Abstract 抽象
Endocytosis and lysosomal trafficking of cell surface receptors can be triggered by endogenous ligands. Therapeutic approaches such as lysosome-targeting chimaeras1,2 (LYTACs) and cytokine receptor-targeting chimeras3 (KineTACs) have used this to target specific proteins for degradation by fusing modified native ligands to target binding proteins. Although powerful, these approaches can be limited by competition with native ligands and requirements for chemical modification that limit genetic encodability and can complicate manufacturing, and, more generally, there may be no native ligands that stimulate endocytosis through a given receptor. Here we describe computational design approaches for endocytosis-triggering binding proteins (EndoTags) that overcome these challenges. We present EndoTags for insulin-like growth factor 2 receptor (IGF2R) and asialoglycoprotein receptor (ASGPR), sortilin and transferrin receptors, and show that fusing these tags to soluble or transmembrane target protein binders leads to lysosomal trafficking and target degradation. As these receptors have different tissue distributions, the different EndoTags could enable targeting of degradation to different tissues. EndoTag fusion to a PD-L1 antibody considerably increases efficacy in a mouse tumour model compared to antibody alone. The modularity and genetic encodability of EndoTags enables AND gate control for higher-specificity targeted degradation, and the localized secretion of degraders from engineered cells. By promoting endocytosis, EndoTag fusion increases signalling through an engineered ligand–receptor system by nearly 100-fold. EndoTags have considerable therapeutic potential as targeted degradation inducers, signalling activators for endocytosis-dependent pathways, and cellular uptake inducers for targeted antibody–drug and antibody–RNA conjugates.
细胞表面受体的内吞作用和溶酶体运输可由内源性配体触发。溶酶体靶向嵌合体1,2 (LYTAC) 和细胞因子受体靶向嵌合体3 (KineTAC) 等治疗方法通过将修饰的天然配体融合到靶向结合蛋白来靶向特定蛋白进行降解。尽管功能强大,但这些方法可能会受到与天然配体的竞争和化学修饰要求的限制,这些限制了遗传可编码性,并使制造复杂化,并且更普遍地说,可能没有通过给定受体刺激内吞作用的天然配体。在这里,我们描述了克服这些挑战的内吞触发结合蛋白 (EndoTags) 的计算设计方法。我们提出了胰岛素样生长因子 2 受体 (IGF2R) 和去唾液酸糖蛋白受体 (ASGPR)、sortilin 和转铁蛋白受体的 EndoTags,并表明将这些标签与可溶性或跨膜靶蛋白结合物融合会导致溶酶体运输和靶标降解。由于这些受体具有不同的组织分布,因此不同的 EndoTag 可以靶向降解到不同的组织。与单独使用抗体相比,EndoTag 与 PD-L1 抗体的融合可显著提高小鼠肿瘤模型中的疗效。EndoTags 的模块化和遗传可编码性使 AND 门控制能够实现更高特异性的靶向降解,以及工程细胞中降解剂的局部分泌。通过促进内吞作用,EndoTag 融合通过工程配体-受体系统将信号转导增加近 100 倍。 EndoTags 具有相当大的治疗潜力,可作为靶向降解诱导剂、内吞依赖性途径的信号激活剂以及靶向抗体-药物和抗体-RNA 偶联物的细胞摄取诱导剂。
Similar content being viewed by others
其他人正在查看的类似内容
Main 主要
The endocytosis of many cell surface receptors is triggered by binding of their endogenous ligands, which can shift the conformational or oligomerization state of the receptor4 and induce receptor clustering and recruitment of adaptor proteins5,6. Native endocytosis-inducing ligands have been utilized to target extracellular and membrane proteins to the lysosome for degradation1,2,3. Although powerful, these approaches have the limitations that native ligands can trigger off-target signalling3,7, their binding sites may be occupied by existing ligands8, and instability and—in some cases—the need for modification can complicate manufacturing9. Bio-orthogonal inducers of endocytosis could have therapeutic utility for targeted degradation or for initiating signalling through pathways involving endocytosis4, and could provide powerful tools for investigating the association between cellular trafficking and receptor conformational and oligomerization state. Antibodies have been identified that stimulate endocytosis, but this can require considerable empirical screening for any target receptor10,11.
许多细胞表面受体的内吞作用是由其内源性配体的结合触发的,这可以改变受体的构象或寡聚化状态4 并诱导受体聚集和接头蛋白的募集5,6。天然内吞作用诱导配体已被用于将细胞外蛋白和膜蛋白靶向溶酶体进行降解 1,2,3。尽管功能强大,但这些方法具有局限性,即天然配体可以触发脱靶信号转导3,7,它们的结合位点可能被现有配体占据8,并且不稳定性和在某些情况下需要修饰会使制造复杂化9。内吞作用的生物正交诱导剂可能具有靶向降解或通过涉及内吞作用的途径启动信号传导的治疗效用4,并且可以为研究细胞运输与受体构象和寡聚化状态之间的关联提供强大的工具。已经确定了刺激内吞作用的抗体,但这可能需要对任何靶受体进行大量的经验性筛选10,11。
We reasoned that de novo protein design could enable the creation of bio-orthogonal endocytosis-inducing proteins that avoid the above limitations using strategies customized for the target receptor. To enable tissue-specific control over endocytosis for downstream applications, we selected target receptors with distinct tissue expression profiles: IGF2R is expressed in most tissues, asialoglycoprotein receptor (ASGPR) is expressed primarily in the liver, transferrin receptor (TfR) is expressed in the brain, liver and muscles, and sortilin is expressed in the brain and spinal cord12,13,14. For receptors such as sortilin and TfR that constitutively traffic between the cell surface and the endosome–lysosome, binding to a site on the receptor that does not overlap with the native ligands could be sufficient (Fig. 1a). For receptors such as IGF2R, for which conformational change triggers endocytosis, binding must induce rearrangement of receptor extracellular domains, whereas for others, such as ASGPR, for which endocytosis is stimulated by clustering, binding should induce oligomerization. Fusion of designed proteins with these properties to a second target-binding protein could promote endocytosis and lysosomal trafficking of the target. We set out to design such endocytosis-targeting proteins, which we call EndoTags, for all four receptor systems (IGF2R, ASGPR, sortilin and TfR), and to explore their utility for modulating protein degradation and cellular signalling.
我们推断,从头蛋白质设计可以创建生物正交内吞诱导蛋白,使用为目标受体定制的策略来避免上述限制。为了在下游应用中实现对内吞作用的组织特异性控制,我们选择了具有不同组织表达谱的靶受体:IGF2R 在大多数组织中表达,唾液酸糖蛋白受体 (ASGPR) 主要在肝脏中表达,转铁蛋白受体 (TfR) 在大脑、肝脏和肌肉中表达,sortilin 在大脑和脊髓中表达12,13,14. 对于在细胞表面和内体-溶酶体之间组成性运输的受体,如 sortilin 和 TfR,与受体上与天然配体不重叠的位点结合就足够了(图 D)。对于构象变化触发内吞作用的 IGF2R 等受体,结合必须诱导受体胞外结构域的重排,而对于其他受体,如 ASGPR,其内吞作用由聚集刺激,结合应诱导寡聚化。具有这些特性的设计蛋白与第二个靶标结合蛋白的融合可以促进靶标的内吞作用和溶酶体运输。我们着手为所有四种受体系统(IGF2R、ASGPR、sortilin 和 TfR)设计这种靶向内吞作用的蛋白质,我们称之为 EndoTags,并探索它们在调节蛋白质降解和细胞信号传导方面的效用。
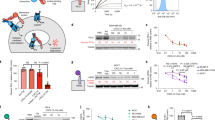
图 1:内吞作用触发 EndoTag 的设计策略。
a, Schema of designed endocytosis mechanisms. Top, design of binding to constitutively cycling receptors at sites that do not overlap with binding sites for natural ligands to avoid competition. Middle, design of binders that trigger endocytosis by eliciting conformational changes in the receptor. The EndoTag binds at two distinct epitopes on the target and actively triggers the conformational change. Bottom, designed endocytosis via receptor clustering. The multivalent EndoTag clusters multiple copies of the target receptor and induces endocytosis. b, Design strategy for sortilin48 and TfR49 EndoTags. c, Cellular uptake of 100 nM AF647-labelled Sort_EndoTags, TfR-EndoTags or LHDB22 scaffold control for 2 h in U-251MG cells. Data were normalized to the 100 nM AF647-labelled LHDB group (no endocytosis). MFI, mean fluorescence intensity. d, Confocal imaging of Sort_EndoTag (red) and lysosomal marker (green, AF488-labelled LysoTracker) after 24 h incubation in U-251MG cells. e, Design strategy for IGF_EndoTags. f, Cellular uptake of IGF_EndoTags in Jurkat cells with biotinylated 100 nM IGF_EndoTags or IGF2 and 33 nM Streptavidin–AF647 for 24 h. Data were normalized with the control group treated with 33 nM Streptavidin–AF647 alone. g, Fluorescence microscopy showing IGF_EndoTag1 (pink) co-localization with lysosomes (green, anti-LAMP2A) in HeLa cells. h, Design strategy for ASGPR EndoTags. i, Cellular uptake of ASGPR EndoTags. Hep3B cells were treated with 100 nM AF647-conjugated ASGPR EndoTags for 24 h followed with flow cytometry. Data were normalized to 100 nM AF647-labelled LHDB group (no endocytosis). j, Confocal imaging of AS_EndoTag (red) with lysosome (green, AF488-labelled LysoTracker); 500 nM AF647-labelled AS_EndoTag/AS_EndoTag-2C/AS_EndoTag-3C was incubated with Hep3B cells for 24 h. In c,f,i, data are mean ± s.e.m. of three biological replicates. In d,g,j, images are representative of three independently replicated samples. Scale bars, 20 µm.
a,设计的内吞机制图式。上图,在与天然配体的结合位点不重叠的位点与组成型循环受体结合的设计,以避免竞争。中间,通过引发受体构象变化来触发内吞作用的结合剂设计。EndoTag 在靶标上的两个不同表位处结合,并主动触发构象变化。底部,通过受体聚集设计内吞作用。多价 EndoTag 聚集靶受体的多个拷贝并诱导内吞作用。b,sortilin48 和 TfR49 EndoTags 的设计策略。c,在 U-251MG 细胞中细胞摄取 100 nM AF647 标记的 Sort_EndoTags、TfR-EndoTags 或 LHDB22 支架对照 2 小时。将数据归一化为 100 nM AF647 标记的 LHDB 组 (无内吞作用)。MFI,平均荧光强度。d,在 U-251MG 细胞中孵育 24 小时后 Sort_EndoTag(红色)和溶酶体标志物(绿色,AF488 标记的 LysoTracker)的共聚焦成像。e,IGF_EndoTags设计策略。f,用生物素化的 100 nM IGF_EndoTags 或 IGF2 和 33 nM 链霉亲和素-AF647 在 Jurkat 细胞中细胞IGF_EndoTags摄取 24 小时。用单独用 33 nM 链霉亲和素-AF647 处理的对照组对数据进行归一化。g,荧光显微镜显示 HeLa 细胞中 IGF_EndoTag1(粉红色)与溶酶体(绿色,抗 LAMP2A)共定位。h,ASGPR EndoTags 的设计策略。i,ASGPR EndoTags 的细胞摄取。Hep3B 细胞用 100 nM AF647 偶联的 ASGPR EndoTags 处理 24 小时,然后进行流式细胞术。将数据归一化为 100 nM AF647 标记的 LHDB 组 (无内吞作用)。 j,AS_EndoTag(红色)与溶酶体(绿色,AF488 标记的 LysoTracker)的共聚焦成像;将 500 nM AF647 标记的 AS_EndoTag/AS_EndoTag-2C/AS_EndoTag-3C 与 Hep3B 细胞孵育 24 小时。在 c,f,i 中,数据是三个生物学重复的平均 ± s.e.m.。在 d,g,j 中,图像代表三个独立复制的样本。比例尺,20 μm。
EndoTags orthogonal to native ligands
与天然配体正交的 EndoTags
TfR and sortilin constitutively cycle between the cell surface and intracellular compartments. Thus, for these receptors, the challenge is not to actively induce endocytosis, but to bind to the receptor at a site that does not compete for the natural ligand, which could have undesired side effects and reduce efficiency. De novo protein design has the advantage of being able to target binders to specific sites of interest on a target15,16,17, and is thus well suited to designing protein binders that target receptor sites that do not overlap with those of native ligands.
