肝脏炎症进展[14,15,16 ] 。 AAM巨噬细胞在ALI中发挥保肝作用[ 11,17,18 ],例如通过抑制坏死性凋亡-S100A9-坏死性炎症轴来保护急性和慢性肝衰竭中的肝脏[19 ] 。在 APAP-ALI 实验模型中,AAM 巨噬细胞减少肝细胞坏死和中性粒细胞浸润,同时减少肝脏炎症 [ 20 ]。因此,M1巨噬细胞和AAM之间的平衡可能是ALI的有效治疗靶点。 ALI 导致肝内细胞(包括肝细胞和巨噬细胞)显着损伤和死亡[ 21 ]。目前尚不清楚 M1 巨噬细胞和 AAM 是否具有不同的死亡率模式,这可能会影响它们的平衡。氧化应激导致多种细胞死亡模式[22,23,24,25 ] 。由于抗氧化相关蛋白(包括诱导型一氧化氮合酶(iNOS))的不同表达,M1巨噬细胞和AAM具有不同的抗氧化防御能力[ 26 ]。 因此,我们假设 M1 巨噬细胞和 AAM 对一种类型的氧化应激相关细胞死亡的敏感性不同,可能导致两种表型之间的不平衡,并加剧 ALI 中的肝脏炎症进展。阐明 M1 巨噬细胞和 AAM 的潜在差异死亡机制可能会导致开发治疗 ALI 的有效药物的新策略。
Ferroptosis is a newly discovered oxidative, non-apoptotic form of cell death that differs in morphology, biochemistry, and genetics from other types of cell death [27]. Ferroptosis has been linked to various liver diseases, including drug-induced liver injury and nonalcoholic fatty liver disease [28,29,30]. Macrophages express key genes involved in iron uptake, processing, export, and lipid metabolism [31]. Several studies have revealed that inhibiting macrophage ferroptosis can reduce pulmonary inflammatory injury in acute lung injury. However, the underlying mechanisms are unknown [32]. During ALI pathogenesis, M1 macrophages dominate intrahepatic macrophages [14], and the number of AAM is lower than normal levels [17]. AAMs are also highly sensitive to ferroptosis [33]. Therefore, we hypothesized that reactive oxygen species (ROS) accumulation during ALI pathogenesis triggers AAM ferroptosis, reduces AMM numbers, and disrupts the balance between M1 macrophages and AAM, aggravating the hepatic inflammatory injury. Therefore, inhibiting AAM ferroptosis in ALI would be beneficial in restoring macrophage phenotype balance.
铁死亡是一种新发现的氧化性、非凋亡形式的细胞死亡,其在形态、生物化学和遗传学方面与其他类型的细胞死亡不同[ 27 ]。铁死亡与多种肝脏疾病有关,包括药物性肝损伤和非酒精性脂肪肝病[28,29,30 ] 。巨噬细胞表达参与铁吸收、加工、输出和脂质代谢的关键基因[ 31 ]。多项研究表明,抑制巨噬细胞铁死亡可以减轻急性肺损伤时的肺部炎症损伤。然而,潜在的机制尚不清楚[ 32 ]。在ALI发病过程中,M1型巨噬细胞在肝内巨噬细胞中占主导地位[ 14 ],AAM的数量低于正常水平[ 17 ]。 AAM 对铁死亡也高度敏感[ 33 ]。因此,我们推测ALI发病过程中活性氧(ROS)的积累会引发AAM铁死亡,减少AMM数量,破坏M1巨噬细胞和AAM之间的平衡,加重肝脏炎症损伤。因此,抑制ALI中的AAM铁死亡将有利于恢复巨噬细胞表型平衡。
Traditional Chinese medicine (TCM) is a result of the traditional culture and wisdom of China. TCMs have been linked to fewer toxic side effects, several therapeutic targets, and significant therapeutic efficacy. Natural product bioactive components are important drug sources and have the potential to be used in drug screening [34]. Several TCMs have been found to influence ferroptosis regulation [35] significantly. Therefore, developing treatment for ALI by identifying novel medications from TCM monomer molecules is feasible. A mouse model of lipopolysaccharide (LPS)/D-GalN-induced ALI was used to determine M1 macrophages and AAM sensitivity to ferroptosis. TCM monomer compound library was used to screen for and assess small-molecule compounds that can effectively inhibit AAM ferroptosis and evaluate their potential therapeutic value. This study established a scientific basis for research and development of hepatoprotective drugs against AAM ferroptosis and provides novel insights into the mechanism of Lie against ALI.
中医药是中华民族传统文化和智慧的结晶。中药具有较少的毒副作用、多个治疗靶点和显着的治疗效果。天然产物生物活性成分是重要的药物来源,具有用于药物筛选的潜力[ 34 ]。已发现几种中药可显着影响铁死亡调节[ 35 ]。因此,通过从中药单体分子中鉴定新药来开发治疗 ALI 的方法是可行的。使用脂多糖 (LPS)/D-GalN 诱导的 ALI 小鼠模型来确定 M1 巨噬细胞和 AAM 对铁死亡的敏感性。利用中药单体化合物库筛选和评价能够有效抑制AAM铁死亡的小分子化合物并评价其潜在的治疗价值。该研究为抗AAM铁死亡的保肝药物研发奠定了科学基础,并为Lie抗ALI的作用机制提供了新的见解。
Results 结果
Sensitivity of alternatively activated macrophages to lipid peroxidation-driven ferroptosis
替代激活的巨噬细胞对脂质过氧化驱动的铁死亡的敏感性
We assessed the sensitivity of different types of macrophages to determine different forms of cell death. Unstimulated RAW264.7 macrophages were polarized to the AAM or M1 macrophage states after treatment with IL-4 or LPS/IFN-γ, respectively (Fig. S1a). The M0 macrophages, M1 macrophages, and AAM were then treated with the appropriate IC50 doses of apoptosis, pyroptosis, ferroptosis, and necrosis inducers (Fig. S1b–f) to observe cell viability changes. The ability of the apoptosis, pyroptosis, and necrosis inducers to inhibit cell viability in the M0 macrophages, M1 macrophages, and AAM did not differ significantly (Fig. 1a–c). However, RSL3, a ferroptosis inducer, significantly inhibited M0 macrophages and AAM viability compared to that of the M1 macrophages (Fig. 1d). Further, similar results were observed when the M0 macrophages, M1 macrophages, and AAM were treated with erastin (a ferroptosis inducer) (Fig. 1e). We then treated AAM with Fer-1 combined with RSL3 and found that Fer-1 could reverse RSL3-induced AAM death (Fig. 1f), reduce Fe2+ (Fig. 1g) and ROS levels (Fig. 1h), and inhibit intracellular lipid oxidation product generation (Fig. 1i, j). These findings suggest that AAMs are ferroptosis-sensitive, whereas M1 macrophages are ferroptosis-resistant.
我们评估了不同类型巨噬细胞的敏感性以确定不同形式的细胞死亡。未刺激的RAW264.7巨噬细胞分别用IL-4或LPS/IFN-γ处理后极化为AAM或M1巨噬细胞状态(图S1a )。然后用适当的IC 50剂量的细胞凋亡、焦亡、铁死亡和坏死诱导剂处理M0巨噬细胞、M1巨噬细胞和AAM(图S1b-f )以观察细胞活力的变化。细胞凋亡、焦亡和坏死诱导剂抑制 M0 巨噬细胞、M1 巨噬细胞和 AAM 细胞活力的能力没有显着差异(图1a-c )。然而,与M1巨噬细胞相比,RSL3(一种铁死亡诱导剂)显着抑制M0巨噬细胞和AAM活力(图1d )。此外,当用erastin(铁死亡诱导剂)处理M0巨噬细胞、M1巨噬细胞和AAM时,观察到类似的结果(图1e )。然后我们用Fer-1联合RSL3处理AAM,发现Fer-1可以逆转RSL3诱导的AAM死亡(图1f ),降低Fe 2+ (图1g )和ROS水平(图1h ),并抑制细胞内脂质氧化产物的产生(图1i,j )。这些发现表明 AAM 对铁死亡敏感,而 M1 巨噬细胞对铁死亡具有抗性。
图 1:替代激活的巨噬细胞对脂质过氧化驱动的铁死亡的敏感性。
a–e CCK-8 assay for cell viability after the treatment of macrophages (M0, M1, AAM) with the apoptosis inducer (20 μM, 24 h), pyroptosis inducers (7 μg/ml, 24 h), necrosis inducer (4×, 24 h), RSL3 inducer (5 μM, 5 h), and erastin inducer (60 μM, 24 h). f–i AAM was treated with RSL3 (5 μM, 5 h) in the presence or absence of Fer-1 (400 nM). f PI staining to assess cell death, Scale bar = 200 µm. g Live cell fluorescence imaging to detect lipid peroxide production using Liperfloo, Scale bar = 100 µm. h Live cell fluorescence imaging of FerroOrange (red), Scale bar = 100 µm. i Fluorescence imaging of the superoxide anion fluorescence detection probe Dihydroethidium (DHE), Scale bar = 100 µm. j Expression of the 4-hydroxynonenal (4-HNE) protein was measured by immunofluorescence, Scale bar = 50 µm. Results were presented as mean ± SD. (n = 3, ****p < 0.0001. Con control, NS not significant).
a – e用细胞凋亡诱导剂(20 μM,24 小时)、焦亡诱导剂(7 μg/ml,24 小时)、坏死诱导剂(4)处理巨噬细胞(M0、M1、AAM)后细胞活力的 CCK-8 测定×,24 小时)、RSL3 诱导剂(5 μM,5 小时)和erastin 诱导剂(60 μM,24 小时)。在存在或不存在 Fer-1 (400 nM) 的情况下,使用 RSL3(5 μM,5 小时)处理f – i AAM。 f PI 染色评估细胞死亡,比例尺 = 200 µm。 g使用 Liperfloo 进行活细胞荧光成像检测脂质过氧化物的产生,比例尺 = 100 µm。 h FerroOrange(红色)的活细胞荧光成像,比例尺 = 100 µm。 i超氧阴离子荧光检测探针二氢乙锭 (DHE) 的荧光成像,比例尺 = 100 µm。 j通过免疫荧光测量 4-羟基壬烯醛 (4-HNE) 蛋白的表达,比例尺 = 50 µm。结果以平均值±标准差表示。 ( n = 3,**** p < 0.0001。Con 对照,NS 不显着)。
Relationship between Fer-1 treatment of LPS/D-GalN-induced liver injury in mice and alternatively activated macrophages
Fer-1治疗LPS/D-GalN诱导的小鼠肝损伤与替代激活巨噬细胞的关系
We then explored the role of ferroptosis in ALI. We examined the LPS/D-GalN-induced histopathological changes in the liver tissues of Fer-1 treated and untreated mice and examined serum transaminases and inflammatory factor levels. The histological analysis and H&E staining results indicated that Fer-1 alleviated histopathological changes in the livers of the LPS/D-GalN-induced mice, including inflammatory infiltration, disorganization, and hepatocyte swelling (Fig. 2a). Furthermore, results of the serum transaminases and inflammatory factors using ELISA showed that when the mice were injured by LPS/D-GalN treatment, AST (Fig. 2b), ALT (Fig. 2c), and inflammatory factors (Fig. 2d–g) increased significantly but decreased significantly in the Fer-1 + LPS/D-GalN Group mice (Fig. 2b–g). In addition, in the LPS/D-GalN-treated mice, Fer-1 partially reversed liver mitochondrial damage (Fig. 2h) and inhibited ROS production (Fig. 2i). Furthermore, MDA and 4-HNE production were reduced by Fer-1 combined with LPS/D-GalN-treated mice compared to LPS/D-GalN-treated mice (Figs. 2j and S2), where GSH production was elevated (Fig. 2k). Immunohistochemistry was used to further examine changes in M1 macrophages and AAM levels. In LPS/D-GalN-treated mice, the M1 macrophage marker (iNOS) level significantly increased, whereas that of CD206 of AAM significantly decreased, which was partially reversed by the Fer-1 treatment (Fig. 2l).