TfR 和 sortilin 在细胞表面和细胞内区室之间组成性循环。因此,对于这些受体,挑战不在于主动诱导内吞作用,而在于在不竞争天然配体的位点与受体结合,这可能会产生不良的副作用并降低效率。从头蛋白质设计的优势在于能够将结合物靶向靶标15,16,17 上感兴趣的特定位点,因此非常适合设计靶向与天然配体不重叠的受体位点的蛋白质结合物。
Sortilin is a rapid trafficking receptor with substantial expression in the neural system, and has a role in lysosomal targeting of neurotensin14,18. We sought to design protein binders of sortilin at binding sites that do not overlap with native ligands, including neurotensin (Fig. 1a,b). We used Rosetta de novo binder design15 and yeast display (Extended Data Fig. 1a) to design and screen molecules that bind sortilin at a site that does not overlap with known interactions or undergo considerable structural change at low pH18 (Extended Data Fig. 1b,c); the highest affinity design (Sort_EndoTag) had a dissociation constant (Kd) for Sortilin of 21 nM (Extended Data Fig. 2g and Extended Data Table 1). Four sortilin variants that introduce N-linked glycans close to the designed Sort_EndoTag interface blocked binding (Supplementary Fig. 19a–f), supporting the computational design model. Following incubation of 200 nM fluorescence-labelled Sort_EndoTag with U-251MG glioblastoma cells for 2 h at 37 °C followed by extensive washing, there was a 90-fold increase in fluorescence compared with fluorophore-conjugated control (Fig. 1c). Confocal imaging indicated co-localization of the Sort_EndoTag with a lysosomal marker after 24 h incubation in U-251MG cells (Fig. 1d and Supplementary Fig. 18a).
Sortilin 是一种快速运输受体,在神经系统中大量表达,在神经降压素的溶酶体靶向中发挥作用14,18。我们试图在与天然配体(包括神经降压素)不重叠的结合位点设计 sortilin 的蛋白质结合剂(图 D)。1a,b)。我们使用了 Rosetta de novo binder design15 和酵母显示(扩展数据图 15)。1a) 设计和筛选在与已知相互作用不重叠或在低 pH值 18 下发生相当大的结构变化的位点结合 sortilin 的分子(扩展数据图 D)。1b,c);最高亲和力设计 (Sort_EndoTag) 对 Sortilin 的解离常数 (Kd) 为 21 nM(扩展数据图 D)。2g 和扩展数据表 1)。四种 sortilin 变体在设计Sort_EndoTag界面附近引入 N-连接聚糖,阻断了结合(补充图 D)。19a-f),支持计算设计模型。将 200 nM 荧光标记的Sort_EndoTag与 U-251MG 胶质母细胞瘤细胞在 37 °C 下孵育 2 小时,然后进行大量洗涤后,与荧光团偶联的对照相比,荧光增加了 90 倍(图 D)。共聚焦成像表明,在 U-251MG 细胞中孵育 24 小时后,Sort_EndoTag与溶酶体标志物共定位(图 D)。1d 和补充图18a)。
We applied a similar orthogonal binding strategy with TfR (Fig. 1b) whose native function is to transport iron-bound transferrin into cells and across the blood–brain barrier13,19, taking advantage of a previously designed binder that binds a site away from the transferrin-binding site20 and further optimized for solubility using ProteinMPNN21 (Supplementary Table 3). We found that this design, referred to here as TfR_EndoTag, was readily endocytosed in U-251MG glioblastoma cells, with a 50-fold increase in cellular uptake over the LHDB scaffold control22 after 2 h incubation (Fig. 1c). Confocal imaging again indicated lysosome targeting of the TfR_EndoTag after 24 h incubation in U-251MG cells (Supplementary Fig. 18b). Co-incubation of TfR_EndoTag with fluorescence-labelled transferrin had no effect on transferrin binding and uptake in HeLa cells (Extended Data Fig. 7d,e).
我们对 TfR 应用了类似的正交结合策略(图 D)。1b) 其天然功能是将铁结合的转铁蛋白转运到细胞中并穿过血脑屏障13,19,利用先前设计的结合剂结合远离转铁蛋白结合位点的位点20,并使用 ProteinMPNN 进一步优化溶解度21(补充表 3).我们发现这种设计,此处称为 TfR_EndoTag,很容易在 U-251MG 胶质母细胞瘤细胞中内吞,孵育 2 小时后,细胞摄取比 LHDB 支架对照22 增加 50 倍(图 2)。共聚焦成像再次表明,在 U-251MG 细胞中孵育 24 小时后,溶酶体靶向TfR_EndoTag(补充图 D)。TfR_EndoTag 与荧光标记的转铁蛋白共孵育对 HeLa 细胞中的转铁蛋白结合和摄取没有影响(扩展数据图 1)。7d,e)。
Triggering conformational change
触发构象变化
IGF2R rapidly transports the endogenous ligands IGF2 and mannose-6-phosphate (M6P) to the lysosome for degradation1. Structural analysis suggests that IGF2 binding induces a conformational change in IGF2R that brings together domain 6 (D6) and domain 11 (D11) and promotes dimerization of the receptor23. Using the ability to design de novo binders at arbitrary interfaces15, we hypothesized that a designed binding protein that brings together domain 6 and domain 11 could similarly trigger IGF2R endocytosis and lysosomal targeting without triggering off-target signalling activation, similar to IGF27.
IGF2R 将内源性配体 IGF2 和甘露糖-6-磷酸 (M6P) 快速转运至溶酶体进行降解1。结构分析表明,IGF2 结合诱导 IGF2R 的构象变化,将结构域 6 (D6) 和结构域 11 (D11) 结合在一起,并促进受体的二聚化23。利用在任意界面15 设计从头结合剂的能力,我们假设将结构域 6 和结构域 11 结合在一起的设计结合蛋白可以类似地触发 IGF2R 内吞作用和溶酶体靶向,而不会触发脱靶信号激活,类似于 IGF27。
We used the Rosetta RIFdock method15,16 to design small proteins (minibinders) that bind to domain 6 and domain 11 (Extended Data Fig. 1d–g and Supplementary Methods). We expressed binding proteins identified by yeast display screening in Escherichia coli and measured the binding affinities using biolayer interferometry (BLI). The tightest binder to domain 6 (D6mb) had an affinity of 41 nM (Extended Data Fig. 2a and Extended Data Table 1), and the tightest binder to domain 11 (D11mb) had an affinity of 190 nM (Extended Data Fig. 2b and Extended Data Table 1), which was improved to 6.5 nM following optimization (D11mb2) (Extended Data Fig. 2c and Extended Data Table 1).
我们使用 Rosetta RIFdock 方法15,16 设计了与结构域 6 和结构域 11 结合的小蛋白(微型结合剂)(扩展数据图 1)。1d-g 和补充方法)。我们在大肠杆菌中表达通过酵母展示筛选鉴定的结合蛋白,并使用生物膜干涉测量法 (BLI) 测量结合亲和力。与结构域 6 (D6mb) 最紧密的结合物具有 41 nM 的亲和力(扩展数据图 D.2a 和扩展数据表 1),并且与结构域 11 最紧密的结合剂 (D11mb) 具有 190 nM 的亲和力(扩展数据图 1)。2b 和扩展数据表 1),优化后提高到 6.5 nM (D11mb2)(扩展数据图 1)。2c 和扩展数据表 1)。
We next sought to develop IGF2R EndoTags (IGF_EndoTags) by fusing the domain 6 and domain 11 binders (Fig. 1e). We first explored flexible fusions between D11mb and D6mb, with different loop lengths and domain orders; we expressed these fusions in E. coli and, following conjugation with Alexa Fluor 647 (AF647), evaluated cellular uptake using flow cytometry. Treatment with a D11mb–GGS–D6mb fusion (IGF_EndoTag1; where GGS is a flexible Gly-Gly-Ser linker) resulted in increased cell-associated fluorescence over native IGF2 or D6mb or D11mb alone in Jurkat cells (Extended Data Fig. 4c). Fluorescence microscopy indicated that IGF_EndoTag1 is targeted to lysosomes (Fig. 1g), recapitulating the trafficking of endogenous IGF2 ligands. Longer linkers decreased the uptake level, whereas a shorter Gly-Ser (GS) linker abolished uptake (Extended Data Fig. 4b), suggesting that the orientation and distance of the two binding domains modulates IGF2R endocytosis. Constructs with two copies of one minibinder (D6mb–linker–D6mb and D11mb–linker–D11mb) were not taken up (Extended Data Fig. 4a); engagement of both domains (which probably drive their reorientation within the receptor structure) appears to be necessary to trigger efficient cellular uptake. Substitution of D11mb with the higher affinity variant D11mb2 in IGF_EndoTag1 (generating IGF_EndoTag2) increased internalization twofold compared with native IGF2 in Jurkat cells (Fig. 1f); IGF_EndoTag2, but not IGF2 or IGF_EndoTag1, was clearly detectable in lysosomes after a 30 min incubation (Extended Data Fig. 4d,e).
接下来,我们试图通过融合结构域 6 和结构域 11 结合剂来开发 IGF2R EndoTags (IGF_EndoTags) (图 1)。我们首先探索了 D11mb 和 D6mb 之间的灵活融合,具有不同的循环长度和域顺序;我们在大肠杆菌中表达这些融合,并在与 Alexa Fluor 647 (AF647) 偶联后,使用流式细胞术评估细胞摄取。用 D11mb-GGS-D6mb 融合处理(IGF_EndoTag1;其中 GGS 是一种灵活的 Gly-Gly-Ser 接头)在 Jurkat 细胞中导致细胞相关荧光比天然 IGF2 或 D6mb 或 D11mb 单独增加(扩展数据图 1)。荧光显微镜表明IGF_EndoTag1靶向溶酶体(图 D)。1g),概括了内源性 IGF2 配体的运输。较长的连接子降低了摄取水平,而较短的 Gly-Ser (GS) 连接子消除了摄取(扩展数据图 1)。4b),表明两个结合域的方向和距离调节 IGF2R 内吞作用。具有一个微型结合剂的两个拷贝(D6mb-接头-D6mb 和 D11mb-接头-D11mb)的构建体未被占用(扩展数据图 D)。4a);两个结构域的参与(可能驱动它们在受体结构内的重新定位)似乎是触发有效细胞摄取所必需的。与 Jurkat 细胞中的天然 IGF2 相比,在 IGF_EndoTag1 中用更高亲和力变体 D11mb2 取代 D11mb(产生 IGF_EndoTag2)使内化增加了两倍(图 D11mb)。1f);孵育 30 分钟后,在溶酶体中可清楚地检测到 IGF_EndoTag2,但未检测到 IGF2 或 IGF_EndoTag1(扩展数据图 .4d,e)。
We reasoned that more potent stimulation of endocytosis could be achieved by using two domain constructs in which the individual domains are rigidly fused to each other to drive specific conformational changes in receptors. We aligned the major interface helix of D11mb and the two interface helices of D6mb (Fig. 1e and Extended Data Fig. 3) on the basis of their binding modes to IGF2R, sampled the rigid-body orientation between the domain 11- and domain 6-binding elements to explore a range of induced receptor conformations, and connected the two chains were using RFInpainting24. The sequence of the fusions was designed in the context of IGF2R using ProteinMPNN21, keeping residues that are in contact with the receptor constant. We tested 170 designs for which AlphaFold225 predictions matched the intended structures for binding to both IGF2R domains, and expressed eight designs that interacted with both domains in E. coli. Different designs had distinct affinities for domain 6 and domain 11; for example, IGF_EndoTag3 possessed strong binding affinity to both domains (6 nM for domain 6 and 190 nM for domain 11; Extended Data Fig. 2d and Extended Data Table 1), whereas EndoTag4 bound more tightly to domain 6 (15 nM for domain 6 and 4.3 µM for domain 11; Extended Data Fig. 2e). In cellular uptake assays, IGF_EndoTag3 was internalized similarly to IGF2 and twofold more efficiently than IGF_EndoTag4 (Extended Data Fig. 4c), and both designs co-localized with lysosomes within 30 min (Extended Data Fig. 4f).
我们推断,通过使用两个结构域构建体可以实现更有效的内吞刺激,其中各个结构域彼此刚性融合以驱动受体的特定构象变化。我们对齐了 D11mb 的主界面螺旋和 D6mb 的两个界面螺旋(图 D)。1e 和扩展数据图3) 根据它们与 IGF2R 的结合模式,对结构域 11 和结构域 6 结合元件之间的刚体取向进行采样,以探索一系列诱导受体构象,并使用 RFInpainting24 将两条链连接起来。融合序列是在 IGF2R 的背景下使用 ProteinMPNN21 设计的,保持与受体接触的残基恒定。我们测试了 170 种设计,其中 AlphaFold225 预测与两个 IGF2R 结构域结合的预期结构相匹配,并表达了与大肠杆菌中两个结构域相互作用的 8 种设计。不同的设计对域 6 和域 11 具有不同的亲和力;例如,IGF_EndoTag3 对两个结构域都具有很强的结合亲和力(结构域 6 为 6 nM,结构域 11 为 190 nM;扩展数据 图2d 和扩展数据表 1),而 EndoTag4 与结构域 6 的结合更紧密(结构域 6 为 15 nM,结构域 11 为 4.3 μM;扩展数据 图在细胞摄取测定中,IGF_EndoTag3 的内化方式与 IGF2 类似,并且比 IGF2 的效率高两倍IGF_EndoTag4(扩展数据图 2)。4c),并且两种设计在 30 分钟内与溶酶体共定位(扩展数据图 D)。4f)。
The M6P-binding site on IGF2R is largely occupied by M6P-tagged lysosomal hydrolases, limiting the maximal degradation capacity through this receptor, and knockout of the M6P biosynthesis enzyme GNPTAB increased binding of M6P-conjugated proteins to the cell surface8. The IGF_EndoTags were designed to bind to an orthogonal binding site to M6P, thus competition with M6P-tagged enzymes should not be an issue. Indeed, the extent of binding of IGF_EndoTag2 was not altered by knockout of GNPTAB in UMRC2 cells (Extended Data Fig. 7c), confirming that competition with M6P-tagged endogenous proteins does not limit EndoTag function, an advantage over the original direct M6P conjugation strategy. Although the binding site of IGF_EndoTag4 is proximal to the IGF2-binding site on IGF2R domain 11, pre-incubation of cells with IGF_EndoTag4 did not inhibit IGF2 uptake or transport, whereas IGF_EndoTag2 did reduce IGF2 uptake (Extended Data Fig. 7a,b), indicating that orthogonality with IGF2 interaction can be achieved by modulating the binding affinity to IGF2R domain 11 while preserving the binding to domain 6.
IGF2R 上的 M6P 结合位点主要被 M6P 标记的溶酶体水解酶占据,限制了通过该受体的最大降解能力,而 M6P 生物合成酶 GNPTAB 的敲除增加了 M6P 偶联蛋白与细胞表面的结合8。IGF_EndoTags 被设计为与 M6P 的正交结合位点结合,因此与 M6P 标记的酶的竞争应该不是问题。事实上,在 UMRC2 细胞中敲除 GNPTAB 并未改变 IGF_EndoTag2 的结合程度(扩展数据图 .7c),证实与 M6P 标记的内源性蛋白竞争不会限制 EndoTag 功能,这是相对于原始直接 M6P 偶联策略的优势。尽管 IGF_EndoTag4 的结合位点靠近 IGF2R 结构域 11 上的 IGF2 结合位点,但细胞与 IGF_EndoTag4 的预孵育不会抑制 IGF2 的摄取或转运,而 IGF_EndoTag2 确实会减少 IGF2 的摄取(扩展数据图 1)。7a,b),表明可以通过调节与 IGF2R 结构域 11 的结合亲和力,同时保持与结构域 6 的结合来实现与 IGF2 相互作用的正交性。
Clustering receptors 聚集受体
The endocytosis of receptors such as ASGPR and EGFR is stimulated through dimerization or oligomerization26. ASGPR is a liver-specific receptor that transports N-acetylgalactosamine (GalNAc)-labelled proteins into lysosomes for clearance27. Multivalent GalNAc ligands have been used for multiple liver-specific degradation applications2,28 and RNA delivery platforms29. However, these require chemical modification and thus are not genetically encodable and must compete with native ligands.
ASGPR 和 EGFR 等受体的内吞作用通过二聚化或寡聚化来刺激26。ASGPR 是一种肝脏特异性受体,可将 N-乙酰半乳糖胺 (GalNAc) 标记的蛋白质转运到溶酶体中以进行清除27。多价 GalNAc 配体已用于多种肝脏特异性降解应用 2,28 和 RNA 递送平台29。然而,这些需要化学修饰,因此不能进行基因编码,必须与天然配体竞争。
We designed binders to ASGPR that do not overlap with the glycan-binding sites (Extended Data Fig. 1h,i) using an updated version of the Rosetta design approach described above that uses ProteinMPNN21 for sequence design and AlphaFold225 for design evaluation. We used yeast display for 2,689 designs that were predicted to bind ASGPR by AlphaFold225 followed by fluorescence-activated cell sorting (FACS) and next-generation sequencing to reveal four highly enriched designs. These were expressed in E. coli, and following purification, BLI showed that the design with the highest affinity, ASmb1, bound to ASGPR with an affinity of 2.7 µM (Extended Data Fig. 2f and Extended Data Table 1).
我们设计了不与聚糖结合位点重叠的 ASGPR 结合物(扩展数据图 .1 小时,i)使用上述 Rosetta 设计方法的更新版本,该方法使用 ProteinMPNN21 进行序列设计,使用 AlphaFold225 进行设计评估。我们对 2,689 个设计使用酵母展示,这些设计通过 AlphaFold225 预测会结合 ASGPR,然后进行荧光激活细胞分选 (FACS) 和下一代测序,以揭示四个高度富集的设计。这些在大肠杆菌中表达,纯化后,BLI 显示具有最高亲和力的设计 ASmb1 以 2.7 μM 的亲和力与 ASGPR 结合(扩展数据图 .2f 和扩展数据表 1)。
To stimulate ASGPR endocytosis through clustering, we connected two or three ASmb1 domains with GS linkers to generate ASGPR EndoTags, which we refer to as AS_EndoTag-2C and AS_EndoTag-3C (Fig. 1h). Following a 2 h incubation with Hep3B cells and extensive washing, 2.5-fold and 4.5-fold more fluorescence was associated with cells for AS_EndoTag-2C and AS_EndoTag-3C, respectively, compared with monomeric ASmb1 (Fig. 1i). Confocal imaging showed that AS_EndoTag-3C strongly co-localized with lysosomes after 24 h (Fig. 1j and Supplementary Fig. 18c). To determine the oligomerization state induced by AS_EndoTag, we mixed ASGPR and AS_EndoTag-3C at a 1:3 ratio and separated the generated species by size-exclusion chromatography (SEC); AS_EndoTag induced formation of a trimeric ASGPR complex as expected given the three ASGPR-binding sites (Supplementary Fig. 23).
为了通过聚类刺激 ASGPR 内吞作用,我们将两个或三个 ASmb1 结构域与 GS 接头连接起来,以生成 ASGPR EndoTags,我们称之为 AS_EndoTag-2C 和 AS_EndoTag-3C(图1h)。在与 Hep3B 细胞孵育 2 小时并充分洗涤后,与单体 ASmb1 相比,AS_EndoTag-2C 和 AS_EndoTag-3C 细胞的荧光分别增加了 2.5 倍和 4.5 倍(图 D)。共聚焦成像显示 24 小时后 AS_EndoTag-3C 与溶酶体强烈共定位(图 D)。1j 和补充图为了确定 AS_EndoTag 诱导的寡聚化状态,我们以 1:3 的比例混合 ASGPR 和 AS_EndoTag-3C,并通过尺寸排阻色谱 (SEC) 分离生成的物种;AS_EndoTag 如预期的那样,给定三个 ASGPR 结合位点(补充图 D)。23)。
Cell surface receptor degradation
细胞表面受体降解
LYTACs utilize mannose-6-phosphonate (M6Pn) ligands that trigger lysosomal delivery and degradation of the targeted proteins through the IGF2R1,30, or GalNAc to trigger the ASGPR lysosomal trafficking pathway2. Although promising, the LYTAC approach is hindered by the reliance on existing native ligands8 and by the sophisticated chemistry required to generate multivalent modifications that increase endocytosis potency, complicating their manufacturing. Given their potent and rapid endocytosis and lysosome-targeting ability, we hypothesized that the fusion of EndoTags with target-specific binders to generate protein–LYTACs (pLYTAC) (Fig. 2a) could provide an orthogonal and genetically encoded approach for efficient extracellular protein degradation, and the different tissue distributions of the different receptors could enable targeting of degradation to distinct tissues.
LYTAC 利用甘露糖-6-膦酸盐 (M6Pn) 配体,通过 IGF2R 1,30 或 GalNAc 触发溶酶体递送和靶蛋白降解,以触发 ASGPR 溶酶体运输途径2。尽管前景广阔,但 LYTAC 方法受到对现有天然配体8 的依赖以及产生增加内吞效力的多价修饰所需的复杂化学成分的阻碍,使其制造复杂化。鉴于它们有效和快速的内吞作用和溶酶体靶向能力,我们假设 EndoTag 与靶标特异性结合剂的融合会产生蛋白质-LYTAC (pLYTAC)(图 D)。2a) 可以为有效的细胞外蛋白质降解提供一种正交和遗传编码的方法,并且不同受体的不同组织分布可以针对不同的组织进行降解。
图 2:组织特异性 pLYTAC 的表面受体降解。
a, Schema of tissue-specific pLYTACs for receptor degradation. POI, protein of interest. b, Western blot analysis of total EGFR in Hep3B cells after treatment with 200 nM EGFRn or EGFRn–AS_EndoTag for 48 h. c, Western blot analysis of total EGFR levels in wild-type (WT) or TfR-knockout (KO) HeLa cells after treatment with 200 nM EGFRn or EGFRn–TfR_EndoTag for 48 h. d, Western blot analysis of total EGFR in wild-type or sortilin-knockout HeLa cells after treatment with 200 nM EGFRn or EGFRn–Sort_EndoTag for 48 h. e, Western blot analysis of total EGFR in H1975 cells after treatment with 200 nM of EGFRn or CTX with or without fusion to EndoTag1 or M6Pn for 48 h. f, Western blot analysis of total EGFR in wild-type or IGF2R-knockout HeLa cells after treatment with 200 nM EGFRn or EGFRn–IGF_EndoTags for 48 h. g, Quantitative proteomics analysis of protein abundance in H1975 cells. Data are mean of three biological replicates. Two-tailed unpaired t-test with Welch’s correction. EGFRmb, EGFR minibinder. h, Western blot analysis of PD-L1 in MDA-MB-231 cells after treatment with 200 nM ATZ or ATZ–pLYTACs for 48 h. i–m, Schema (i) of in vivo study. Five million A20 tumour cells were inoculated subcutaneously into BALB/c mice on day 0, then 5 mg kg−1 of indicated reagent (n = 6) was administered intratumourally on days 10, 13 and 16 (j–m). j, Tumour growth curve over time. One-way ANOVA. k, Tumour mass measured at day 21. l, Overall survival of treated mice. P value by log-rank (Mantel–Cox) test. m, Body weight of treated mice. One-way ANOVA. In j–l, data are mean ± s.e.m. of n = 6 biological independent samples. NS, not significant.