然后我们探讨了铁死亡在 ALI 中的作用。我们检查了 Fer-1 治疗和未治疗小鼠肝组织中 LPS/D-GalN 诱导的组织病理学变化,并检查了血清转氨酶和炎症因子水平。组织学分析和H&E染色结果表明,Fer-1减轻了LPS/D-GalN诱导的小鼠肝脏的组织病理学变化,包括炎症浸润、解体和肝细胞肿胀(图2a )。此外,ELISA检测血清转氨酶和炎症因子的结果显示,当小鼠受到LPS/D-GalN处理损伤时,AST(图2b )、ALT(图2c )和炎症因子(图2d-g) )在 Fer-1 + LPS/D-GalN 组小鼠中显着增加,但显着下降(图2b-g )。此外,在LPS/D-GalN处理的小鼠中,Fer-1部分逆转了肝线粒体损伤(图2h )并抑制ROS产生(图2i )。此外,与LPS/D-GalN处理的小鼠相比,Fer-1联合LPS/D-GalN处理的小鼠的MDA和4-HNE产生减少(图2j和S2 ),其中GSH产生升高(图2j和S2)。 2k )。使用免疫组织化学进一步检查 M1 巨噬细胞和 AAM 水平的变化。在LPS/D-GalN处理的小鼠中,M1巨噬细胞标记物(iNOS)水平显着升高,而AAM的CD206水平显着降低,Fer-1处理部分逆转了这种情况(图2l )。
图 2:Fer-1 减轻 LPS/D-GalN 引起的小鼠肝损伤,同时增加替代激活的巨噬细胞数量。
a Liver injury was assessed using histopathology and hematoxylin and eosin (H&E) staining, Scale bar = 100 µm. b, c The levels of AST and ALT in serum. d–g Serum content of TNF-α, IL-6, IL-1β, and HMGB1. h Transmission electron microscopy (TEM) shows a representative liver tissue image, Scale bar = 2 μm. i Representative fluorescence imaging of the superoxide anion fluorescence detection probe dihydroethidium (DHE) in liver tissue, Scale bar = 50 µm. j, k Assessment of MDA and GSH contents in liver tissue. l Expression of iNOS and CD206 was detected using immunocytochemistry in the liver tissue, Scale bar = 100 µm. Results were presented as mean ± SD (n = 5). (##p < 0.01, vs. Con. **p < 0.01, vs. D-GalN/LPS. Con Control, NS not significant).
a使用组织病理学和苏木精和伊红 (H&E) 染色评估肝损伤,比例尺 = 100 µm。 b 、 c血清中 AST 和 ALT 的水平。 d – g TNF-α、IL-6、IL-1β 和 HMGB1 的血清含量。 h透射电子显微镜 (TEM) 显示代表性肝脏组织图像,比例尺 = 2 μm。 i肝组织中超氧阴离子荧光检测探针二氢乙锭 (DHE) 的代表性荧光成像,比例尺 = 50 µm。 j , k肝组织中MDA和GSH含量的评估。 l使用免疫细胞化学检测肝组织中 iNOS 和 CD206 的表达,比例尺 = 100 µm。结果以平均值±标准差( n = 5)表示。 ( ## p < 0.01,与 Con 相比。 ** p < 0.01,与 D-GalN/LPS 相比。Con 对照,NS 不显着)。
Screening TCM library for active compounds against alternatively activated macrophages ferroptosis
筛选中药库中针对替代激活巨噬细胞铁死亡的活性化合物
In ALI, AAM plays a crucial role in repairing and inhibiting inflammation [10]; therefore, promoting AAM survival may be a promising therapeutic strategy. We screened the TCM library for small-molecule compounds that could inhibit ferroptosis in AAM. Cell viability was examined by treating AAM for 5 h with 1488 monomers obtained from the TCM library combined with RSL3 (Fig. 3a). Figure 3b shows that Lie was the most effective product. However, the Lie treatment did not significantly affect the M0 macrophages, M1 macrophages, or AAM (Fig. S3a–d); Fig. 1c depicts its chemical structure. Its effect on RSL3-induced ferroptosis in AAM was evaluated by treating the cells with varying concentrations (1, 5, and 10 μM) of Lie combined with RSL3. Lie increased cell viability in a dose-dependent manner (Fig. 3c). In addition, LDH level decreased in a dose-dependent manner (Fig. 3d). A Lie concentration of 10 μM was the most effective dose that promoted the survival of the AAM. The PI staining results showed that AAM treated with 10 μM Lie had a significantly lower RSL3-induced death rate (Fig. 3f). Immunofluorescence results revealed that Lie inhibited the synthesis of Fe2+ in the RSL3-treated AAM (Fig. 3g) and reduced the production of intracellular ROS (Fig. 3h) and lipid oxidation products (Fig. 3i, j). The electron microscopy results showed that exposure to Lie and RSL3-treated AAM reduced the number of damaged mitochondria and those with reduced or missing mitochondrial cristae (Fig. 3k). Lie also partially reversed erastin-induced ferroptosis in AAM (Fig. S3e–g).
在ALI中,AAM在修复和抑制炎症方面发挥着至关重要的作用[ 10 ];因此,促进 AAM 存活可能是一种有前途的治疗策略。我们在中药库中筛选了可以抑制 AAM 铁死亡的小分子化合物。通过用从TCM文库获得的1488个单体与RSL3结合处理AAM 5小时来检查细胞活力(图3a )。图3b显示 Lie 是最有效的产品。然而,Lie 处理并没有显着影响 M0 巨噬细胞、M1 巨噬细胞或 AAM(图S3a-d );图1c描述了它的化学结构。通过用不同浓度(1、5 和 10 μM)的 Lie 与 RSL3 组合处理细胞来评估其对 AAM 中 RSL3 诱导的铁死亡的影响。细胞活力以剂量依赖性方式增加(图3c )。此外,LDH水平以剂量依赖性方式降低(图3d )。 10μM的Lie浓度是促进AAM存活的最有效剂量。 PI染色结果显示,用10μM Lie处理的AAM具有显着较低的RSL3诱导的死亡率(图3f )。免疫荧光结果显示,Lie抑制RSL3处理的AAM中Fe 2+的合成(图3g ),并减少细胞内ROS(图3h )和脂质氧化产物(图3i,j )的产生。电子显微镜结果表明,暴露于Lie和RSL3处理的AAM减少了受损线粒体以及线粒体嵴减少或缺失的线粒体数量(图3k )。 Lie 还部分逆转了 AAM 中erastin 诱导的铁死亡(图S3e-g )。
图3:中药莲心碱在体外对抗替代激活的巨噬细胞铁死亡的作用。
a Schematic of the drug-screening program. b AAM was treated with the 1488 candidates in combination with RSL3 (5 μM) for 5 h, and cell viability was measured using the CCK-8 assay kit. Each point represents the percentage of cell viability for a concentration of 10 μM of the candidate compound. c Chemical structure diagram of liensinine (Lie). d CCK-8 assay for cell viability. e Amount of LDH in the cell supernatant. f PI staining to assess cell death, Scale bar = 200 µm. g Live cell fluorescence imaging of FerroOrange (red), Scale bar = 100 µm. h Fluorescence imaging of the superoxide anion fluorescence detection probe dihydroethidium (DHE), Scale bar = 100 µm. i Live cell fluorescence imaging using Liperfloo, Scale bar = 100 µm. j Expression of the 4-HNE protein was measured by immunofluorescence, Scale bar = 50 µm. k Representative electron micrograph image of cells, Scale bar = 2 μm. Results were presented as mean ± SD. (n = 3, ####p < 0.0001, vs. Con. *p < 0.05, ***p < 0.001, vs. RSL3. Con Control, NS not significant).
药物筛选计划示意图。 b将 1488 个候选物与 RSL3 (5 μM) 组合处理 AAM 5 小时,并使用 CCK-8 检测试剂盒测量细胞活力。每个点代表浓度为 10 μM 的候选化合物的细胞活力百分比。 c莲心碱(Lie)的化学结构图。 d CCK-8 细胞活力测定。 e细胞上清液中 LDH 的量。 f PI 染色评估细胞死亡,比例尺 = 200 µm。 g FerroOrange(红色)的活细胞荧光成像,比例尺 = 100 µm。 h超氧阴离子荧光检测探针二氢乙锭 (DHE) 的荧光成像,比例尺 = 100 µm。 i使用 Liperfloo 进行活细胞荧光成像,比例尺 = 100 µm。 j通过免疫荧光测量 4-HNE 蛋白的表达,比例尺 = 50 µm。 k细胞的代表性电子显微图像,比例尺 = 2 μm。结果以平均值±标准差表示。 ( n = 3, #### p < 0.0001,与Con 相比。* p < 0.05,*** p < 0.001,与 RSL3 相比。Con 对照,NS 不显着)。
In vivo attenuation of liver injury and the inflammatory response using liensinine
使用莲心宁体内减轻肝损伤和炎症反应
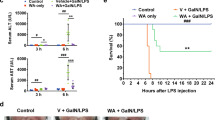
We examined LPS/D-GalN-induced survival in Lie-treated and untreated mice to determine whether Lie may offer ALI protection in vivo. Findings revealed that LPS/D-GalN caused a high mortality rate, with 100% mortality occurring within 24 h. Lie treatment increased survival in a dose-dependent manner. For example, 60% of the Lie-treated (60 mg/kg) mice group survived after 24 h (Fig. 4a). We then examined the effects of Lie on serum transaminase levels in LPS/D-GalN-treated mice. Our findings revealed that Lie significantly inhibited the activities of ALT and AST in the mice sera (Fig. 4b, c). H&E staining results revealed that Lie partially alleviated LPS/D-GalN-induced hepatotoxicity (Fig. 4d). The ELISA results showed that Lie treatment significantly reduced serum TNF-α, IL-6, HMGB1, and IL-1β levels in the LPS/D-GalN-treated mice (Fig. 4e–h). Furthermore, the administration of Lie alone had no significant effect on the liver tissue or inflammatory cytokine levels (Fig. 4a–h), implying that the Lie doses used in this study had no adverse effects on the liver (Fig. 4d). We also used immunofluorescence to examine the changes in the M1 macrophage and AAM content. Lie treatment significantly inhibited iNOS expression while increasing CD206 expression (Fig. 4i).
我们检查了 LPS/D-GalN 诱导的 Lie 治疗和未治疗小鼠的存活率,以确定 Lie 是否可以在体内提供 ALI 保护。结果显示,LPS/D-GalN 造成很高的死亡率,24 小时内死亡率为 100%。谎言治疗以剂量依赖性方式增加存活率。例如,60%的Lie治疗(60mg/kg)小鼠组在24小时后存活(图4a )。然后我们检查了 Lie 对 LPS/D-GalN 治疗小鼠血清转氨酶水平的影响。我们的研究结果表明,Lie 显着抑制小鼠血清中 ALT 和 AST 的活性(图4b、c )。 H&E染色结果显示,Lie部分减轻了LPS/D-GalN诱导的肝毒性(图4d )。 ELISA结果显示,Lie治疗显着降低了LPS/D-GalN治疗小鼠的血清TNF-α、IL-6、HMGB1和IL-1β水平(图4e-h )。此外,单独使用Lie对肝组织或炎症细胞因子水平没有显着影响(图4a-h ),这意味着本研究中使用的Lie剂量对肝脏没有不良影响(图4d )。我们还使用免疫荧光检查M1巨噬细胞和AAM含量的变化。 Lie处理显着抑制iNOS表达,同时增加CD206表达(图4i )。
图 4:莲心碱治疗可减轻 LPS/D-GalN 诱导的小鼠病理性肝损伤和炎症反应。
a Survival was monitored over a 24 h period (n = 10/group). b, c Serum ALT and AST levels. d Histopathology, hematoxylin, and eosin (H&E) staining, Scale bar = 100 µm. e–h Determination of the levels of the inflammatory factors HMGB1, TNF-α, IL-6, and IL-1β in mice sera. i Expression of iNOS and CD206 in liver tissue was detected by immunofluorescence, Scale bar = 50 µm. Results were presented as mean ± SD (n = 5). (##p < 0.01, vs. Con. *p < 0.05, **p < 0.01, ****p < 0.0001, vs. LPS/D-GalN. Con control, NS not significant).
a在 24 小时内监测存活率( n = 10/组)。 b 、 c血清 ALT 和 AST 水平。 d组织病理学、苏木精和伊红 (H&E) 染色,比例尺 = 100 µm。 e – h测定小鼠血清中炎症因子 HMGB1、TNF-α、IL-6 和 IL-1β 的水平。 i通过免疫荧光检测肝组织中 iNOS 和 CD206 的表达,比例尺 = 50 µm。结果以平均值±标准差( n = 5)表示。 ( ## p < 0.01,与 Con 相比。* p < 0.05,** p < 0.01,**** p < 0.0001,与 LPS/D-GalN 相比。Con 对照,NS 不显着)。
In vivo possible inhibition of alternatively activated macrophage ferroptosis using liensinine
使用莲心碱体内可能抑制替代激活的巨噬细胞铁死亡
Ferroptosis plays an important role in ALI, and Lie can partially inhibit RSL3-induced ferroptosis in AAM in vitro. Owing to the fact that Lie also increased the number of AAM in the LPS/D-GalN-induced mice, we hypothesized that Lie could increase the number of AAM in ALI by inhibiting ferroptosis. Lie partially reversed the decreased GSH content in LPS/D-GalN-induced mice (Fig. 5a), partially inhibited MDA production (Fig. 5b), and inhibited ROS production (Fig. 5c). We also used TEM to investigate mitochondrial morphology and found that the mitochondrial cristae were reduced or even absent in the livers of the LPS/D-GalN-treated group, the outer mitochondrial membrane was ruptured, and Lie treatment partially reversed the LPS/D-GalN-induced mitochondrial damage in the liver (Fig. 5d). These findings revealed that Lie could be used to treat ferroptosis induced by LPS/D-GalN. Immunofluorescence results showed that 4-HNE expression was significantly increased in the CD206-positive cells in liver tissues of LPS/D-GalN-treated mice, and it was lower in the iNOS-positive cells. In the Lie- and LPS/D-GalN-treated mice, the iNOS-positive cells and 4-HNE production in the CD206-positive cells were significantly reduced.