a,用于受体降解的组织特异性 pLYTAC 图式。POI,目标蛋白。b,用 200 nM EGFRn 或 EGFRn-AS_EndoTag 处理 48 小时后 Hep3B 细胞中总 EGFR 的蛋白质印迹分析,用 200 nM EGFRn 或 EGFRn-TfR_EndoTag 处理 48 小时后野生型 (WT) 或 TfR 敲除 (KO) HeLa 细胞中总 EGFR 水平的蛋白质印迹分析,用 200 nM EGFRn 或 EGFRn-Sort_EndoTag 处理后野生型或分选蛋白敲除的 HeLa 细胞中总 EGFR 的蛋白质印迹分析48 小时,用 200 nM EGFRn 或 CTX 处理 48 小时后 H1975 细胞中总 EGFR 的蛋白质印迹分析,不带与 EndoTag1 或 M6Pn 融合后,野生型或 IGF2R 敲除的 HeLa 细胞中总 EGFR 的蛋白质印迹分析200 nM EGFRn 或 EGFRn-IGF_EndoTags 处理 48 小时后, H1975 细胞中蛋白质丰度的定量蛋白质组学分析。数据是 3 次生物学重复的平均值。双尾未配对 t 检验与 Welch 校正。EGFRmb,EGFR 微型粘合剂。h,用 200 nM ATZ 或 ATZ-pLYTAC 处理 48 小时后 MDA-MB-231 细胞中 PD-L1 的蛋白质印迹分析。在第 0 天将 500 万个 A20 肿瘤细胞皮下接种到 BALB/c 小鼠中,然后在第 10 天、第 13 天和第 16 天 (j-m) 瘤内注射 5 mg kg-1 的指定试剂 (n = 6)。j,肿瘤随时间变化的生长曲线。单因子方差分析。k,第 21 天测量的肿瘤质量。l,治疗小鼠的总生存期。对数秩 (Mantel-Cox) 检验的 P 值。m,治疗小鼠的体重。单因子方差分析。 在 j-l 中,数据是 n = 6 个生物独立样本的平均 ± sem。NS,不显著。
We began by investigating the ability of EndoTags to target and degrade EGFR, which is frequently overexpressed in cancers and has an important role in regulating cell proliferation31. We first assessed the degradation efficiency of the liver-specific AS_EndoTags. Consistent with the cellular uptake results (Fig. 1i), introduction of fusions of AS_EndoTags-2C of AS_EndoTags-3C with a minibinder targeting the N terminus of EGFR (EGFRn) resulted in a 40% decrease in total EGFR levels, whereas fusions to the monomeric ASGPR binder had little effect (Fig. 2b). Thus, the ASGPR EndoTags function as liver-specific targeted degraders. To generate EGFR–pLYTACs targeting the brain, we fused TfR_EndoTag or Sort_EndoTag with EGFRn15. We observed efficient clearance of EGFR in wild-type HeLa cells after 48 h incubation, with EGFRn-TfR_EndoTag resulting in a 55% reduction of EGFR (Fig. 2c) and EGFRn–Sort_EndoTag resulting in a 78% reduction of EGFR (Fig. 2d).
我们首先研究了 EndoTags 靶向和降解 EGFR 的能力,EGFR 在癌症中经常过表达,在调节细胞增殖中具有重要作用31。我们首先评估了肝脏特异性 AS_EndoTags 的降解效率。与细胞摄取结果一致(图 D)。1i),将 AS_EndoTags-2C 的 AS_EndoTags-3C 与靶向 EGFR 的 N 末端的微型粘合剂 (EGFRn) 融合的引入导致总 EGFR 水平降低 40%,而与单体 ASGPR 粘合剂的融合几乎没有影响(图 D)。因此,ASGPR EndoTags 起到肝脏特异性靶向降解剂的作用。为了产生靶向大脑的 EGFR-pLYTACs,我们将 TfR_EndoTag 或 Sort_EndoTag 与 EGFRn15 融合。我们在野生型 HeLa 细胞中观察到 EGFR 在孵育 48 小时后有效清除,EGFRn-TfR_EndoTag导致 EGFR 降低 55%(图 D)。2c) 和 EGFRn-Sort_EndoTag导致 EGFR 降低 78%(图 D)。2d)。
To confirm that the EndoTags function through their target receptor, we carried out parallel experiments in sortilin-knockout or TfR-knockout HeLa cells (Supplementary Fig. 22). Knockout of these receptors eliminated EGFR degradation by the corresponding EndoTags, demonstrating that degradation is dependent on the target receptors (Fig. 2c,d). As a further test, we introduced mutations into Sort_EndoTag to eliminate sortilin binding based on the design model and mutation scanning data (EGFRn–Sort_EndoTagKO). The EGFR-degradation capacity of EGFRn–Sort_EndoTagKO was largely ablated compared with EGFRn–Sort_EndoTag, further confirming that EGFR degradation requires sortilin engagement (Supplementary Fig. 20). Given the abundant expression of the corresponding receptors in the brain, both TfR_EndoTag and Sort_EndoTag could function as pLYTACs for applications in neurodegenerative disease.
为了确认 EndoTags 通过其靶受体发挥作用,我们在 sortilin 敲除或 TfR 敲除 HeLa 细胞中进行了平行实验(补充图 D)。22). 敲除这些受体消除了相应 EndoTag 的 EGFR 降解,表明降解取决于靶受体(图 D)。2c,d)。作为进一步的测试,我们将突变引入 Sort_EndoTag 中,以根据设计模型和突变扫描数据 (EGFRn-Sort_EndoTagKO) 消除 sortilin 结合。与 EGFRn-Sort_EndoTag 相比,EGFRn-Sort_EndoTagKO 的 EGFR 降解能力在很大程度上被消融,进一步证实了 EGFR 降解需要 sortilin 参与(补充图 D)。鉴于相应受体在大脑中的丰富表达,TfR_EndoTag 和 Sort_EndoTag 都可以作为 pLYTAC 在神经退行性疾病中的应用发挥作用。
We next sought to make systemically active pLYTACs that act through the ubiquitously expressed IGF2R. Addition of IGF_EndoTag–EGFRn fusions to H1975 or HeLa cells reduced EGFR levels (Fig. 2e,f, Extended Data Fig. 5b and Supplementary Fig. 21); EGFRn–IGF_EndoTag2 was the most effective opf these fusions, leading to more than 80% clearance of EGFR. Mass spectrometry-based proteomic analyses showed that EGFRn–IGF_EndoTag2 and EGFRn–IGF_EndoTag1 reduced EGFR levels in HeLa and H1975 cells without affecting IGF2R levels (Fig. 2g, Extended Data Fig. 5e and Supplementary Table 5); EGFRn without EndoTag had no effect on EGFR levels (Fig. 2g and Extended Data Fig. 5d). The EGFR reduction induced by EGFRn–IGF_EndoTag2 in HeLa cells was eliminated by IGF2R knockout (Fig. 2f), further confirming the receptor dependence of the degradation mechanism. Consistent with this, mutations in IGF_EndoTag2 predicted to eliminate IGF2R binding largely ablated the EGFR degradation-inducing activity (Supplementary Fig. 20). To investigate the functional consequences of EGFR knockdown, HeLa cells pre-treated with 100 nM EGFRn–IGF_EndoTag or EGFRn control for 24 h were stimulated with EGF and downstream phospho-ERK signalling was detected by phosphorylation flow cytometry. Compared with EGFRn control, pre-incubation of EGFRn–IGF_EndoTag largely ablated EGF signalling (Extended Data Fig. 5i).
接下来,我们试图制造具有系统活性的 pLYTACs,它们通过普遍表达的 IGF2R 起作用。向 H1975 或 HeLa 细胞中添加 IGF_EndoTag-EGFRn 融合降低了 EGFR 水平(图 D)。2e,f,扩展数据图5b 和补充图21);EGFRn-IGF_EndoTag2 是这些融合中最有效的 OPF,导致 EGFR 清除率超过 80%。基于质谱的蛋白质组学分析表明,EGFRn-IGF_EndoTag2 和 EGFRn-IGF_EndoTag1 降低了 HeLa 和 H1975 细胞中的 EGFR 水平,而不会影响 IGF2R 水平(图 D)。2g,扩展数据图5e 和补充表 5);不含 EndoTag 的 EGFRn 对 EGFR 水平没有影响(图 D)。2g 和扩展数据图5d). EGFRn-IGF_EndoTag2 在 HeLa 细胞中诱导的 EGFR 降低被 IGF2R 敲除消除(图 D)。2f),进一步证实了降解机制的受体依赖性。与此一致,预测会消除 IGF2R 结合的 IGF_EndoTag2 突变在很大程度上消融了 EGFR 降解诱导活性(补充图 1)。为了研究 EGFR 敲低的功能后果,用 EGF 刺激用 100 nM EGFRn-IGF_EndoTag 或 EGFRn 对照预处理 24 小时的 HeLa 细胞,并通过磷酸化流式细胞术检测下游磷酸化 ERK 信号传导。与 EGFRn 对照相比,EGFRn-IGF_EndoTag 的预孵育在很大程度上消融了 EGF 信号传导(扩展数据图 D)。5i)。
To compare these systemically active pLYTACs with the original M6P-based LYTACs, we generated genetic fusions of EndoTag1 with cetuximab (CTX), a clinically approved therapeutic antibody that targets EGFR with high affinity32. In H1975 cells, CTX–IGF_EndoTag1 led to more effective degradation of EGFR than the M6P-based LYTAC1 (Fig. 2e). Proteomic analyses demonstrated that CTX–IGF_EndoTag1 elicited a significantly greater reduction in EGFR levels than CTX alone (Extended Data Fig. 5f,g), with little effect on the amount of IGF2R; incubation with 10 nM CTX–IGF_EndoTag1 led to an 85% reduction in EGFR (Extended Data Fig. 5a).
为了将这些具有全身活性的 pLYTAC 与原始的基于 M6P 的 LYTAC 进行比较,我们生成了 EndoTag1 与西妥昔单抗 (CTX) 的基因融合,西妥昔单抗是一种临床批准的治疗性抗体,以高亲和力靶向 EGFR32。在 H1975 细胞中,CTX-IGF_EndoTag1 比基于 M6P 的 LYTAC1 更有效地降解 EGFR(图 D)。蛋白质组学分析表明,CTX-IGF_EndoTag1 引起的 EGFR 水平降低明显大于单独使用 CTX(扩展数据图 1)。5f,g),对 IGF2R 的量影响不大;与 10 nM CTX-IGF_EndoTag1 一起孵育导致 EGFR 降低 85%(扩展数据图 D)。5a)。
We next investigated targeted degradation of PD-L1, an immune checkpoint used in cancer immunotherapy33. We genetically fused pLYTACs to the C terminus of the PD-L1 antibody atezolizumab34,35,36 (ATZ) and tested the ability of this construct to clear PD-L1. The ATZ–EndoTag fusions reduced the amount of PD-L1 in MDA-MB-231 cells within 4 h (Extended Data Fig. 5h), and 77% of the PD-L1 in the cells was eliminated after 48 h (Fig. 2h). Similar to PD-L1, CTLA4 is an immune checkpoint component for which inhibitors have shown promising anti-tumour effects18,19. A fusion of EndoTag1 and a minibinder against CTLA437 (CTLA4mb) resulted in a 45% decrease of CTLA4 in Jurkat cells expressing CTLA4 (Jurkat-CTLA4) cells after 3 h (Extended Data Fig. 5c).
我们接下来研究了 PD-L1 的靶向降解,PD-L1 是癌症免疫疗法中使用的免疫检查点33。我们将 pLYTAC 基因融合到 PD-L1 抗体 atezolizumab34、35、36 (ATZ) 的 C 端,并测试了该构建体清除 PD-L1 的能力。ATZ-EndoTag 融合在 4 小时内减少了 MDA-MB-231 细胞中 PD-L1 的量(扩展数据图 D)。5 小时),细胞中 77% 的 PD-L1 在 48 小时后被消除(图 D)。与 PD-L1 类似,CTLA4 是一种免疫检查点成分,抑制剂已显示出有希望的抗肿瘤作用18,19。EndoTag1 和针对 CTLA437 (CTLA4mb) 的微结合剂的融合导致 3 小时后表达 CTLA4 (Jurkat-CTLA4) 细胞的 Jurkat 细胞中 CTLA4 减少 45%(扩展数据图 1)。5c)。
To evaluate EndoTag function in vivo, we compared the efficacy of the ATZ–EndoTag fusions described above in a mouse tumour model compared with ATZ alone. BALB/c mice were inoculated subcutaneously with A20 cells to initiate tumour growth, and were treated intratumourally with EndoTag constructs when the tumour size reached around 100 mm3. The proteins were administered every 3 days for 9 days at 5 mg kg−1 per injection. When the tumour volume in the isotype control group reached around 1,000 mm3, the mice were euthanized and the tumours were weighed and collected for western blot analysis (Fig. 2i). ATZ–IGF_EndoTag3 and ATZ–IGF_EndoTag4 were considerably more effective than ATZ alone in reducing tumour size and mass (Fig. 2j,k). ATZ–IGF_EndoTag4 markedly increased overall survival compared with ATZ alone or isotype control: after 55 days, half of the mice treated with the EndoTag fusion remained alive, whereas all mice treated with ATZ alone had died (Fig. 2l). Western blot analysis showed that ATZ–IGF_EndoTag3 and ATZ–IGF_EndoTag4 triggered significant degradation of PD-L1 compared with ATZ alone or isotype control (Extended Data Fig. 8). Body weight assessment showed that the EndoTag treatments were well-tolerated (Fig. 2m). Thus, the efficacy of antagonistic antibodies can be enhanced by fusion to EndoTags; such fusions not only block disease-asociated interactions of the target (like the unfused antibody) but also induce cellular uptake and degradation of the target in the lysosome.
为了评估体内 EndoTag 功能,我们比较了上述 ATZ-EndoTag 融合在小鼠肿瘤模型中与单独使用 ATZ 的疗效。用 A20 细胞皮下接种 BALB/c 小鼠以启动肿瘤生长,并在肿瘤大小达到约 100 mm3 时用 EndoTag 构建体进行瘤内处理。蛋白质每 3 天给药一次,持续 9 天,每次注射 5 mg kg-1。当同种型对照组的肿瘤体积达到 1,000 mm3 左右时,对小鼠实施安乐死,称量并收集肿瘤用于蛋白质印迹分析(图 D)。ATZ-IGF_EndoTag3 和 ATZ-IGF_EndoTag4 在减少肿瘤大小和质量方面比单独使用 ATZ 有效得多(图 D)。2j,k)。与单独使用 ATZ 或同种型对照相比,ATZ-IGF_EndoTag4 显著提高了总生存期:55 天后,接受 EndoTag 融合治疗的小鼠中有一半仍然存活,而所有单独接受 ATZ 治疗的小鼠都已死亡(图 D)。Western blot 分析显示,与单独使用 ATZ 或同种型对照相比,ATZ-IGF_EndoTag3 和 ATZ-IGF_EndoTag4 触发了 PD-L1 的显着降解(扩展数据图 2)。体重评估显示 EndoTag 治疗耐受性良好(图 D)。因此,通过与 EndoTags 融合可以增强拮抗抗体的疗效;这种融合不仅阻断了靶标(如未融合的抗体)的疾病相关相互作用,而且还诱导溶酶体中靶标的细胞摄取和降解。
Clearance of soluble proteins
清除可溶性蛋白质
We next investigated the ability of EndoTags to degrade targeted soluble proteins (Fig. 3a). As a proof of concept, we used the nanomolar affinity de novo designed protein heterodimer pair LHD101A (LHDA) and LHD101B (LHDB) used as the basis for synthetic signalling systems22,38. We fused LHDA to IGF_EndoTags and LHDB to AF647, and found that the EndoTags significantly enhanced the uptake of LHDB–AF647 in Jurkat and K562 cells (Fig. 3b and Extended Data Fig. 6a,b), with IGF_EndoTag3 producing a remarkable 40-fold increase in mean fluorescence intensity compared with LHDB–AF647 alone. Incubation of Jurkat cells with 100 nM LHDA–IGF_EndoTag3 resulted in 50% clearance of 100 nM LHDB from the solution after 48 h incubation in Jurkat cells (Fig. 3c; clearance may be limited by the number of available receptors).
接下来,我们研究了 EndoTags 降解目标可溶性蛋白的能力(图 D)。作为概念验证,我们使用纳摩尔亲和力从头设计的蛋白质异二聚体对 LHD101A (LHDA) 和 LHD101B (LHDB) 作为合成信号系统的基础22,38。我们将 LHDA 与 IGF_EndoTags 融合,将 LHDB 与 AF647 融合,发现 EndoTags 显着增强了 Jurkat 和 K562 细胞对 LHDB-AF647 的摄取(图 D)。图 3b 和扩展数据图6a,b),与单独的 LHDB-AF647 相比,IGF_EndoTag3 的平均荧光强度显着增加 40 倍。在 Jurkat 细胞中孵育 48 小时后,Jurkat 细胞与 100 nM LHDA-IGF_EndoTag3 一起孵育导致 100 nM LHDB 从溶液中清除 50%(图 D)。3c;清除率可能受到可用受体数量的限制)。
图 3:IGF2R pLYTAC 清除可溶性蛋白。
a, Schema for the use of soluble pLYTACs with IGF_EndoTags. b, Cellular uptake of LHDB–AF647 via LHDA–IGF_EndoTags in Jurkat cells. Cells were incubated with 33 nM LHDB–AF647 with or without 1 μM LHDA–IGF_EndoTags for 24 h, washed twice with cold PBS and analysed by flow cytometry. c, Remaining supernatant LHDB–AF647 levels in Jurkat cells. Jurkat cells were incubated with 100 nM LHDB–AF647 with or without 500 nM LHDA–IGF_EndoTags. At timepoints 24 h and 48 h, the cells were pelleted down, and IgG in the supernatant was quantified using a Neo2 plate reader. IgG level was normalized to the IgG-alone control group. d, Cellular uptake of IgG–AF647 via protein G–IGF_EndoTags in K562 cells. Cells were incubated with 33 nM IgG–AF647 with or without 1 μM protein G–IGF_EndoTag3 for 24 h, washed twice with cold PBS and analysed by flow cytometry. The fold change in MFI was calculated by normalizing to the IgG–AF647-alone group. e, Remaining IgG–AF647 levels in the supernatant of Jurkat cells. Jurkat cells were incubated with 133 nM IgG–AF647 with or without 100 nM protein G–IGF_EndoTag3. At timepoints 24 h and 48 h, the cells were pelleted down, and IgG–AF647 in the supernatant was quantified using a Neo2 plate reader. The IgG–AF647 level was normalized to the IgG–AF647-alone control group at each timepoint. f, Confocal imaging of IgG–AF647 co-localization with lysosome in HeLa cells. g, Confocal imaging of IgG–AF647 co-localization with lysosome in HeLa (IGF2R-knockout) cells. Representative images of three replicated samples. f,g, Cells were incubated with 200 nM IgG–AF647 and 1 μM protein G–IGF_EndoTag3 for 24 h, washed and stained with LAMP2A antibody followed by AF488-labelled secondary antibody. Scale bars, 20 µm. Data in b–e are mean ± s.e.m. of n = 3 biologically independent samples.
a,可溶性 pLYTAC 与 IGF_EndoTags 的使用方案。b, Jurkat 细胞中通过 LHDA-IGF_EndoTags 细胞摄取 LHDB-AF647。将细胞与 33 nM LHDB-AF647 一起孵育,含或不含 1 μM LHDA-IGF_EndoTags 24 小时,用冷 PBS 洗涤两次,并通过流式细胞术分析。c,Jurkat 细胞中剩余的上清液 LHDB-AF647 水平。Jurkat 细胞与 100 nM LHDB-AF647 一起孵育,含或不含 500 nM LHDA-IGF_EndoTags。在 24 h 和 48 h 的时间点,将细胞沉淀下来,并使用 Neo2 读板器定量上清液中的 IgG。IgG 水平正常化为单独 IgG 对照组。d,K562 细胞中通过蛋白 G-IGF_EndoTags 细胞摄取 IgG-AF647。将细胞与 33 nM IgG-AF647 一起孵育,含或不含 1 μM 蛋白 G-IGF_EndoTag3 24 小时,用冷 PBS 洗涤两次,并通过流式细胞术分析。通过归一化为 IgG-AF647 单独组来计算 MFI 的倍数变化。e,Jurkat 细胞上清液中剩余的 IgG-AF647 水平。Jurkat 细胞与 133 nM IgG-AF647 一起孵育,含或不含有 100 nM 蛋白 G-IGF_EndoTag3。在 24 h 和 48 h 的时间点,将细胞沉淀下来,并使用 Neo2 读板器定量上清液中的 IgG-AF647。在每个时间点,IgG-AF647 水平标准化为单独 IgG-AF647 对照组。f,HeLa 细胞中 IgG-AF647 与溶酶体共定位的共聚焦成像。g,IgG-AF647 与 HeLa(IGF2R 敲除)细胞中溶酶体共定位的共聚焦成像。三个复制样本的代表性图像。 f,g,将细胞与 200 nM IgG-AF647 和 1 μM 蛋白 G-IGF_EndoTag3 一起孵育 24 小时,洗涤并用 LAMP2A 抗体染色,然后用 AF488 标记的二抗染色。比例尺,20 μm。b-e 中的数据是 n = 3 个生物学独立样本的均值 ± s.e.m.。
Autoantibodies that recognize self-antigens have been linked to multiple autoimmune diseases39. We tested whether the fusion of EndoTags with the IgG-binding protein G40 could clear IgG in solution. For comparison, we used protein G–M6Pn, generated by conjugating protein G with azido-NHS ester followed by M6Pn–BCN peptide. The EndoTag- and M6Pn-coupled protein G constructs triggered substantial uptake of IgG in Jurkat and K562 cells (Extended Data Fig. 6c,e). Protein G–IGF_EndoTag3 elicited twofold higher cellular uptake of IgG than protein G–M6Pn, leading to an overall 80-fold increase in IgG in K562 cells and a 360-fold increase in Jurkat cells (Extended Data Fig. 6e) compared with protein G alone. To quantify the clearance of IgG in the solution, we measured the fluorescence intensity in the cell culture supernatant normalized to the supernatant of control cells treated with IgG–AF647 alone. Incubation of Jurkat cells with 100 nM protein G–IGF_EndoTag3 and 133 nM IgG for 48 h resulted in depletion of 70% of the IgG (Fig. 3e); less clearance was observed with the other IGF EndoTags (Extended Data Fig. 6d,f,h). Utilizing confocal microscopy of HeLa cells, we observed enhanced co-localization of IgG with lysosomes following treatment with protein G–IGF_EndoTag3 for 24 h (Fig. 3f and Extended Data Fig. 9a); similar co-localization was not observed in HeLa cells that did not express IGF2R (Fig. 3g and Extended Data Fig. 9b).