铁死亡在 ALI 中发挥重要作用,Lie 在体外可以部分抑制 RSL3 诱导的 AAM 铁死亡。由于Lie还增加了LPS/D-GalN诱导的小鼠中AAM的数量,我们推测Lie可以通过抑制铁死亡来增加ALI中AAM的数量。 Lie部分逆转了LPS/D-GalN诱导的小鼠中GSH含量的降低(图5a ),部分抑制MDA产生(图5b ),并抑制ROS产生(图5c )。我们还使用TEM研究了线粒体形态,发现LPS/D-GalN治疗组的肝脏中线粒体嵴减少甚至消失,线粒体外膜破裂,Lie治疗部分逆转了LPS/D- GalN 诱导的肝脏线粒体损伤(图5d )。这些发现表明,Lie 可用于治疗 LPS/D-GalN 诱导的铁死亡。免疫荧光结果显示,LPS/D-GalN处理小鼠肝组织中CD206阳性细胞中4-HNE表达显着升高,iNOS阳性细胞中4-HNE表达降低。在 Lie 和 LPS/D-GalN 处理的小鼠中,iNOS 阳性细胞和 CD206 阳性细胞中 4-HNE 的产生显着减少。
图 5:莲心碱抑制 LPS/D-GalN 诱导的小鼠巨噬细胞铁死亡。
a, b Assessment of GSH and MDA contents in liver tissues. (##p < 0.01, vs. Con. **p < 0.01, vs. LPS/D-GalN. Con Control). c Superoxide anion fluorescence detection probe dihydroethidium (DHE) was used to assess the level of ROS in the liver, Scale bar = 50 µm. d Representative electron micrograph of liver tissue, Scale bar = 2 µm. e Fluorescence analysis showing the co-localization of iNOS (green) with 4-HNE (red) in the liver, Scale bar = 10 µm. f Fluorescence analysis showing the co-localization of CD206 (green) with 4-HNE (red) in the liver, Scale bar = 10 µm. f–h Macrophages were depleted by intraperitoneal injections of clodronate liposomes (CLs) for 48 h. Mice-depleted macrophages were treated with Lie (60 mg/kg) intraperitoneally for 2 h and then injected with LPS/D-GalN for 6 h, after which the liver and blood were collected for subsequent experiments (n = 5/group). f Histopathology, hematoxylin, and eosin (H&E) staining, Scale bar = 100 µm. g, h Assessment of GSH and MDA contents in the mice sera. i–m Assessment of serum inflammatory factors IL-1β, IL-6, HMGB1, and TNF-α. Results were presented as mean ± SD (n = 5). (*p < 0.05, **p < 0.01. Con control).
a 、 b肝组织中 GSH 和 MDA 含量的评估。 ( ## p < 0.01,与 Con 相比。** p < 0.01,与 LPS/D-GalN 相比。Con 对照)。 c超氧阴离子荧光检测探针二氢乙锭(DHE)用于评估肝脏中ROS的水平,比例尺= 50 µm。 d肝组织的代表性电子显微照片,比例尺 = 2 µm。 e荧光分析显示 iNOS(绿色)与 4-HNE(红色)在肝脏中共定位,比例尺 = 10 µm。 f荧光分析显示 CD206(绿色)与 4-HNE(红色)在肝脏中共定位,比例尺 = 10 µm。 f – h通过腹腔注射氯膦酸盐脂质体 (CL) 48 小时来耗尽巨噬细胞。腹腔注射Lie (60 mg/kg)处理小鼠巨噬细胞2 h,然后注射LPS/D-GalN 6 h,然后收集肝脏和血液用于后续实验( n = 5/组)。 f组织病理学、苏木精和伊红 (H&E) 染色,比例尺 = 100 µm。 g 、 h小鼠血清中 GSH 和 MDA 含量的评估。 i – m评估血清炎症因子 IL-1β、IL-6、HMGB1 和 TNF-α。结果以平均值±标准差( n = 5)表示。 (* p < 0.05,** p < 0.01。Con 对照)。
We further assessed whether the effect of Lie in inhibiting LPS/D-GalN-induced pathological liver injury and inflammatory responses in mice was partly attributed to AAM. Mice were depleted of macrophages using intraperitoneal injections of clodronate liposomes, followed by LPS/D-GalN injections. H&E staining of samples revealed that the effects of Lie on LPS/D-GalN-induced pathological liver injury in the macrophage-depleted mice were significantly reduced (Fig. 5f). Furthermore, clodronate liposomes were found to be effective in LPS/D-GalN-treated mice as they partially reversed the effects of Lie on serum transaminases (Fig. 5g, h). Furthermore, the efficacy of Lie to suppress hepatic inflammatory responses in the LPS/D-GalN-treated mice was significantly reduced in macrophage-depleted mice (Fig. 5i–m). However, Lie partially inhibited ROS and 4-HNE production in the liver of macrophage-depleted mice (Fig. S4a, b), suggesting that Lie partially ameliorates LPS/D-GalN-induced hepatocyte ferroptosis. These findings suggest that the effects of Lie on LPS/D-GalN-induced pathological liver injury and inflammatory responses in mice are partially related to AAM survival.
我们进一步评估了 Lie 抑制 LPS/D-GalN 诱导的小鼠病理性肝损伤和炎症反应的作用是否部分归因于 AAM。腹腔注射氯膦酸盐脂质体,然后注射 LPS/D-GalN,以清除小鼠的巨噬细胞。样品的H&E染色显示,Lie对巨噬细胞耗竭小鼠中LPS/D-GalN诱导的病理性肝损伤的影响显着降低(图5f )。此外,发现氯膦酸盐脂质体对LPS/D-GalN治疗的小鼠有效,因为它们部分逆转了Lie对血清转氨酶的影响(图5g,h )。此外,在巨噬细胞耗尽的小鼠中,Lie 抑制 LPS/D-GalN 治疗小鼠肝脏炎症反应的功效显着降低(图5i-m )。然而,Lie 部分抑制巨噬细胞耗尽小鼠肝脏中 ROS 和 4-HNE 的产生(图S4a,b ),表明 Lie 部分改善 LPS/D-GalN 诱导的肝细胞铁死亡。这些发现表明,Lie 对 LPS/D-GalN 诱导的小鼠病理性肝损伤和炎症反应的影响与 AAM 存活部分相关。
Inhibition of ferritinophagic flux by liensinine increases resistance of alternatively activated macrophages to ferroptosis
莲心碱对铁蛋白吞噬通量的抑制增加了替代激活的巨噬细胞对铁死亡的抵抗力
We investigated the mechanisms by which Lie inhibits ferroptosis in AAM. Ferroptosis is an iron-dependent mode of cell death [36]. By degrading ferritin, an intracellular iron storage protein, autophagy promotes ferroptosis by releasing stored iron and increasing the quantity of unstable iron in cells [37]. Lie has been found to play a role in autophagy regulation [38]. Owing to the fact that Lie can increase AAM resistance to ferroptosis, we explored whether Lie increases AAM resistance to ferroptosis by modulating ferritinophagy. The laser confocal scanning microscopy analysis revealed increased autophagosome accumulation in the presence of Lie and in the RSL3-induced AAM than in the RSL3 group (Fig. 6a). The ultrastructural alterations in these cells were observed using TEM to further characterize the autophagic properties of AAM following RSL3 and Lie treatment. The autophagic vacuole numbers increased in the Lie + RSL3 group compared to that in the RSL3 group (Fig. 6b). Western blot analysis revealed that Lie significantly enhanced LC3B/GADPH and P62/GADPH expression (Fig. 6c). Owing to the fact that p62 is the link between LC3 and autophagic substrates, autophagy inhibition may correspond with increasing P62 expression levels [39]. Therefore, Lie may be a potent autophagic flux inhibitor, increasing AAM resistance to ferroptosis by suppressing ferritinophagic flux. Using the Fe2+-selective fluorescent probe FerroOrange and lysosomal probe LysoTracker Green, we investigated whether Lie could alter the levels of Fe2+ in the lysosomes of RSL3-treated AAM. Hence, when AAM was treated with RSL3 combined with Lie, Fe2+ levels in the lysosomes (coexistence of FerroOrange and LysoTracker Green) reduced compared to that in the RSL3 group. Bafilomycin A1, an autophagy inhibitor, was used as a positive control, showing almost the same effect as Lie.
我们研究了 Lie 抑制 AAM 中铁死亡的机制。铁死亡是一种铁依赖性细胞死亡模式[ 36 ]。通过降解铁蛋白(一种细胞内铁储存蛋白),自噬释放储存的铁并增加细胞中不稳定铁的数量,从而促进铁死亡[ 37 ]。已发现Lie在自噬调节中发挥作用[ 38 ]。由于Lie可以增加AAM对铁死亡的抵抗力,我们探讨了Lie是否通过调节铁蛋白自噬来增加AAM对铁死亡的抵抗力。激光共聚焦扫描显微镜分析显示,在存在Lie的情况下以及在RSL3诱导的AAM中,自噬体积累比RSL3组增加(图6a )。使用 TEM 观察这些细胞的超微结构变化,以进一步表征 RSL3 和 Lie 处理后 AAM 的自噬特性。与RSL3组相比,Lie+RSL3组的自噬泡数量增加(图6b )。 Western blot分析显示,Lie显着增强了LC3B/GADPH和P62/GADPH的表达(图6c )。由于p62是LC3和自噬底物之间的纽带,自噬抑制可能与P62表达水平的增加相对应[ 39 ]。因此,Lie 可能是一种有效的自噬流抑制剂,通过抑制铁蛋白自噬流来增强 AAM 对铁死亡的抵抗力。使用 Fe 2+选择性荧光探针 FerroOrange 和溶酶体探针 LysoTracker Green,我们研究了 Lie 是否可以改变 RSL3 处理的 AAM 溶酶体中 Fe 2+的水平。 因此,当AAM用RSL3联合Lie处理时,溶酶体中的Fe 2+水平(FerroOrange和LysoTracker Green共存)与RSL3组相比降低。自噬抑制剂巴弗洛霉素A1作为阳性对照,表现出与Lie几乎相同的效果。
图 6:莲心碱抑制铁蛋白自噬,从而抑制替代激活的巨噬细胞铁死亡。
a EGFP-LC3 spot aggregation was observed under confocal microscopy, Scale bar = 10 µm (n = 3). b Expression of autophagy-related proteins LC3B-I, LC3B-II, and P62 was detected using western blots. c Representative electron micrograph image, Scale bar = 2 µm (n = 3). d Representative immunofluorescence images of FerroOrange (red) and LysoTracker Green (green) were used to examine the subcellular localization of Fe2+ in cells, Scale bar = 10 µm. Results were presented as mean ± SD. (n = 3, ##p < 0.01, #p < 0.05, vs. Con. **p < 0.01, ***p < 0.001, ****p < 0.0001, vs. RSL3. Con Control, NS not significant).