识别自身抗原的自身抗体与多种自身免疫性疾病有关39。我们测试了 EndoTags 与 IgG 结合蛋白 G40 的融合是否可以清除溶液中的 IgG。为了进行比较,我们使用了蛋白质 G-M6Pn,它是通过将蛋白质 G 与叠氮基-NHS 酯偶联,然后是 M6Pn-BCN 肽生成的。EndoTag 和 M6Pn 偶联的蛋白 G 构建体在 Jurkat 和 K562 细胞中触发了 IgG 的大量摄取(扩展数据图 1)。6c,e)。蛋白 G-IGF_EndoTag3 引起的细胞对 IgG 的摄取比蛋白 G-M6Pn 高两倍,导致 K562 细胞中 IgG 总体增加 80 倍,Jurkat 细胞中 IgG 增加 360 倍(扩展数据图 D)。6e) 与单独的蛋白 G 相比。为了量化溶液中 IgG 的清除率,我们测量了细胞培养上清液中的荧光强度,该上清液标准化为单独用 IgG-AF647 处理的对照细胞的上清液。将 Jurkat 细胞与 100 nM 蛋白 G-IGF_EndoTag3 和 133 nM IgG 一起孵育 48 小时,导致 70% 的 IgG 耗尽(图 D)。3e);使用其他 IGF EndoTag 观察到较少的清除率(扩展数据图 1)。6d,f,h)。利用 HeLa 细胞的共聚焦显微镜,我们观察到用蛋白 G-IGF_EndoTag3 处理 24 小时后 IgG 与溶酶体的共定位增强(图 D)。3f 和扩展数据图9a);在未表达 IGF2R 的 HeLa 细胞中未观察到类似的共定位(图 D)。3g 和扩展数据图9b)。
Logic-gated and secretable degradation factors
逻辑门控和可分泌降解因子
Target degradation conditioned on the presence of a specific marker in the tumour microenvironment could help to avoid undesired effects on healthy cells. Such logic-gated targeted degradation has not been achieved with current extracellular protein-degradation systems. To address this limitation, we utilized the co-localization-dependent protein switch (Co-LOCKR) system41,42, which functions as an AND logic gate by only exposing a recruitment motif when two target cell markers are present on the same cell (Fig. 4a). We utilized Co-LOCKR to selectively degrade EGFR only when HER2 was also present on the surface of cancer cells41. We fused EndoTag with BCL2, which binds the Bim peptide that is exposed upon coincident binding in this version of Co-LOCKR, and evaluated EGFR degradation in HER2+ and HER2− cells. In K562 cells overexpressing both EGFR and HER2, addition of BCL2–IGF_EndoTag2 resulted in 80% degradation of EGFR, whereas in K562 cells expressing EGFR without HER2, the EGFR level remained unchanged (Fig. 4b). Thus, the EndoTag system can be precisely targeted to specific cells based on combinations of surface markers.
以肿瘤微环境中存在特定标志物为条件的靶标降解有助于避免对健康细胞的不良影响。目前的细胞外蛋白质降解系统尚未实现这种逻辑门控靶向降解。为了解决这一限制,我们利用了共定位依赖性蛋白质开关 (Co-LOCKR) 系统41,42,该系统通过仅在同一细胞上存在两个靶细胞标记物时暴露募集基序来充当 AND 逻辑门(图 D)。4a). 仅当 HER2 也存在于癌细胞表面时,我们才利用 Co-LOCKR 选择性降解 EGFR41。我们将 EndoTag 与 BCL2 融合,BCL2 结合在此版本的 Co-LOCKR 中重合结合时暴露的 Bim 肽,并评估 HER2+ 和 HER2− 细胞中的 EGFR 降解。在过表达 EGFR 和 HER2 的 K562 细胞中,添加 BCL2-IGF_EndoTag2 导致 EGFR 降解 80%,而在表达 EGFR 而没有 HER2 的 K562 细胞中,EGFR 水平保持不变(图 D)。因此,EndoTag 系统可以根据表面标志物的组合精确靶向特定细胞。
图 4:逻辑门控靶向降解和局部可分泌降解剂。
a, Schematic illustration of AND gate logic for EGFR degradation in the presence of HER2. EGFR–Key, designed fusion protein composed of an EGFR-binding domain and a ‘key’ domain; HER2–LOCKR, designed fusion protein composed of an HER2-binding domain and a ‘LOCKR’ domain with a BCL2-recognizing peptide designed to bind to LOCKR domain at weak affinity. At close proximity, the key domain will bind to LOCKR and release the BCL2-recognizing peptide. b, Flow cytometry quantification of EGFR on the cell surface. K562 cells overexpressing EGFR only (K562-HER2−) or K562 cells overexpressing both EGFR and HER2 (K562-HER2+) were incubated with combinations of 100 nM of EGFRn–Key, HER2–LOCKR and BCL2–EndoTag2 for 24 h. c, Schematic of the use of EGFR–pLYTAC secretion to degrade EGFR in target cells. d, Flow cytometry quantification of cell surface EGFR in cells treated with cell supernatant or exogenous EGFRn–IGF_EndoTag1 for 24 h. For the secretion groups, IGF2R-knockout HeLa cells were transfected with viral vectors encoding EGFRn–IGF_EndoTag1 or LHDA–pLYTACs. Cell supernatants were collected and incubated with K562 cells overexpressing EGFR. Data in b,d are mean ± s.e.m. of n = 3 biologically independent samples.
a,在 HER2 存在下 EGFR 降解的 AND 门逻辑示意图。EGFR – 关键,由 EGFR 结合结构域和“关键”结构域组成的设计融合蛋白;HER2-LOCKR 设计的融合蛋白由 HER2 结合结构域和“LOCKR”结构域组成,BCL2 识别肽旨在以弱亲和力结合 LOCKR 结构域。在附近,关键结构域将与 LOCKR 结合并释放 BCL2 识别肽。b,流式细胞术定量细胞表面的 EGFR。将仅过表达 EGFR 的 K562 细胞 (K562-HER2−) 或过表达 EGFR 和 HER2 的 K562 细胞 (K562-HER2+) 与 100 nM EGFRn-Key、HER2-LOCKR 和 BCL2-EndoTag2 的组合一起孵育 24 小时。使用 EGFR-pLYTAC 分泌降解靶细胞中 EGFR 的示意图。d,流式细胞术定量用细胞上清液或外源性 EGFRn-IGF_EndoTag1 处理 24 小时的细胞表面 EGFR。对于分泌组,用编码 EGFRn-IGF_EndoTag1 或 LHDA-pLYTAC 的病毒载体转染 IGF2R 敲除的 HeLa 细胞。收集细胞上清液并与过表达 EGFR 的 K562 细胞一起孵育。b,d 中的数据是 n = 3 个生物学独立样本的均值 ± s.e.m.。
Local secretion of an EndoTag fusion introduced via mRNA delivery or as part of an adoptive cell therapy could focus degradation activity where needed and overcome depletion from lysosomal targeting. Unlike the M6P-based LYTAC system, EndoTags can be deployed in this way, as they are fully protein-based and consequently can be secreted locally with high specificity and efficiency43,44. To investigate this possibility, we transiently transfected IGF2R-knockout HeLa cells with plasmid encoding EGFRn–IGF_EndoTag and incubated the supernatants with EGFR+ K562 cells (Fig. 4c). Cell supernatants containing EGFRn–IGF_EndoTag1 and EGFRn–IGF_EndoTag2 cleared EGFR as efficiently as the purified proteins (Fig. 4d). Thus, EndoTags remain functional when secreted from cells, providing a means for adoptive cell therapies to degrade proteins in the surrounding environment for greater efficacy, and to degrade self proteins for feedback control.
通过 mRNA 递送引入的 EndoTag 融合的局部分泌或作为过继细胞疗法的一部分,可以将降解活性集中在需要的地方,并克服溶酶体靶向引起的耗竭。与基于 M6P 的 LYTAC 系统不同,EndoTag 可以通过这种方式部署,因为它们完全基于蛋白质,因此可以以高特异性和高效局部分泌43,44。为了研究这种可能性,我们用编码 EGFRn-IGF_EndoTag 的质粒瞬时转染 IGF2R 敲除的 HeLa 细胞,并将上清液与 EGFR+ K562 细胞一起孵育(图 D)。含有 EGFRn-IGF_EndoTag1 和 EGFRn-IGF_EndoTag2 的细胞上清液清除 EGFR 的效率与纯化的蛋白质一样有效(图 D)。因此,EndoTags 在从细胞分泌时仍保持功能,为过继细胞疗法提供了一种在周围环境中降解蛋白质以获得更高疗效的方法,并降解自身蛋白质以进行反馈控制。
Activation of cell signalling
细胞信号传导的激活
Transmembrane signalling resulting from extracellular ligands binding to plasma membrane receptors is frequently accompanied by endocytosis of the ligand, and in some cases, signalling may take place in part or primarily in the endosome45. We reasoned that in such cases, EndoTags could enhance signalling by increasing the fraction of the ligand–receptor complex that is in the endosome. As a model system, we used a minimalist Notch-derived synthetic signalling system, ortho-SNIPR38, which inhibitor experiments suggested was primarily activated in the endosome. Ortho-SNIPR is based on de novo-designed LHDA–LHDB heterodimer pair22, with an LHDA-containing synthetic ligand and a receptor comprising of a LHDB extracellular domain fused to the Notch transmembrane segment; binding of the ligand to the receptor results in cleavage and release of an intracellular transcription factor domain, which activates downstream BFP expression (Fig. 5a). We found that fusion of IGF_EndoTags to LHDA resulted in an increase of up to 100-fold (in the case of IGF_EndoTag2) in signal activation (Fig. 5b,c). EndoTag-enhanced signalling was not affected by small molecules that block engagement with cell surface proteases, but was blocked by chloroquine, which disrupts endosomal acidification, suggesting that EndoTag-enhanced signalling occurs in the endosome (inhibition of γ-secretase, which carries out the proteolytic cleavage needed to free the transcription factor, also blocked signalling) (Fig. 5d). Confocal microscopy showed rapid lysosomal targeting of the LHDA–IGF_EndoTag2 construct (Fig. 5e). This marked enhancement of signalling, together with the ability to localize responses to specific target cells using tissue-specific EndoTags or Co-LOCKR targeting, should make the ortho-SNIPR system a powerful tool for synthetic biology and adoptive cell therapy applications. Further studies will be required to determine whether EndoTags can enhance signalling through endogenous pathways. Conversely, for pathways that are downregulated by endocytosis, EndoTags could be used to shorten the signalling half-life, which could have utility in applications such as T cell receptor signalling, in which overstimulation can lead to exhaustion and reduction of downstream signalling.
细胞外配体与质膜受体结合产生的跨膜信号传导通常伴随着配体的内吞作用,在某些情况下,信号传导可能部分或主要发生在内体中 45。我们推断,在这种情况下,EndoTags 可以通过增加内体中配体-受体复合物的分数来增强信号传导。作为模型系统,我们使用了极简的 Notch 衍生的合成信号系统 ortho-SNIPR38,抑制剂实验表明它主要在内体中被激活。Ortho-SNIPR 基于从头设计的 LHDA-LHDB 异二聚体对22,具有含 LHDA 的合成配体和受体,该受体由与 Notch 跨膜片段融合的 LHDB 胞外结构域组成;配体与受体的结合导致细胞内转录因子结构域的切割和释放,从而激活下游 BFP 表达(图 D)。我们发现 IGF_EndoTags 与 LHDA 的融合导致信号激活增加高达 100 倍(在 IGF_EndoTag2 的情况下)(图 D)。5b,c)。EndoTag 增强的信号传导不受阻断与细胞表面蛋白酶结合的小分子的影响,但被氯喹阻断,氯喹破坏了内体酸化,表明 EndoTag 增强的信号传导发生在内体中(抑制 γ-分泌酶,它执行释放转录因子所需的蛋白水解切割,也阻断了信号传导)(图 D。共聚焦显微镜显示 LHDA-IGF_EndoTag2 构建体的快速溶酶体靶向(图 D)。5e)。 这种信号传导的显著增强,以及使用组织特异性 EndoTags 或 Co-LOCKR 靶向定位对特定靶细胞的反应的能力,应该使 ortho-SNIPR 系统成为合成生物学和过继细胞治疗应用的强大工具。需要进一步的研究来确定 EndoTags 是否可以通过内源性途径增强信号传导。相反,对于因内吞作用下调的通路,EndoTags 可用于缩短信号传导半衰期,这可能在 T 细胞受体信号传导等应用中有用,其中过度刺激会导致下游信号传导耗尽和减少。
图 5:EndoTags 增强信号传导。
a, Schematic illustrating the designed SNIPR system consisting of an extracellular LHDB protein that recognizes the LHDA ligand, a cleavable membrane proximal domain and an intracellular domain that releases transcription factor, which induces expression of blue fluorescent protein (BFP). Upon ligand binding of LHDB to LHDA SNIPR, the IGF_EndoTag triggers the endocytosis of the complex; signalling activation is quantified by BFP fluorescence intensity. b, Activation of an LHDA-responsive SNIPR driving a BFP reporter circuit in Jurkat T cells by a non-EndoTag ligand (LHDA-C2, a homodimer composed of two LHDA molecules in C2 rotational symmetry) is much weaker than by a similarly flexibly linked EndoTag-containing ligand (IGF_EndoTag1) (n = 3; mean ± s.e.m.). c, Dose–response curve of signal activation with LHDA fusion with IGF_EndoTags. b,c, Jurkat T cells expressing LHDB SNIPR were incubated with IGF_EndoTags at the titrated concentration. n = 3 replicates. Data are mean ± s.e.m. d, Relative activation of Jurkat T cells by IGF_EndoTag1 ligand in the presence of chemical inhibitors. Data are mean ± s.e.m. of measurements normalized to the activation of an inhibitor-free vehicle control sample; n = 3 biologically independent samples. e, Left, live confocal imaging of lysosome co-localization with EndoTag ligands and SNIPR receptors at 24 h. The lysosomes were stained with AF488-labelled LysoTracker. Right, images were acquired at 0.25 h, 3 h, 6 h and 24 h for the experiment in c and analysed for co-localization between the labelled EndoTag ligand and LysoTracker signal. Scale bars, 20 µm for e. n = 4 images per timepoint with at least 10 cells per image; data are mean ± s.e.m.
a,示意图说明了所设计的 SNIPR 系统,该系统由识别 LHDA 配体的细胞外 LHDB 蛋白、可切割膜近端结构域和释放转录因子的胞内结构域组成,转录因子诱导蓝色荧光蛋白 (BFP) 的表达。当 LHDB 与 LHDA SNIPR 的配体结合时,IGF_EndoTag触发复合物的内吞作用;信号激活通过 BFP 荧光强度进行量化。b,非 EndoTag 配体(LHDA-C2,由两个 LHDA 分子组成的 C2 旋转对称性同源二聚体)激活驱动 Jurkat T 细胞中 BFP 报告电路的 LHDA 反应性 SNIPR 比类似灵活连接的含有 EndoTag 的配体 (IGF_EndoTag1) 弱得多(n = 3;平均值 ± sem)。c,LHDA 与 IGF_EndoTags 融合信号激活的剂量-反应曲线。b,c,表达 LHDB SNIPR 的 Jurkat T 细胞与滴定浓度的 IGF_EndoTags 一起孵育。n = 3 个重复。数据是 s.e.m. d ±平均值,在化学抑制剂存在下IGF_EndoTag1配体对 Jurkat T 细胞的相对激活。数据是测量值的平均± sem,标准化为无抑制剂载体对照样品的激活;n = 3 个生物学独立样本。e,左图,24 小时溶酶体与 EndoTag 配体和 SNIPR 受体共定位的实时共聚焦成像。溶酶体用 AF488 标记的 LysoTracker 染色。右图,在 0.25 小时、3 小时、6 小时和 24 小时采集图像,用于 c 中的实验,并分析标记的 EndoTag 配体和 LysoTracker 信号之间的共定位。比例尺,20 μm for e. n = 每个时间点 4 张图像,每张图像至少 10 个单元格;数据均为 S.E.M. ±平均值。
Conclusion 结论
The designed EndoTag approach considerably expands the possibilites for targeted degradation as a therapeutic modality. First, whereas native ligands can trigger off-target signalling and competition with endogenous proteins can reduce potency8, the designed EndoTags, as illustrated by the sortilin, TfR and ASGPR models (Fig. 1b,e,h), can be targeted to sites on endocytosing receptors that are not bound by native ligands. Second, high-valency chemical modification1,2,46 has been used to enhance endocytosis, but this complicates manufacturing1,14,23; as illustrated by the multidomain ASGPR EndoTags, small synthetic domains can be readily combined to create all protein receptor clustering and endocytosis stimulating proteins. The all-protein nature of our pLYTACs simplifies manufacturing and enables deployment of targeted degradation approaches in adoptive cell therapies using secretion from engineered cells (Fig. 4c). Although a de novo designed IL2 mimic has been shown to be not strongly immunogenic in humans47, as with any new therapeutic agent, it will be important to assess and, if necessary, reduce the immunogenicity of EndoTags, and catalytic versions that recycle to the plasma membrane following delivery of target to the lysosome could enable considerable dose sparing. The small, stable and readily producible EndoTags could be useful both for therapeutic applications and as molecular tools for probing how receptor conformational and oligomerization state modulates cellular trafficking.
设计的 EndoTag 方法大大扩展了靶向降解作为一种治疗方式的可能性。首先,虽然天然配体可以触发脱靶信号传导,并且与内源性蛋白质的竞争会降低效力8,但所设计的 EndoTags,如 sortilin、TfR 和 ASGPR 模型所示(图 D)。1b,e,h) 可以靶向内吞受体上不受天然配体结合的位点。其次,高价化学修饰 1,2,46 已被用于增强内吞作用,但这会使制造复杂化1,14,23;如多结构域 ASGPR EndoTag 所示,小合成结构域可以很容易地组合起来,以产生所有蛋白受体聚集和内吞刺激蛋白。我们的 pLYTAC 的全蛋白性质简化了生产,并能够利用工程细胞的分泌物在过继细胞疗法中部署靶向降解方法(图 D)。尽管从头设计的 IL2 模拟物已被证明在人类中不具有很强的免疫原性47,但与任何新的治疗剂一样,评估并在必要时降低 EndoTag 的免疫原性非常重要,并且在将靶标递送到溶酶体后循环到质膜的催化版本可以实现相当大的剂量节省。小而稳定且易于生产的 EndoTag 可用于治疗应用,并可用作探测受体构象和寡聚化状态如何调节细胞运输的分子工具。
There are many avenues for future work using our computational design approach to generating EndoTag-stimulated enhancers of cell surface receptor endocytosis and trafficking. First, there are likely to be many more receptor targets that can undergo rapid endocytosis upon suitable triggering at the cell surface; the ability to design endocytosis stimulators without requiring native ligands or identification of chemical modifications should enable utilization of the full range of these receptors to achieve more tissue-restricted targeting and modulatable intracellular trafficking (different receptors are likely to have different intracellular compartment residence times and transition dynamics). The increase in survival of mice treated with EndoTag–anti-PD-L1 fusions compared with the antibody alone (Fig. 2l) highlights the potential of EndoTag fusion to enhance the activity of antagonistic therapeutic antibodies. In addition to the targeted degradation application pursued here, such designed endocytosis stimulators could be of great utility for enhancing uptake of nucleic acids (such as short interfering RNAs) and small-molecule drug conjugates. Second, there are likely to be natural signalling pathways which, like the synthetic ortho-SNIPR system, can be more potently activated by promotion of receptor endocytosis. We achieved a 100-fold enhancement of maximum signalling effect by fusing the SNIPR ligand to EndoTags—if similar levels of signalling enhancement or modulation can be achieved by fusing natural signalling molecules to EndoTags, there could be many applications in therapeutics and biotechnology. Third, as illustrated by the use of the co-LOCKR system to make logic-gated protein-degradation systems and the secretion of EndoTag-degrader constructs from cells, the robustness and modularity of de novo designed proteins and capability for logic-gated activation and cell-based expression open the door to a wide range of more precise and controllable targeted degradation strategies for protein and cell-based therapies.
使用我们的计算设计方法生成 EndoTag 刺激的细胞表面受体内吞作用和运输增强子,未来工作有许多途径。首先,可能有更多的受体靶标在细胞表面适当触发后可以进行快速内吞作用;在不需要天然配体或识别化学修饰的情况下设计内吞刺激剂的能力应该能够利用这些受体的全部范围来实现更多组织限制性靶向和可调节的细胞内运输(不同的受体可能具有不同的细胞内区室停留时间和过渡动力学)。与单独使用抗体相比,接受 EndoTag-抗 PD-L1 融合治疗的小鼠存活率增加(图 D)。2l) 强调了 EndoTag 融合增强拮抗治疗性抗体活性的潜力。除了这里追求的靶向降解应用外,这种设计的内吞刺激剂对于增强核酸(如短干扰 RNA)和小分子药物偶联物的摄取可能具有很大的用途。其次,可能存在天然信号通路,如合成的 Ortho-SNIPR 系统,可以通过促进受体内吞作用来更有效地激活。通过将 SNIPR 配体与 EndoTag 融合,我们实现了 100 倍的最大信号传导效果增强——如果通过将天然信号分子与 EndoTags 融合可以实现类似水平的信号增强或调节,那么在治疗和生物技术中可能有许多应用。 第三,正如使用 co-LOCKR 系统制造逻辑门控蛋白质降解系统和从细胞中分泌 EndoTag 降解剂构建体所说明的那样,从头设计蛋白质的稳健性和模块化以及逻辑门控激活和基于细胞的表达的能力为蛋白质和基于细胞的疗法打开了一扇更精确和可控的靶向降解策略的大门。
Methods 方法
Computational design of sortilin minibinders as Sort_EndoTags
sortilin 微型粘合剂的计算设计作为 Sort_EndoTags
Using a Rosetta-based binder design protocol15, 21,000 binders were generated to each of 2 sites on the sortilin. The epitope of site1 comprises five amino acids (UniProt numbering: F92, V93, T546, T559 and T561), chosen since it provided a modest patch of exposed hydrophobicity while avoiding any sites of known interactions. The selected epitope has the added feature that a binder to this location would be pH-dependent, since this region undergoes considerable structural change at low pH18. As previously described15, we used a set of scaffold libraries to generate several million docks to each of the sites. As in the protocol, 100,000 docks were sub-selected and sequence was designed. Helical motifs were extracted, and 3,000 designs were selected, grafted and subjected to further design. Designs were filtered based on their Rosetta ddG and ContactMolecularSurface to the hydrophobic residues listed above. This resulted in 42,000 designs that were tested experimentally. The final designed sequences for Sort_EndoTag are provided in Supplementary Table 2.