在共焦显微镜下观察到EGFP -LC3 斑点聚集,比例尺 = 10 µm ( n = 3)。 b使用蛋白质印迹法检测自噬相关蛋白 LC3B-I、LC3B-II 和 P62 的表达。 c代表性电子显微图像,比例尺 = 2 µm ( n = 3)。 d使用 FerroOrange(红色)和 LysoTracker Green(绿色)的代表性免疫荧光图像来检查细胞中 Fe 2+的亚细胞定位,比例尺 = 10 µm。结果以平均值±标准差表示。 ( n = 3, ## p < 0.01, # p < 0.05,与 Con 相比。** p < 0.01,*** p < 0.001,**** p < 0.0001,与 RSL3 相比。Con 对照,NS 不是重要的)。
Induction of ferritinophagic flux alterations by liensinine via blocking autophagosome-lysosome fusion in alternatively activated macrophages
莲心碱通过阻断替代激活的巨噬细胞中的自噬体-溶酶体融合来诱导铁蛋白吞噬通量改变
To determine whether the effects of Lie were due to ferritinophagic flux inhibition, AAM was treated with RSL3 combined with or without Lie, and ferritin punctate co-localization with the lysosomal marker LAMP1 was examined. Ferritin and LAMP1 co-localization was reduced following Lie treatment (Fig. 7a), suggesting that Lie may affect the ferritin autophagosome formation or block autophagosome fusion with lysosomes. We then evaluated LC3 co-localization with ferritin after treating AAM using RSL3 with or without Lie and found that the latter increased LC3-ferritin co-localization (Fig. 7b). These findings imply that Lie had no effect on ferritin autophagosomes formation. Fusing autophagosomes with lysosomes in the late stages of autophagy leads to autophagolysosome formation, and inhibiting this process impairs ferritin degradation. Therefore, we hypothesized that suppressing autophagosome-lysosome fusion could explain the impairment of Lie-induced ferritinophagic flux. To determine whether Lie inhibits the fusion of ferritin autophagosomes with lysosomes, we used immunofluorescence to investigate the co-localization of the autophagosome marker LC3 and the lysosomal membrane marker LAMP1. Lie significantly inhibited LC3-LAMP1 co-localization (Fig. 7c). Similarly, Lie reduced LC3 co-localization and the lysosomal probe LysoTracker was reduced (Fig. 7d), implying that Lie prevented autophagosome-lysosome fusion from inducing impaired ferritinophagic flux; use of bafilomycin A1, an autophagy inhibitor that prevents autophagosome-lysosome fusion, had similar effects (Fig. 7a–d).
为了确定 Lie 的影响是否是由于铁蛋白吞噬流抑制所致,用 RSL3 联合或不联合 Lie 处理 AAM,并检查铁蛋白点状与溶酶体标记物 LAMP1 的共定位。 Lie处理后铁蛋白和LAMP1共定位减少(图7a ),表明Lie可能影响铁蛋白自噬体形成或阻止自噬体与溶酶体融合。然后,我们在使用RSL3(有或没有Lie)处理AAM后评估了LC3与铁蛋白的共定位,发现后者增加了LC3-铁蛋白的共定位(图7b )。这些发现表明 Lie 对铁蛋白自噬体的形成没有影响。在自噬后期,自噬体与溶酶体融合导致自噬溶酶体形成,抑制这一过程会损害铁蛋白降解。因此,我们假设抑制自噬体-溶酶体融合可以解释 Lie 诱导的铁蛋白吞噬流的损伤。为了确定 Lie 是否抑制铁蛋白自噬体与溶酶体的融合,我们使用免疫荧光来研究自噬体标记物 LC3 和溶酶体膜标记物 LAMP1 的共定位。 Lie显着抑制LC3-LAMP1共定位(图7c )。类似地,Lie减少了LC3共定位,并且溶酶体探针LysoTracker减少了(图7d ),这意味着Lie阻止了自噬体-溶酶体融合诱导受损的铁蛋白吞噬通量;使用巴弗洛霉素A1(一种防止自噬体-溶酶体融合的自噬抑制剂)也有类似的效果(图7a-d )。
图 7:莲心碱通过阻断替代激活的巨噬细胞中的自噬体-溶酶体融合来诱导铁蛋白吞噬通量改变。
a Confocal microscopy images of the co-localization of ferritin (red) with LAMP1 (green), Scale bar = 100 µm (n = 3). b Confocal microscopy images of the co-localization of ferritin (red) with GFP-LC3 (green), Scale bar = 10 µm (n = 3). c Confocal microscopy images of immunostained GFP-LC-3 (green) and LAMP1 (red), Scale bar = 10 µm (n = 3). d Confocal microscopy images of LysoTracker Green (red) and GFP-LC3 (green), Scale bar = 10 µm (n = 3).
a铁蛋白(红色)与 LAMP1(绿色)共定位的共聚焦显微镜图像,比例尺 = 100 µm( n = 3)。 b铁蛋白(红色)与 GFP-LC3(绿色)共定位的共聚焦显微镜图像,比例尺 = 10 µm( n = 3)。 c免疫染色 GFP-LC-3(绿色)和 LAMP1(红色)的共聚焦显微镜图像,比例尺 = 10 µm( n = 3)。 d LysoTracker Green(红色)和 GFP-LC3(绿色)的共聚焦显微镜图像,比例尺 = 10 µm( n = 3)。
Discussion 讨论
ALI is a multi-etiological disease with high morbidity and mortality rates [5]. During ALI progression, Macrophages play critical roles in coordinating tissue destruction and repair following an acute liver injury [12]. However, macrophages are highly malleable and, depending on the stimuli, can develop into phenotypes with diverse properties and effects. Therefore, exerting different regulatory functions on the physiological and pathological activities of the body [13]. Macrophages are mainly polarized into two phenotypes: pro-inflammatory M1 and anti-inflammatory AAM. Many pro-inflammatory cytokines, including IL-1b, iNOS, and TNF-a, are secreted by M1 macrophages, whereas AAM macrophages mainly produce anti-inflammatory factors, such as IL-10, transforming growth factor-b, and arginase 1 [14, 17]. Studies revealed that pro-inflammatory cytokines secreted by M1 macrophages aggravate ALI. In contrast, M2 macrophages were found to enhance tissue damage repair and secrete anti-inflammatory cytokines, facilitating inflammation regression and ALI remission [16,17,18,19,20]. Therefore, increasing AAM numbers may be an effective strategy for ameliorating ALI. Two strategies can be used to increase the number of AAM in ALI: one requires increasing AAM, and the other involves lowering AAM depletion. However, current studies have focused almost exclusively on improving ALI by regulating macrophage reprogramming or increasing the AAM via chemotaxis [11, 14, 17, 20]. Drugs have rarely been reported to ameliorate ALI by lowering AAM depletion and enhancing AAM survival. This could be because drugs that reduce the damage and consumption of AAM also reduce that of M1 macrophages, making it challenging to maintain an equilibrium between the two phenotypes.
ALI是一种具有高发病率和死亡率的多病因性疾病[ 5 ]。在 ALI 进展过程中,巨噬细胞在协调急性肝损伤后的组织破坏和修复中发挥着关键作用 [ 12 ]。然而,巨噬细胞具有高度可塑性,并且根据刺激,可以发育成具有不同特性和作用的表型。因此对机体的生理和病理活动发挥不同的调节功能[ 13 ]。巨噬细胞主要极化为两种表型:促炎性M1和抗炎性AAM。许多促炎细胞因子,包括 IL-1b、iNOS 和 TNF-a,由 M1 巨噬细胞分泌,而 AAM 巨噬细胞主要产生抗炎因子,如 IL-10、转化生长因子-b 和精氨酸酶 1。 14、17 ] 。研究表明,M1 巨噬细胞分泌的促炎细胞因子会加重 ALI。相反, M2巨噬细胞被发现可以增强组织损伤修复并分泌抗炎细胞因子,促进炎症消退和ALI缓解[16,17,18,19,20 ] 。因此,增加 AAM 数量可能是改善 ALI 的有效策略。可以使用两种策略来增加 ALI 中的 AAM 数量:一种需要增加 AAM,另一种涉及降低 AAM 消耗。然而,目前的研究几乎完全集中在通过调节巨噬细胞重编程或通过趋化性增加AAM 来改善 ALI [ 11,14,17,20 ]。很少有药物通过降低 AAM 消耗和增强 AAM 存活来改善 ALI 的报道。 这可能是因为减少 AAM 损伤和消耗的药物也会减少 M1 巨噬细胞的损伤和消耗,从而难以维持两种表型之间的平衡。
M1 macrophages expressing iNOS were recently found to be highly resistant to ferroptosis. However, AAM lacking iNOS expression was highly susceptible to ferroptosis [33]. In il-4-driven macrophage differentiation, loss of Gpx4 activity leads to ferroptotic cell death, but not in M1 macrophages. [40]. Our findings are consistent with those of previous studies. We exposed M1 macrophages and AAM to various cell-death inducers and found that AAM was ferroptosis-sensitive, whereas M1 macrophages were ferroptosis-resistant. Ferroptosis is a new cell death type triggered by excessive Fe2+ ion accumulation in the cell [27]. Excessive intracellular Fe2+ ion accumulation induces oxidative stress, which promotes lipid peroxidation of cell membranes, protein oxidation, and DNA damage [27]. Clinical studies have revealed that increased hepatic irons reserves and elevated serum ferritin concentrations are typical characteristics of various liver diseases [28]. In vivo studies have shown that iron overload can cause liver damage in mice [41, 42]. In APAP-induced hepatotoxicity, lysosomal iron was also observed to translocate to mitochondria and promote oxidation, and this harmful effect was antagonized by iron chelators [30]. These findings suggest that ferroptosis plays a role in ALI progression. Accordingly, we hypothesized that Fer-1 (a potent ferroptosis inhibitor) could alleviate ALI, possibly rescue ferroptosis, and increase AAM survival in ALI. Our findings confirm that Fer-1 alleviates histopathological changes in the liver and increases the expression of the AAM marker CD206 levels in LPS/D-GalN-treated mice. These findings suggest that ferroptosis is an important factor in ALI pathogenesis, and inhibiting it can partially alleviate LPS/D-GalN-induced ALI while increasing the number of AAM.
最近发现表达 iNOS 的 M1 巨噬细胞对铁死亡具有高度抵抗力。然而,缺乏 iNOS 表达的 AAM 极易发生铁死亡[ 33 ]。在 il-4 驱动的巨噬细胞分化中,Gpx4 活性的丧失会导致铁死亡,但在 M1 巨噬细胞中则不然。 [ 40 ]。我们的研究结果与之前的研究结果一致。我们将 M1 巨噬细胞和 AAM 暴露于各种细胞死亡诱导剂,发现 AAM 对铁死亡敏感,而 M1 巨噬细胞对铁死亡具有抗性。铁死亡是一种新的细胞死亡类型,由细胞内过量的 Fe 2+离子积累引发[ 27 ]。细胞内过量的Fe 2+离子积累会诱发氧化应激,从而促进细胞膜的脂质过氧化、蛋白质氧化和DNA损伤[ 27 ]。临床研究表明,肝脏铁储备增加和血清铁蛋白浓度升高是各种肝脏疾病的典型特征[ 28 ]。体内研究表明铁超载会导致小鼠肝损伤[ 41 , 42 ]。在APAP诱导的肝毒性中,还观察到溶酶体铁易位至线粒体并促进氧化,这种有害作用可被铁螯合剂拮抗[ 30 ]。这些发现表明铁死亡在 ALI 进展中发挥作用。因此,我们假设 Fer-1(一种有效的铁死亡抑制剂)可以缓解 ALI,可能挽救铁死亡,并增加 ALI 中 AAM 的存活率。我们的研究结果证实,Fer-1 可减轻 LPS/D-GalN 治疗小鼠肝脏的组织病理学变化,并增加 AAM 标记 CD206 的表达水平。 这些发现表明铁死亡是ALI发病机制中的一个重要因素,抑制它可以部分缓解LPS/D-GalN诱导的ALI,同时增加AAM的数量。
Our findings revealed that Fer-1 partially alleviates LPS/D-GalN-induced ALI and increases the number of AAM. However, its limited clinical use is attributed to its in vivo instability. Hence, a more effective drug is needed. The bioactive components of natural products are a significant source of drugs. A practical strategy for developing ALI treatments is the identification of novel medications from herbal monomeric compounds [34]. We identified that Lie effectively inhibits RSL3-induced ferroptosis in AAM after screening the herbal library monomers for small-molecule compounds. Lie was found to reduce lipid peroxides and Fe2+ production in RSL3-induced AAM in vitro. These findings suggest that Lie may boost AAM resistance to ferroptosis. In addition, Lie was found to partially protect mice from LPS/D-GalN-induced pathological liver injury and inflammation, attenuate LPS/D-GalN-induced lipid peroxidation, and increase the amount of AAM. Notably, it is hypothesized that the accumulation of iron-dependent lipid peroxidation products is a key factor in the onset of ferroptosis [27]. Further, when the balance between lipid peroxidation product synthesis and clearance are disrupted, such as by inhibiting GPX4, iron-dependent lipid peroxidation product accumulation triggers ferroptosis [29]. 4-HNE is one of the end products of iron-dependent lipid peroxidation and is frequently used as an indicator of ferroptosis [27]. 4-HNE expression was found to be significantly increased in acute liver injury induced by APAP or erastin [30]. In this study, we found that iNOS-positive cells in LPS/D-GalN-treated mice had less 4-HNE than CD206-positive cells and that 4-HNE synthesis in CD206-positive cells was significantly reduced in Lie-treated LPS/D-GalN-induced acute liver injury. These findings suggest M1 macrophages are resistant to ferroptosis in ALI. In contrast, AAM are susceptible to ferroptosis, which may result in an imbalance between M1 macrophages and AAM, and promote the development of intrahepatic inflammation. In addition, the efficacy of Lie to inhibit the hepatic inflammatory response in LPS/D-GalN-treated mice was significantly reduced in macrophage-deficient mice. These findings suggest that Lie inhibits ferroptosis in ALI and is protective in mice against pathological liver injury. Inflammatory responses induced by LPS/D-galactosamine may be partly related to AAM ferroptosis inhibition.
我们的研究结果表明,Fer-1 可以部分缓解 LPS/D-GalN 诱导的 ALI 并增加 AAM 的数量。然而,由于其体内不稳定,其临床应用受到限制。因此,需要一种更有效的药物。天然产物的生物活性成分是药物的重要来源。开发 ALI 治疗的实用策略是从草药单体化合物中鉴定新药物 [ 34 ]。在筛选草药库单体中的小分子化合物后,我们发现 Lie 可有效抑制 AAM 中 RSL3 诱导的铁死亡。 Lie 被发现可以减少体外 RSL3 诱导的 AAM 中脂质过氧化物和 Fe 2+ 的产生。这些发现表明 Lie 可能会增强 AAM 对铁死亡的抵抗力。此外,Lie 还被发现可以部分保护小鼠免受 LPS/D-GalN 诱导的病理性肝损伤和炎症,减弱 LPS/D-GalN 诱导的脂质过氧化,并增加 AAM 的量。值得注意的是,据推测铁依赖性脂质过氧化产物的积累是铁死亡发生的关键因素[ 27 ]。此外,当脂质过氧化产物合成和清除之间的平衡被破坏时,例如通过抑制 GPX4,铁依赖性脂质过氧化产物积累会引发铁死亡[ 29 ]。 4-HNE 是铁依赖性脂质过氧化的最终产物之一,经常用作铁死亡的指标[ 27 ]。发现4-HNE表达在APAP或erastin诱导的急性肝损伤中显着增加[ 30 ]。 在这项研究中,我们发现 LPS/D-GalN 处理的小鼠中的 iNOS 阳性细胞比 CD206 阳性细胞具有更少的 4-HNE,并且在 Lie 处理的 LPS/D-GalN 处理的小鼠中,CD206 阳性细胞中的 4-HNE 合成显着减少。 D-GalN 诱导的急性肝损伤。这些发现表明 M1 巨噬细胞对 ALI 中的铁死亡具有抵抗力。相反,AAM容易发生铁死亡,这可能导致M1巨噬细胞和AAM之间的不平衡,并促进肝内炎症的发展。此外,Lie 抑制 LPS/D-GalN 治疗小鼠肝脏炎症反应的功效在巨噬细胞缺陷小鼠中显着降低。这些发现表明,Lie 可抑制 ALI 中的铁死亡,并保护小鼠免受病理性肝损伤。 LPS/D-半乳糖胺诱导的炎症反应可能部分与 AAM 铁死亡抑制有关。
Lie is a bioactive ingredient extracted from lotus seeds that play an important role in preventing and treating various diseases [43]. Moreover, recent studies have revealed that it regulates autophagy. For example, Lie enhances doxorubicin-mediated apoptosis by inhibiting autophagy [44] and suppresses non-small cell lung cancer progression in vitro and in vivo by blocking autophagic flux [45]. Moreover, it preserves beige adipocyte properties by inhibiting mitotic phagocytosis, thereby reducing obesity [46]. To date, the effects of Lie on the relationship between ferroptosis and autophagy have not been well studied. Ferroptosis is an iron-dependent form of oxidative cell death. The Fenton reaction is a chemical reaction that occurs when ferrous iron reacts with hydrogen peroxide to form ferric iron. This results in ROS production, which, if not immediately scavenged, damages lipid membranes and causes cell death [27]. The nuclear receptor coactivator 4, which binds to FTH1 in the autophagosome, transfers the ferritin autophagosome to the lysosome to degrade ferritin and then releases free iron, is an important source of intracellular ferrous ions [36, 47]. Ferroptosis can be prevented by inhibiting ferritinophagy. For example, inhibiting RANKL-induced ferritinophagy can protect osteoclasts from ferroptosis [48], whereas inhibiting ferritinophagy can reduce intracellular iron levels and lipid peroxidation, attenuating ZnONP-induced ferroptosis [49]. Therefore, we hypothesized that Lie might inhibit ferroptosis in AAM by regulating ferritinophagy. Our in vitro experiments revealed that autophagic flux changed following Lie treatment. Further, when AAM was treated with RSL3 and Lie combination, the amount of Fe2+ in the lysosomes was significantly reduced compared to the RSL3 group, suggesting that Lie may inhibit the ferritinophagy flux and reduce Fe2+ levels to increase AAM resistance to ferroptosis. In addition, we found that treating AAM with RSL3 in conjunction with Lie significantly reduced the co-localization of ferritin and the lysosomal marker LAMP1. Nonetheless, the combination did not inhibit the co-localization of LC3 with ferritin. These findings suggest that Lie may attenuate ferroptosis in AAM in ALI by blocking autophagosome-lysosome fusion and inhibiting ferritinophagy.
李是从莲子中提取的一种生物活性成分,在预防和治疗多种疾病方面发挥着重要作用[ 43 ]。此外,最近的研究表明它调节自噬。例如,Lie 通过抑制自噬增强阿霉素介导的细胞凋亡 [ 44 ],并通过阻断自噬流在体外和体内抑制非小细胞肺癌进展 [ 45 ]。此外,它通过抑制有丝分裂吞噬作用保留米色脂肪细胞特性,从而减少肥胖[ 46 ]。迄今为止,Lie 对铁死亡和自噬之间关系的影响尚未得到充分研究。铁死亡是一种铁依赖性细胞氧化死亡形式。芬顿反应是二价铁与过氧化氢反应形成三价铁时发生的化学反应。这会导致 ROS 的产生,如果不立即清除,则会损害脂质膜并导致细胞死亡 [ 27 ]。核受体共激活因子4与自噬体中的FTH1结合,将铁蛋白自噬体转移至溶酶体降解铁蛋白,然后释放游离铁,是细胞内亚铁离子的重要来源[ 36 , 47 ]。可以通过抑制铁蛋白自噬来预防铁死亡。例如,抑制 RANKL 诱导的铁蛋白自噬可以保护破骨细胞免于铁死亡[ 48 ],而抑制铁蛋白自噬可以降低细胞内铁水平和脂质过氧化,从而减弱 ZnONP 诱导的铁死亡[ 49 ]。因此,我们假设Lie可能通过调节铁蛋白自噬来抑制AAM中的铁死亡。我们的体外实验表明,Lie 治疗后自噬通量发生了变化。 此外,当 AAM 用 RSL3 和 Lie 组合处理时,与 RSL3 组相比,溶酶体中 Fe2+ 的量显着减少,表明 Lie 可能抑制铁蛋白自噬通量并降低 Fe2+ 水平,从而增加 AAM 对铁死亡的抵抗力。此外,我们发现用 RSL3 与 Lie 联合处理 AAM 显着减少了铁蛋白和溶酶体标记物 LAMP1 的共定位。尽管如此,该组合并没有抑制 LC3 与铁蛋白的共定位。这些发现表明,Lie 可能通过阻断自噬体-溶酶体融合和抑制铁蛋白自噬来减轻 ALI 中 AAM 的铁死亡。
In conclusion, we revealed that Lie, a novel ferroptosis inhibitor, may inhibit AAM ferroptosis in ALI, maintain the balance between M1 macrophages and AAM, and reduce acute liver damage, potentially by partially inhibiting autophagosome-lysosome fusion and ferritin degradation. Therefore, Lie may be proposed as a novel candidate for treating patients with ALI.
总之,我们发现Lie是一种新型铁死亡抑制剂,可能通过部分抑制自噬体-溶酶体融合和铁蛋白降解来抑制ALI中的AAM铁死亡,维持M1巨噬细胞和AAM之间的平衡,并减少急性肝损伤。因此,Lie 可能被提议作为治疗 ALI 患者的新候选药物。
This study had some limitations. We did not identify other potential molecular targets for Lie; hence, whether Lie could bind to multi-target proteins involved in ferroptosis or autophagy remains unknown. In future studies, the following should be considered: (1) the specific target proteins of Lie, (2) mechanisms by which it inhibits ferritinophagy, and (3) whether the findings of this study may be used as a reference point in developing treatments for other types of acute organ injury with comparable pathogenesis to ALI.
这项研究有一些局限性。我们没有确定 Lie 的其他潜在分子靶点;因此,Lie 是否能够与参与铁死亡或自噬的多靶蛋白结合仍然未知。在未来的研究中,应考虑以下因素:(1)Lie的特定靶蛋白,(2)它抑制铁蛋白自噬的机制,以及(3)本研究的结果是否可以作为开发治疗方法的参考点用于与 ALI 发病机制相似的其他类型的急性器官损伤。
Materials and methods 材料和方法
Antibodies and reagents 抗体和试剂
Antibodies against iNOS (ab178945), ferritin (ab75973), LC3B (ab48394), p62 (ab109012), and horseradish peroxidase-conjugated secondary antibodies (ab6721) were purchased from Abcam (Cambridge, MA, USA). Anti-CD206 (24595) antibody was purchased from Cell Signaling Technology (Danvers, MA, USA). An anti-4-hydroxynonenal (4-HNE) (BS-6313R) antibody was purchased from Bioss (Beijing, China); an anti-LAMP-1 (AF4320-SP) antibody was purchased from R&D Systems (Minnesota, USA); and anti-GAPDH (60004-1-1g) antibody was purchased from Proteintech (Wuhan, China). Liensinine (Lie), a TCM library, RSL3, erastin, apoptosis inducers, ferrostatin-1 (Fer-1), and bafilomycin A1 (Ba1) were purchased from Selleck Chemicals (Houston, TX, USA). A necroptosis inducer kit with TSZ was purchased from Beyotime (Shanghai, China). Clodronate liposomes (CLs) were purchased from FormuMax (Shanghai, China).
iNOS (ab178945)、铁蛋白 (ab75973)、LC3B (ab48394)、p62 (ab109012) 和辣根过氧化物酶缀合二抗 (ab6721) 的抗体购自 Abcam (Cambridge, MA, USA)。抗 CD206 (24595) 抗体购自 Cell Signaling Technology(美国马萨诸塞州丹弗斯)。抗4-羟基壬烯醛(4-HNE)(BS-6313R)抗体购自Bioss(中国北京);抗LAMP-1(AF4320-SP)抗体购自R&D Systems(美国明尼苏达州);抗GAPDH(60004-1-1g)抗体购自Proteintech(中国武汉)。 Liensinine (Lie)、TCM 文库、RSL3、erastin、细胞凋亡诱导剂、铁他汀-1 (Fer-1) 和巴弗洛霉素 A1 (Ba1) 购自 Selleck Chemicals(休斯顿,德克萨斯州,美国)。含有 TSZ 的坏死性凋亡诱导试剂盒购自 Beyotime(中国上海)。氯膦酸盐脂质体(CL)购自FormuMax(中国上海)。
RAW264.7 cell culture, polarization, and treatment
RAW264.7细胞培养、极化和处理
RAW264.7 cells were purchased from Procell Life Science&Technology (Wuhan, China) and authenticated by STR profiling. The cells were cultured in high-glucose Dulbecco’s modified Eagle medium (Gibco, USA) with 10% fetal bovine serum (Australian origin; Gibco) and 1% penicillin-streptomycin (Gibco, USA) at 37 °C with 5% CO2.
RAW264.7细胞购自Procell生命科技(中国武汉)并通过STR分析验证。将细胞在含有10%胎牛血清(澳大利亚产;Gibco)和1%青霉素-链霉素(Gibco,美国)的高糖Dulbecco改良Eagle培养基(Gibco,美国)中于37℃和5%CO 2下培养。
RAW264.7 macrophages were unstimulated to create M0 macrophages. For M1 macrophage creation, RAW264.7 cells were treated with LPS (10 ng/ml; Sigma-Aldrich, St. Louis, MO, USA) and interferon (IFN)-γ (100 ng/ml; PeproTech) for 24 h. For the AAM, RAW264.7 cells were treated with IL-4 (20 ng/ml; PeproTech) for 24 h.
RAW264.7 巨噬细胞未受刺激产生 M0 巨噬细胞。对于 M1 巨噬细胞的创建,用 LPS(10 ng/ml;Sigma-Aldrich,St. Louis,MO,USA)和干扰素 (IFN)-γ(100 ng/ml;PeproTech)处理 RAW264.7 细胞 24 小时。对于 AAM,用 IL-4(20 ng/ml;PeproTech)处理 RAW264.7 细胞 24 小时。
Apoptosis inducer (20 μM, 24 h), pyroptosis inducers (7 μg/ml, 24 h), necrosis inducer (4 x, 24 h), RSL3 inducer (5 μM, 5 h), and erastin inducer (60 μM, 24 h) were used to treat macrophages (M0, M1, and AAM). For 5 h, AAM was treated with RSL3 (5 μM) in the presence or absence of Fer-1 (400 nM). AAM was treated with the 1488 candidates combined with RSL3 (5 μM) for 5 h. AAM was treated with Lie (1, 5, and 10 μM) combined with RSL3 (5 μM) for 5 h. AAM was treated with RSL3 (5 μM) in the presence or absence of Lie (10 μM) for 5 h. AAM was treated with RSL3 (5 μM) in the presence or absence of Lie (10 μM) or bafilomycin A1 (25 nM) for 5 h.
凋亡诱导剂(20 μM,24 小时)、焦亡诱导剂(7 μg/ml,24 小时)、坏死诱导剂(4 x,24 小时)、RSL3 诱导剂(5 μM,5 小时)和erastin 诱导剂(60 μM,24 小时) h) 用于处理巨噬细胞(M0、M1 和 AAM)。在存在或不存在 Fer-1 (400 nM) 的情况下,用 RSL3 (5 μM) 处理 AAM 5 小时。将 1488 种候选药物与 RSL3 (5 μM) 联合处理 AAM 5 小时。 AAM 用 Lie(1、5 和 10 μM)结合 RSL3(5 μM)处理 5 小时。在存在或不存在 Lie (10 μM) 的情况下用 RSL3 (5 μM) 处理 AAM 5 小时。在存在或不存在 Lie (10 μM) 或巴弗洛霉素 A1 (25 nM) 的情况下,用 RSL3 (5 μM) 处理 AAM 5 小时。
Cell Counting Kit-8 (CCK-8) and lactate dehydrogenase (LDH) cytotoxicity assays
Cell Counting Kit-8 (CCK-8) 和乳酸脱氢酶 (LDH) 细胞毒性测定
Cell viability was determined using the CCK-8 kit (Beyotime, Shanghai, China). In addition, LDH levels were measured using LDH Cytotoxicity Assay Kit (Beyotime, Shanghai, China) following the protocol of the manufacturer.
使用CCK-8试剂盒(Beyotime,上海,中国)测定细胞活力。此外,按照制造商的方案,使用LDH细胞毒性测定试剂盒(Beyotime,上海,中国)测量LDH水平。
Propidium iodide (PI) staining
碘化丙啶 (PI) 染色
Approximately 1/10th of the medium volume of PI (KGA214-50; Nanjing, China) working solution (20 μM) was added to the culture medium. The cells were incubated at 37 °C for 15 min. DAPI staining was performed for 5 min. The cells were observed under a fluorescence microscope and photographed.
将大约培养基体积的 1/10 PI(KGA214-50;南京,中国)工作溶液(20 μM)添加到培养基中。将细胞在 37°C 下孵育 15 分钟。 DAPI染色进行5分钟。在荧光显微镜下观察细胞并拍照。
Transmission electron microscopy (TEM)
透射电子显微镜 (TEM)
Cells or liver tissues were collected and fixed in 2.5% Gluta fixative (Solarbio, Beijing, China) for 30 min in the dark at 25 °C before being stored at 4 °C. Dehydrated samples were embedded in epoxy resin and cut into ultrathin sections. The samples were then examined and photographed using an HT7800 TEM (Hitachi, Japan).
收集细胞或肝组织,并在 2.5% Gluta 固定剂(Solarbio,北京,中国)中在 25°C 下避光固定 30 分钟,然后在 4°C 下保存。将脱水样品包埋在环氧树脂中并切成超薄切片。然后使用 HT7800 TEM(日本日立)对样品进行检查和拍照。
Glutathione (GSH) and malondialdehyde (MDA) assays
谷胱甘肽 (GSH) 和丙二醛 (MDA) 测定
MDA and GSH levels in mouse liver tissues collected were determined using a commercial assay kit (Beyotime, Shanghai, China).
使用商业检测试剂盒(Beyotime,上海,中国)测定收集的小鼠肝组织中的 MDA 和 GSH 水平。
Divalent iron ion probe detection
二价铁离子探针检测
FerroOrange working solution (1 mM; Dojindo Laboratories, Kyushu, Japan) was added to the samples and incubated for 30 min before being photographed under a fluorescence microscope.
将 FerroOrange 工作溶液(1 mM;Dojindo Laboratories,九州,日本)添加到样品中并孵育 30 分钟,然后在荧光显微镜下拍照。
Active oxygen determination
活性氧测定
Dihydroethidium (Beyotime, Shanghai, China) was used to measure superoxide anion levels in cells and liver tissues. Under different experimental conditions, the cells or tissue sections were placed on coverslips. They were then incubated for 30 min at 37 °C with a 5-µM dihydroethidium working solution before being photographed under a fluorescence microscope.
二氢乙锭(Beyotime,上海,中国)用于测量细胞和肝组织中的超氧阴离子水平。在不同的实验条件下,将细胞或组织切片置于盖玻片上。然后将它们与 5 µM 二氢乙锭工作溶液一起在 37 °C 下孵育 30 分钟,然后在荧光显微镜下拍照。
Liperfluo assay Liperfluo测定
Liperfluo (Dojindo Laboratories, Kyushu, Japan) is a Spy-LHP analog that can be used to detect lipid peroxides. Liperfluo working solution (1 mM) was added to the samples and incubated for 30 min at 37 °C before being photographed using a fluorescence microscope.
Liperfluo(日本九州同仁化学研究所)是一种 Spy-LHP 类似物,可用于检测脂质过氧化物。将 Liperfluo 工作溶液 (1 mM) 添加到样品中,并在 37 °C 下孵育 30 分钟,然后使用荧光显微镜拍照。
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and inflammatory factor measurements
丙氨酸转氨酶 (ALT)、天冬氨酸转氨酶 (AST) 和炎症因子测量
ALT, AST, and inflammatory factor levels in mice sera were measured with the analytical assay kits. Inflammatory factor assay kits were purchased from Solarbio Science & Technology (Beijing, China). AST and ALT assay kits were purchased from JINGMEI BIOTECHNOLOGY (Suzhou, China).
使用分析检测试剂盒测量小鼠血清中的 ALT、AST 和炎症因子水平。炎症因子测定试剂盒购自Solarbio Science & Technology(中国北京)。 AST和ALT检测试剂盒购自精美生物科技(中国苏州)。
Western blotting 蛋白质印迹法
RIPA buffer (Beyotime, Shanghai, China) was used to extract proteins from liver tissues. A bicinchoninic acid assay kit was used to determine the protein levels. The proteins were denatured by boiling for 10 min. Protein samples (containing 50 μg per well) were dispensed into the gel and were treated to an initial voltage of 80 V for ~30 min. The voltage was then raised to 120 V for 60 min. After incubation at 26 °C for 2 h, the separated proteins in the gel were transferred onto polyvinylidene difluoride membranes (0.45 μm) and blocked in skimmed milk (5%) at 100 mA/90 min. The corresponding primary antibodies (LC3, 1:1000; P62, 1:1000) were added and incubated overnight at 4 °C. The secondary antibody (1:5000) was then added and incubated for 2 h at 26 °C before being washed four times in TBST for 5 min. The protein bands were then examined.
使用RIPA缓冲液(Beyotime,上海,中国)从肝组织中提取蛋白质。使用二辛可宁酸测定试剂盒测定蛋白质水平。煮沸 10 分钟使蛋白质变性。将蛋白质样品(每孔含 50 μg)分配到凝胶中,并在 80 V 的初始电压下处理约 30 分钟。然后将电压升至120V,保持60分钟。 26℃孵育2小时后,将凝胶中分离的蛋白质转移到聚偏二氟乙烯膜(0.45μm)上,并在脱脂牛奶(5%)中以100mA/90分钟封闭。添加相应的一抗(LC3,1:1000;P62,1:1000)并在4℃下孵育过夜。然后加入二抗(1:5000)并在 26°C 下孵育 2 小时,然后在 TBST 中洗涤四次,每次 5 分钟。然后检查蛋白质条带。
Immunohistochemistry and hematoxylin and eosin (H&E) staining
免疫组织化学和苏木精和伊红 (H&E) 染色
The paraffin-embedded sections were oven dried at 60 °C for 60 min, dewaxed, and then rehydrated. H&E stains were applied for 3 min and 30 s, respectively. Antigen repair was performed under high pressure for 15 min for immunohistochemistry analysis. H2O2 (3%) in phosphate-buffered saline was applied for 15 min to remove endogenous peroxidase. The tissue was covered with 5% goat serum at 26 °C for 1 h. Dropwise additions of primary antibodies (iNOS, 1:100; CD206, 1:100; 4-HNE, 1:100) were conducted overnight at 4 °C. Fluorescent secondary antibodies (1:200) or horseradish peroxidase-labeled secondary antibodies (1:200) were then added and incubated for 30 min at 26 °C; DAPI staining was used for the fluorescent secondary antibody for 5 min while in the horseradish peroxidase-labeled secondary antibody, staining was continued with diaminobenzidine or tyramide signal amplification for 10 min, followed by hematoxylin re-staining.
将石蜡包埋的切片在 60°C 下烘干 60 分钟,脱蜡,然后再水化。 H&E 染色分别应用 3 分钟和 30 秒。高压下进行抗原修复15分钟以进行免疫组织化学分析。使用磷酸盐缓冲盐水中的 H 2 O 2 (3%) 15 分钟以去除内源性过氧化物酶。将组织用5%山羊血清在26℃下覆盖1小时。滴加一抗(iNOS,1:100;CD206,1:100;4-HNE,1:100)在 4°C 过夜。然后加入荧光二抗(1:200)或辣根过氧化物酶标记二抗(1:200),26℃孵育30 min;荧光二抗使用 DAPI 染色 5 分钟,而辣根过氧化物酶标记二抗则用二氨基联苯胺或酪酰胺信号放大继续染色 10 分钟,然后苏木精重新染色。
Immunofluorescence 免疫荧光
The cells or tissue sections were placed on coverslips. Lysosomes were stained using LysoTracker Red (Beyotime Biotechnology) or LysoTracker Green (Beyotime Biotechnology). The samples were fixed in 4% paraformaldehyde for 30 min, permeabilized for 10 min in 0.1% Triton X-100, then incubated in 10% goat serum for 30 min. LAMP1 (1:100), ferritin (1:100), and 4-HNE (1:100) were added and incubated overnight at 4 °C. The fluorescent secondary antibody (1:200) was then added and incubated for 2 h at 26 °C. The cells were observed using laser scanning confocal or fluorescence microscopy.
将细胞或组织切片放置在盖玻片上。使用LysoTracker Red(Beyotime Biotechnology)或LysoTracker Green(Beyotime Biotechnology)对溶酶体进行染色。将样品在 4% 多聚甲醛中固定 30 分钟,在 0.1% Triton X-100 中透化 10 分钟,然后在 10% 山羊血清中孵育 30 分钟。添加 LAMP1 (1:100)、铁蛋白 (1:100) 和 4-HNE (1:100),并在 4°C 下孵育过夜。然后加入荧光二抗(1:200),26℃孵育2小时。使用激光扫描共聚焦或荧光显微镜观察细胞。
GFP-LC3B transfection GFP-LC3B 转染
The cells were dispensed into six-well plates at 4 × 105 cells/well at 37 °C with 5% CO2 overnight. The old culture medium was aspirated and replaced with a 1.2 ml/well fresh culture medium. Viral solution (20 multiplicity of infection) was added separately. After 24 h, the culture medium was carefully removed and replaced with 2 ml/well fresh complete culture medium before being incubated for 24 h at 37 °C with 5% CO2.
将细胞以4×10 5 个细胞/孔在37℃和5%CO 2下分配到六孔板中过夜。吸出旧培养基并更换为1.2ml/孔的新鲜培养基。单独添加病毒溶液(20感染复数)。 24小时后,小心除去培养基并更换为2ml/孔的新鲜完全培养基,然后在37℃、5%CO 2下孵育24小时。
Distribution of ferrous iron in LysoTracker staining
LysoTracker 染色中亚铁的分布
The cells were stained using LysoTracker Red staining working solution (50 nM), then incubated at 37 °C for 20 min. Next, the LysoTracker Red staining working solution was carefully removed, replaced with 1 mol/l of FerroOrange working solution, and incubated for 20 min at 37 °C. After carefully removing the FerroOrange working solution, a fresh cell culture medium was added before examining the samples using a laser confocal microscope.
使用 LysoTracker Red 染色工作液 (50 nM) 对细胞进行染色,然后在 37 °C 下孵育 20 分钟。接下来,小心除去LysoTracker Red染色工作液,更换为1 mol/l FerroOrange工作液,并在37℃下孵育20 min。小心除去铁橙工作溶液后,添加新鲜的细胞培养基,然后使用激光共聚焦显微镜检查样品。
Animals and models 动物和模型
Male C57BL/6 mice (6–8 weeks old, weighing 18–23 g) were purchased from Guangdong Experimental Animal Centre (Guangzhou, China). The ALI model was established based on a previous study [50]. Briefly, the mice were intraperitoneally injected with 600 mg/kg of D-GalN (Sigma-Aldrich, Shanghai, China) and 30 μg/kg of LPS (Sigma-Aldrich, Shanghai, China). Mice were treated with different reagents and were randomly assigned to different groups: GalN/LPS + Lie (10, 30, and 60 mg/kg) groups. Mice were administered LPS/D-GalN intraperitoneally to induce acute liver injury before being administered intraperitoneal injections of Lie (10, 30, and 60 mg/kg) 2 h before treatment. Further, survival was monitored over a 24 h period (n = 10/group). In the GalN/LPS + Fer1 groups, mice were administered intraperitoneal injections of Fer-1 (10 mg/kg) 2 h before the LPS/D-GalN injection (n = 5/group) [51]. In the GalN/LPS + Lie groups, mice were pre-treated with Lie (60 mg/kg) intraperitoneally for 2 h before LPS/D-GalN injections (n = 5/group). In the GalN/LPS + Lie + CLs groups, macrophages were depleted by intraperitoneal clodronate liposomes (CLs) injections for 48 h. Mice-depleted macrophages were treated with Lie (60 mg/kg) intraperitoneally for 2 h before being injected with LPS/D-GalN for 6 h (n = 5/group). In the control groups, mice were administered saline solution intraperitoneally, whereas Fer-1 was administered intraperitoneally to mice in the Fer1 groups (10 mg/kg). Mice in the Lie groups were administered Lie (60 mg/kg) intraperitoneally. Sera and liver tissues were harvested for analysis 6 h after acute exposure. All animal experiments were approved by the Humane Animal Care Standard and authorized by the Experimental Animal Ethics Committee of Zhongke Industry Holding (Shenzhen) Co., LTD. The individuals conducting the experiments were blinded to the allocation sequence and group allocation.
雄性C57BL/6小鼠(6-8周龄,体重18-23 g)购自广东省实验动物中心(中国广州)。 ALI模型是根据之前的研究建立的[ 50 ]。简而言之,给小鼠腹腔注射600 mg/kg的D-GalN(Sigma-Aldrich,上海,中国)和30 μg/kg的LPS(Sigma-Aldrich,上海,中国)。用不同的试剂处理小鼠并随机分配到不同的组:GalN/LPS + Lie(10、30 和 60 mg/kg)组。小鼠腹腔注射 LPS/D-GalN 以诱导急性肝损伤,然后在治疗前 2 小时腹腔注射 Lie(10、30 和 60 mg/kg)。此外,在 24 小时内监测存活率( n = 10/组)。在GalN/LPS + Fer1组中,在注射LPS/D-GalN前2小时对小鼠腹腔注射Fer-1(10 mg/kg)( n =5/组)[ 51 ]。在 GalN/LPS + Lie 组中,在注射 LPS/D-GalN 之前,小鼠腹腔内用 Lie (60 mg/kg) 预处理 2 小时( n = 5/组)。在 GalN/LPS + Lie + CLs 组中,通过腹腔内注射氯膦酸盐脂质体 (CLs) 48 小时来耗尽巨噬细胞。腹腔内用 Lie (60 mg/kg) 处理小鼠巨噬细胞 2 小时,然后注射 LPS/D-GalN 6 小时( n = 5/组)。在对照组中,小鼠腹腔内施用生理盐水,而Fer1组的小鼠腹腔内施用Fer-1(10mg/kg)。 Lie组的小鼠腹膜内注射Lie (60 mg/kg)。急性暴露后 6 小时收获血清和肝组织用于分析。 所有动物实验均经人道动物保护标准批准,并经中科实业控股(深圳)有限公司实验动物伦理委员会授权。进行实验的个人对分配顺序和组分配不知情。
Statistical analysis 统计分析
Each experiment was performed at least three times. GraphPad Prism 7.0 software (GraphPad, San Diego, CA, United States) was used to perform all data analyses. The log-rank test was used to examine survival rates. Unpaired Student’s t test was used to compare the two groups. A one-way analysis of variance and Tukey’s multiple comparisons tests were used to compare three or more groups, and statistical significance was set at p < 0.05.
每个实验至少进行三次。 GraphPad Prism 7.0 软件(GraphPad,圣地亚哥,加利福尼亚州,美国)用于执行所有数据分析。对数秩检验用于检查存活率。使用不配对的学生t检验来比较两组。使用单因素方差分析和 Tukey 多重比较检验来比较三个或更多组,统计显着性设定为p < 0.05。
Data availability 数据可用性
All data generated or analyzed during this study are included in this published article and its Supplementary Information files. Additional data are available from the corresponding author on reasonable request.
本研究期间生成或分析的所有数据均包含在本发表的文章及其补充信息文件中。如有合理要求,可从通讯作者处获取更多数据。
References 参考
Kakisaka K, Suzuki Y, Takikawa Y. Disease severity of acute liver injury caused by drug-induced liver injury may affect the response to corticosteroid therapy. Liver Int. 2020;40:1781.
Kakisaka K,Suzuki Y,Takikawa Y。药物性肝损伤引起的急性肝损伤的疾病严重程度可能会影响对皮质类固醇治疗的反应。肝脏国际。 2020;40:1781。Gowda C, Newcomb CW, Liu Q, Carbonari DM, Lewis JD, Forde KA, et al. Risk of acute liver injury with antiretroviral therapy by viral hepatitis status. Open Forum Infect Dis. 2017;4:x12.
Gowda C、Newcomb CW、Liu Q、Carbonari DM、Lewis JD、Forde KA 等。病毒性肝炎状态下抗逆转录病毒治疗引起急性肝损伤的风险。开放论坛感染疾病。 2017;4:x12。Puri P, Lee WM, Fontana RJ, Kim NK, Durkalski V, McGuire BM, et al. Alcohol consumption is associated with the severity and outcome of acute liver injury/failure. Liver Int. 2020;40:360–7.
Puri P、Lee WM、Fontana RJ、Kim NK、Durkalski V、McGuire BM 等。饮酒与急性肝损伤/衰竭的严重程度和结果相关。肝脏国际。 2020;40:360–7。Chen T, Li R, Chen P. Gut microbiota and chemical-induced acute liver injury. Front Physiol. 2021;12:688780.
Chen T,Li R,Chen P。肠道微生物群和化学物质引起的急性肝损伤。前生理学。 2021;12:688780。Perez RDGA, Kortgen A, Leonhardt J, Zipprich A, Bauer M. Critical care hepatology: definitions, incidence, prognosis, and role of liver failure in critically ill patients. Crit Care. 2022;26:289.
Perez RDGA、Kortgen A、Leonhardt J、Zipprich A、Bauer M。重症监护肝病学:重症患者肝衰竭的定义、发生率、预后和作用。危重护理。 2022;26:289。Lemmer P, Pospiech JC, Canbay A. Liver failure-future challenges and remaining questions. Ann Transl Med. 2021;9:734.
Lemmer P、Pospiech JC、Canbay A. 肝功能衰竭——未来的挑战和剩余问题。安翻译医学。 2021;9:734。Saliba F, Bañares R, Larsen FS, Wilmer A, Parés A, Mitzner S, et al. Artificial liver support in patients with liver failure: a modified DELPHI consensus of international experts. Intensive Care Med. 2022;48:1352–67.
Saliba F、Bañares R、Larsen FS、Wilmer A、Parés A、Mitzner S 等。肝功能衰竭患者的人工肝支持:修订版 DELPHI 国际专家共识。重症监护医学。 2022;48:1352–67。Lee CA, Sinha S, Fitzpatrick E, Dhawan A. Hepatocyte transplantation and advancements in alternative cell sources for liver-based regenerative medicine. J Mol Med. 2018;96:469–81.
Lee CA、Sinha S、Fitzpatrick E、Dhawan A。肝细胞移植和肝脏再生医学替代细胞来源的进展。分子医学杂志。 2018;96:469–81。Woolbright BL, Jaeschke H. Sterile inflammation in acute liver injury: myth or mystery? Expert Rev Gastroenterol Hepatol. 2015;9:1027–9.
Woolbright BL,Jaeschke H。急性肝损伤中的无菌性炎症:神话还是神秘?肠胃肝专家牧师。 2015;9:1027–9。van der Heide D, Weiskirchen R, Bansal R. Therapeutic targeting of hepatic macrophages for the treatment of liver diseases. Front Immunol. 2019;10:2852.
van der Heide D、Weiskirchen R、Bansal R。肝巨噬细胞的治疗靶向治疗肝脏疾病。前免疫学。 2019;10:2852。Yang Z, Zhang J, Wang Y, Lu J, Sun Q. Caveolin-1 deficiency protects mice against carbon tetrachloride-induced acute liver injury through regulating polarization of hepatic macrophages. Front Immunol. 2021;12:713808.
Yang Z,Zhang J,Wang Y,Lu J,Sun Q。Caveolin-1缺陷通过调节肝巨噬细胞的极化保护小鼠免受四氯化碳诱导的急性肝损伤。前免疫学。 2021;12:713808。Papachristoforou E, Ramachandran P. Macrophages as key regulators of liver health and disease. Int Rev Cell Mol Biol. 2022;368:143–212.
Papachristoforou E,Ramachandran P。巨噬细胞是肝脏健康和疾病的关键调节因子。 Int Rev 细胞分子生物学。 2022;368:143–212。Jiang P, Li X. Regulatory mechanism of lncRNAs in M1/M2 macrophages polarization in the diseases of different etiology. Front Immunol. 2022;13:835932.
江平,李霞。不同病因疾病中M1/M2巨噬细胞极化中lncRNA的调控机制。前免疫学。 2022;13:835932。Wang Y, Li X, Chen Q, Jiao F, Shi C, Pei M, et al. Histone deacetylase 6 regulates the activation of M1 macrophages by the glycolytic pathway during acute liver failure. J Inflamm Res. 2021;14:1473–85.
王Y,李X,陈Q,焦F,石C,裴M,等。组蛋白脱乙酰酶 6 在急性肝衰竭期间通过糖酵解途径调节 M1 巨噬细胞的激活。 J炎症研究。 2021;14:1473–85。Jin GL, Liu HP, Huang YX, Zeng QQ, Chen JX, Lan XB, et al. Koumine regulates macrophage M1/M2 polarization via TSPO, alleviating sepsis-associated liver injury in mice. Phytomedicine. 2022;107:154484.
金桂林,刘慧波,黄YX,曾QQ,陈JX,兰XB,等。 Koumine 通过 TSPO 调节巨噬细胞 M1/M2 极化,减轻小鼠脓毒症相关的肝损伤。植物药。 2022;107:154484。Tomar S, Zumbrun EE, Nagarkatti M, Nagarkatti PS. Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs. J Pharmacol Exp Ther. 2015;353:369–79.
托马尔 S、祖布伦 EE、纳加卡蒂 M、纳加卡蒂 PS。大麻素受体 2 激活通过调节巨噬细胞极化和 microRNA 在半乳糖胺/脂多糖诱导的急性肝衰竭中发挥保护作用。 J Pharmacol Exp Ther。 2015;353:369–79。Li YW, Zhang C, Sheng QJ, Bai H, Ding Y, Dou XG. Mesenchymal stem cells rescue acute hepatic failure by polarizing M2 macrophages. World J Gastroenterol. 2017;23:7978–88.
李永文,张春,盛庆军,白辉,丁勇,窦新光。间充质干细胞通过极化 M2 巨噬细胞来挽救急性肝衰竭。世界胃肠病学杂志。 2017;23:7978–88。Wang J, Liu Y, Ding H, Shi X, Ren H. Mesenchymal stem cell-secreted prostaglandin E(2) ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization. Stem Cell Res Ther. 2021;12:15.
Wang J,Liu Y,Ding H,Shi X,Ren H。间充质干细胞分泌的前列腺素E(2)通过减弱细胞死亡和调节巨噬细胞极化来改善急性肝衰竭。干细胞研究。 2021 年;12:15。Bai L, Kong M, Duan Z, Liu S, Zheng S, Chen Y. M2-like macrophages exert hepatoprotection in acute-on-chronic liver failure through inhibiting necroptosis-S100A9-necroinflammation axis. Cell Death Dis. 2021;12:93.
Bai L,Kong M,Duan Z,Liu S,Zheng S,Chen Y。M2样巨噬细胞通过抑制坏死性凋亡-S100A9-坏死炎症轴在慢加急性肝衰竭中发挥保肝作用。细胞死亡疾病。 2021;12:93。Starkey LP, Campana L, Aleksieva N, Cartwright JA, Mackinnon A, O’Duibhir E, et al. Alternatively activated macrophages promote resolution of necrosis following acute liver injury. J Hepatol. 2020;73:349–60.
Starkey LP、Campana L、Aleksieva N、Cartwright JA、Mackinnon A、O'Duibhir E 等。另一种激活的巨噬细胞促进急性肝损伤后坏死的消退。 J肝醇。 2020;73:349–60。Bantel H, Schulze-Osthoff K. Mechanisms of cell death in acute liver failure. Front Physiol. 2012;3:79.
Bantel H,Schulze-Osthoff K。急性肝衰竭中细胞死亡的机制。前生理学。 2012;3:79。Zhong X, Zhang Z, Shen H, Xiong Y, Shah YM, Liu Y, et al. Hepatic NF-κB-inducing kinase and inhibitor of NF-κB kinase subunit α promote liver oxidative stress, ferroptosis, and liver injury. Hepatol Commun. 2021;5:1704–20.
钟X,张Z,沉华,熊Y,Shah YM,刘Y,等。肝脏 NF-κB 诱导激酶和 NF-κB 激酶亚基 α 抑制剂可促进肝脏氧化应激、铁死亡和肝损伤。肝公社。 2021;5:1704–20。Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–65.
Pei X, Jiang H, Li C, Li D, Tang S. Oxidative stress-related canonical pyroptosis pathway, as a target of liver toxicity triggered by zinc oxide nanoparticles. J Hazard Mater. 2022;442:130039.
Wang M, Sun J, Yu T, Wang M, Jin L, Liang S, et al. Diacerein protects liver against APAP-induced injury via targeting JNK and inhibiting JNK-mediated oxidative stress and apoptosis. Biomed Pharmacother. 2022;149:112917.
王明,孙杰,余涛,王明,金丽,梁S,等。双醋瑞因通过靶向 JNK 并抑制 JNK 介导的氧化应激和细胞凋亡来保护肝脏免受 APAP 诱导的损伤。生物医学药剂师。 2022;149:112917。Tsai CF, Chen GW, Chen YC, Shen CK, Lu DY, Yang LY, et al. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients. 2021;14:67.
蔡CF,陈国伟,陈永昌,沉CK,卢DY,杨LY,等。槲皮素对 M1/M2 巨噬细胞极化和氧化/抗氧化平衡的调节作用。营养素。 2021 年;14:67。Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Dixon SJ、Lemberg KM、Lamprecht MR、Skouta R、Zaitsev EM、Gleason CE 等。铁死亡:一种铁依赖性非凋亡细胞死亡形式。细胞。 2012;149:1060–72。Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022;29:467–80.
Chen J,Li X,Ge C,Min J,Wang F。铁死亡在肝脏疾病中的多方面作用。细胞死亡不同。 2022;29:467–80。Tong J, Li D, Meng H, Sun D, Lan X, Ni M, et al. Targeting a novel inducible GPX4 alternative isoform to alleviate ferroptosis and treat metabolic-associated fatty liver disease. Acta Pharm Sin B. 2022;12:3650–66.
童杰,李东,孟辉,孙东,兰新,倪明,等。靶向新型诱导型 GPX4 替代亚型来缓解铁死亡并治疗代谢相关的脂肪肝疾病。药学学报 B.2022;12:3650–66。Wang M, Liu CY, Wang T, Yu HM, Ouyang SH, Wu YP, et al. (+)-Clausenamide protects against drug-induced liver injury by inhibiting hepatocyte ferroptosis. Cell Death Dis. 2020;11:781.
王明,刘春云,王涛,于洪明,欧阳秀华,吴永平,等。 (+)-Clausenamide 通过抑制肝细胞铁死亡来防止药物引起的肝损伤。细胞死亡疾病。 2020;11:781。Yang Y, Wang Y, Guo L, Gao W, Tang TL, Yan M. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022;13:355.
杨Y,王Y,郭L,高W,唐TL,严M。巨噬细胞与铁死亡的相互作用。细胞死亡疾病。 2022 年;13:355。He R, Liu B, Xiong R, Geng B, Meng H, Lin W, et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 2022;8:43.
何瑞,刘波,熊瑞,耿波,孟华,林伟,等。衣康酸通过 Nrf2 途径抑制巨噬细胞铁死亡,对抗脓毒症引起的急性肺损伤。细胞死亡发现。 2022 年;8:43。Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16:278–90.
Kapralov AA、Yang Q、Dar HH、Tyurina YY、Anthonymuthu TS、Kim R 等。氧化还原脂质重编程控制巨噬细胞和小胶质细胞对铁死亡的敏感性。自然化学生物。 2020;16:278–90。Sun YK, Zhang YF, Xie L, Rong F, Zhu XY, Xie J, et al. Progress in the treatment of drug-induced liver injury with natural products. Pharmacol Res. 2022;183:106361.
孙YK,张YF,谢L,荣F,朱XY,谢J,等。天然产物治疗药物性肝损伤的进展。药理学研究中心。 2022;183:106361。Gao Q, Yin XD, Zhang F, Zhu YZ, Li ZL. The regulatory effects of traditional Chinese medicine on ferroptosis. Oxid Med Cell Longev. 2022;2022:4578381.
高Q,尹XD,张F,朱YZ,李ZL。中药对铁死亡的调节作用。氧化医学细胞长寿。 2022;2022:4578381。Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89–100.
Zhou B,Liu J,Kang R,Klionsky DJ,Kroemer G,Tang D。铁死亡是一种自噬依赖性细胞死亡。塞明癌症生物学。 2020;66:89–100。Liu MZ, Kong N, Zhang GY, Xu Q, Xu Y, Ke P, et al. The critical role of ferritinophagy in human disease. Front Pharmacol. 2022;13:933732.
刘明志,孔宁,张国勇,徐清,徐勇,柯平,等。铁蛋白自噬在人类疾病中的关键作用。前药理学。 2022;13:933732。Zhou J, Li G, Zheng Y, Shen HM, Hu X, Ming QL, et al. A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy. 2015;11:1259–79.
周杰,李刚,郑Y,沉HM,胡X,明QL,等。一种新型自噬/线粒体自噬抑制剂 Liensinine 通过 DNM1L 介导的线粒体裂变使乳腺癌细胞对化疗敏感。自噬。 2015;11:1259–79。Ylä-Anttila P, Gupta S, Masucci MG. The Epstein-Barr virus deubiquitinase BPLF1 targets SQSTM1/p62 to inhibit selective autophagy. Autophagy. 2021;17:3461–74.
Ylä-Anttila P、Gupta S、Masucci MG。 Epstein-Barr 病毒去泛素化酶 BPLF1 以 SQSTM1/p62 为靶点,抑制选择性自噬。自噬。 2021;17:3461–74。Piattini F, Matsushita M, Muri J, Bretshcer P, Feng X, Freigang S, et al. Differential sensitivity of inflammatory macrophages and alternatively activated macrophages to ferroptosis. Eur J Immunol. 2021;51:2417–29.
Kelly CJ, Couch RK, Ha VT, Bodart CM, Wu J, Huff S, et al. Iron status influences mitochondrial disease progression in Complex I-deficient mice. Elife. 2023;12:e75825.
Abbina S, Abbasi U, Gill A, Leitch H, Kizhakkedathu JN. Active transport nanochelators for the reduction of liver iron burden in iron overload. J Control Release. 2022;350:857–69.
Liang L, Ye S, Jiang R, Zhou X, Zhou J, Meng S. Liensinine alleviates high fat diet (HFD)-induced nonalcoholic fatty liver disease (NAFLD) through suppressing oxidative stress and inflammation via regulating TAK1/AMPK signaling. Int Immunopharmacol. 2022;104:108306.
Liang X, Wang S, Wang L, Ceylan AF, Ren J, Zhang Y. Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of Drp1-mediated maladaptive mitochondrial fission. Pharmacol Res. 2020;157:104846.
Chang M, Ding S, Dong X, Shang X, Li Y, Xie L, et al. Liensinine inhibits cell growth and blocks autophagic flux in nonsmall-cell lung cancer. J Oncol. 2022;2022:1533779.
Xie S, Li Y, Teng W, Du M, Li Y, Sun B. Liensinine inhibits beige adipocytes recovering to white adipocytes through blocking mitophagy flux in vitro and in vivo. Nutrients. 2019;11:1640.
Santana-Codina N, Mancias JD. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals. 2018;11:114.
Ni S, Yuan Y, Qian Z, Zhong Z, Lv T, Kuang Y, et al. Hypoxia inhibits RANKL-induced ferritinophagy and protects osteoclasts from ferroptosis. Free Radic Biol Med. 2021;169:271–82.
Qin X, Zhang J, Wang B, Xu G, Yang X, Zou Z, et al. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy. 2021;17:4266–85.
Zhao E, Ilyas G, Cingolani F, Choi JH, Ravenelle F, Tanaka KE, et al. Pentamidine blocks hepatotoxic injury in mice. Hepatology. 2017;66:922–35.
Liu GZ, Xu XW, Tao SH, Gao MJ, Hou ZH. HBx facilitates ferroptosis in acute liver failure via EZH2 mediated SLC7A11 suppression. J Biomed Sci. 2021;28:67.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was supported by grants from the Shenzhen Science and Technology Project (Nos. JCYJ20170817094901026 and JCYJ20180302173542393).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Huang, Q., Lv, M. et al. Role of liensinine in sensitivity of activated macrophages to ferroptosis and in acute liver injury. Cell Death Discov. 9, 189 (2023). https://doi.org/10.1038/s41420-023-01481-3IF: 6.1 Q1
Subjects
This article is cited by
-
Targeting epigenetic and posttranslational modifications regulating ferroptosis for the treatment of diseases
Signal Transduction and Targeted Therapy (2023)