使用基于 Rosetta 的结合剂设计方案15,在 sortilin 上的 2 个位点中的每一个位点生成了 21,000 个结合剂。位点 1 的表位包含五个氨基酸(UniProt 编号:F92、V93、T546、T559 和 T561),选择它是因为它提供了适度的暴露疏水性片段,同时避免了任何已知相互作用的位点。所选表位具有额外的特征,即该位置的结合剂将依赖于 pH 值,因为该区域在低 pH18 时会发生相当大的结构变化。如前所述15,我们使用一组基架库为每个站点生成数百万个码头。与协议中一样,对 100,000 个码头进行了二次选择并设计了序列。提取螺旋图案,并选择 3,000 个设计,嫁接并进行进一步设计。根据 Rosetta ddG 和 ContactMolecularSurface 对设计进行过滤,以去除上述疏水残基。这导致了 42,000 个设计进行了实验测试。补充表 2 中提供了 Sort_EndoTag 的最终设计序列。
Computational design of IGF2R and ASGPR minibinders
IGF2R 和 ASGPR 微型粘合剂的计算设计
The minibinders against IGF2R domain 6 and domain 11 were computationally designed via a Rosetta-based approach as described15. In brief, the structures of IGF2R domain 6 (Preotein Data Bank (PDB) 6UM2) and IGF2R domain 11 (PDB 1GP0) were refined using Rosetta Fastrelax with coordinate constraints. The residues at the IGF2-binding site for each domain were selected as ‘hotspot’ residues. Helical protein scaffolds were docked against the hotspot residues via the Patchdock followed by the Rifdock protocol. After sequence optimization with Rosetta FastDesign and filtering with Rosetta interface metrics including ddg and contact_molecular_surface, the top candidates were then resamplered with Rosetta Motifgraft44 and FastDesign. Candidates passing previous filters were then filtered again with exposed hydrophobicity (sap_score) and optimized with a net-charge of −7. The final designed sequences for IGF_EndoTag are provided in Supplementary Table 1.
针对 IGF2R 结构域 6 和结构域 11 的微结合剂是通过基于 Rosetta 的方法进行计算设计的,如前所述15。简而言之,IGF2R 结构域 6 (Preotein 数据库 (PDB) 6UM2) 和 IGF2R 结构域 11 (PDB 1GP0) 的结构是使用具有坐标约束的 Rosetta Fastrelax 进行细化的。选择每个结构域的 IGF2 结合位点的残基作为“热点”残基。螺旋蛋白支架通过 Patchdock 对接热点残基,然后是 Rifdock 方案。在使用 Rosetta FastDesign 进行序列优化并使用 Rosetta 接口指标(包括 ddg 和 contact_molecular_surface)进行过滤后,然后使用 Rosetta Motifgraft44 和 FastDesign 对排名靠前的候选者进行重新采样。然后,通过先前过滤器的候选物再次使用暴露的疏水性 (sap_score) 进行过滤,并使用 -7 的净电荷进行优化。补充表 1 中提供了 IGF_EndoTag 的最终设计序列。
The minibinders against ASGPR were designed with a Rosetta-based approach integrated with ProteinMPNN and AlphaFold2. The crystal structure of ASGPR (PDB 5JQ1) was refined and helical protein scaffolds were docked against the exposed hydrophobic residues via Patchdock followed by Rifdock. The sequences were optimized with protein-MPNN and interface scores were calculated with Rosetta Fastrelax. The models were then predicted by AlphaFold2 and scored after Fastrelax. Designs with pae_interaction<10 and relaxed_ddg < −40 were selected for resampling with another round of protein-MPNN prediction followed by Rosetta Fastrelax. After final round filtering with pae_interaction, relaxed_ddg and sap_score, the sequences were further optimized to have a net-charge of −7. The final designed sequences for AS_EndoTag are provided in Supplementary Table 4.
针对 ASGPR 的微结合剂是采用基于 Rosetta 的方法设计的,该方法与 ProteinMPNN 和 AlphaFold2 相结合。对 ASGPR (PDB 5JQ1) 的晶体结构进行精炼,并通过 Patchdock 和 Rifdock 将螺旋蛋白支架对接在暴露的疏水残基上。使用 protein-MPNN 优化序列,并使用 Rosetta Fastrelax 计算界面评分。然后通过 AlphaFold2 预测模型并在 Fastrelax 后评分。pae_interaction<10 and relaxed_ddg < −40 were selected for resampling with another round of protein-MPNN prediction followed by Rosetta Fastrelax. After final round filtering with pae_interaction, relaxed_ddg and sap_score, the sequences were further optimized to have a net-charge of −7. The final designed sequences for AS_EndoTag are provided in Supplementary Table 4 的设计。
Computational design of IGF_EndoTags
IGF_EndoTags 的计算设计
To generate flexible IGF2R agonists, all combinations of GS linkers with various lengths linking D6mb and D11mb were modelled with AlphaFold225. The designs with poor monomer plddt (plddt < 85) were dropped.
为了产生灵活的 IGF2R 激动剂,用 AlphaFold225 对连接 D6mb 和 D11mb 的不同长度的 GS 接头的所有组合进行建模。单体 plddt 较差 (plddt < 85) 的设计被丢弃。
To generate rigid IGF_EndoTags, the major binding helix from D11mb or the native IGF2-binding helix, and two interface helices from D6mb were extracted. Crystal structures obtained for D6mb and D11mb in complex with IGF2 and IGF2R were used as starting points for design. Domains 6 and 11 of the complex structures were aligned with the respective domains of IGF2R in the putative receptor internalizing conformation available in the Protein Data Bank (PDB: 6UM2). In this orientation, the two interface helices from D6mb and the single interface helix from D11mb were extracted and used as motifs to scaffold by protein inpainting24. To increase the likelihood of design success, the D11mb structure was adjusted to form an ideal three helical bundle with the two domain 6 helices. Protein inpainting was implemented such that the interacting residues within 3 Å of the receptor maintained the same identity as in the original minibinders. To increase design diversity, the domain 11 helix motif was randomly perturbed by rigid-body translations (up to 5 Å) and rotations (up to 10 radians) for each design prior to inpainting a scaffold between the motifs. The best inpainting outputs were selected by RosettaFold LDDT metrics (>0.5) for the inpainted region and used for sequence design with ProteinMPNN. ProteinMPNN sequence design was performed on the inpainted outputs in their desired complex orientation (with both domains 6 and 11 present) while fixing the original minibinder identities of interface residues (D6mb: R4, V8, Q11, D15, V20, K24, M25, I27, I31 and E34; D11mb: M1, A4, L7, L8 and W11). After 2,000 sequences were generated for each ProteinMPNN input, designs were filtered by predicted Rosetta ddG. AlphaFold2 structure predictions of the designed sequences were filtered by the pLDDT metric (keeping those with pLDDT > 90), and designs with a sub-angstrom backbone atom root mean squared deviation to the original design models realigned to D6mb and D11mb crystal structures (in complex with the IGF2–M6PR target domains). Finally, the complexes were assessed by Rosetta FastRelax. Designs with ddG metrics less than −40 and spatial aggregation propensity scores less than 35 were selected for expression and experimental assays.
为了产生刚性IGF_EndoTags,提取了来自 D11mb 或天然 IGF2 结合螺旋的主结合螺旋,以及来自 D6mb 的两个界面螺旋。以 D6mb 和 D11mb 与 IGF2 和 IGF2R 复合物的晶体结构为起点进行设计。复合物结构的结构域 6 和 11 与蛋白质数据库 (PDB: 6UM2) 中推定的受体内化构象中 IGF2R 的相应结构域对齐。在这个方向上,提取了来自 D6mb 的两个界面螺旋和来自 D11mb 的单界面螺旋,并用作蛋白质修复支架的基序24。为了增加设计成功的可能性,调整了 D11mb 结构,以形成具有两个结构域 6 螺旋的理想三螺旋束。实施蛋白质修复,使得受体 3 Å 内的相互作用残基保持与原始微结合剂中的相同身份。为了增加设计多样性,在对基体之间的支架进行修复之前,每个设计的 11 螺旋域基序被每个设计的刚体平移(高达 5 Å)和旋转(最多 10 弧度)随机扰动。通过 RosettaFold LDDT 指标 (>0.5) 为修复区域选择最佳修复输出,并用于 ProteinMPNN 的序列设计。在修复界面残基的原始微结合剂身份(D6mb:R4、V8、Q11、D15、V20、K24、M25、I27、I31 和 E34;D11mb:M1、A4、L7、L8 和 W11)。为每个 ProteinMPNN 起始量生成 2,000 个序列后,按预测的 Rosetta ddG 过滤设计。 设计序列的 AlphaFold2 结构预测通过 pLDDT 指标过滤(保持 pLDDT > 90),并且与原始设计模型相比具有亚埃骨架原子根均方偏差的设计重新对齐为 D6mb 和 D11mb 晶体结构(与 IGF2-M6PR 目标域复杂)。最后,通过 Rosetta FastRelax 评估复合物。选择 ddG 指标小于 -40 且空间聚集倾向评分小于 35 的设计用于表达和实验测定。
N-linked glycan verification of epitope
表位的 N-连接聚糖验证
To verify the epitope of the designed binders, an N-linked glycan scan was performed. This was performed to rapidly determine if the computational designed binder was interacting with the chosen interface50. Four engineered N-linked glycan variants (NN-0975, NN-0979, NN-0981 and NN-0977) with mutation close to the Sort_EndoTag-binding site were designed and expressed. For design, the computational models were used as a starting point for the computational screen. All positions 10 Å away from the interface were screened using RosettaMatch51 followed by a design step to introduce the NXS/T motif into the protein. Computational models were minimized and filtered based on geometrical restraints, CST-score <5. Next, the four variants were used as bait in the yeast display assay against the computational designed binder, Sort_EndoTag, which was displayed on the surface of yeast.
为了验证所设计结合物的表位,进行了 N-连接聚糖扫描。执行此操作是为了快速确定计算设计的 binder 是否与所选界面50 交互。设计并表达了四种基因化的 N-连接糖变体 (NN-0975 、 NN-0979 、 NN-0981 和 NN-0977),其突变靠近 Sort_EndoTag 结合位点。在设计方面,计算模型被用作计算屏幕的起点。使用 RosettaMatch51 筛选距离界面 10 Å 的所有位置,然后进行设计步骤,将 NXS/T 基序引入蛋白质中。计算模型根据几何约束、CST 评分 <5 进行最小化和过滤。接下来,在酵母展示测定中,将四种变体用作诱饵,针对计算设计的结合物 Sort_EndoTag,该结合物显示在酵母表面。
Yeast surface display screening with FACS
使用 FACS 进行酵母表面显示筛选
The yeast surface display screening was performed as described15,17. In brief, DNAs encoding the minibinder sequences were transformed into EBY-100 yeast strain. The yeast cells were grown in CTUG medium and induced in SGCAA medium. After washing with PBSF (PBS + 1% BSA), the cells were incubated with 1 μM biotinylated target proteins (IGF2R, ASGPR or sortilin) together with Streptavidin–phycoerythrin (SAPE, Thermo Fisher, 1:100) and anti-Myc fluorescein isothiocyanate (FITC, Miltenyi Biotech, 6.8:100) for 30 min. After washing twice with PBSF, the yeast cells were then resuspended in PBSF and screened via FACS. Only cells with PE and FITC double-positive signals were sorted for next-round screening. After another round of enrichment, the cells were titrated with biotinylated target protein at different concentrations for 30 min, washed, and further stained with both Streptavidin–phycoerythrin (SAPE, Thermo Fisher) and anti-Myc fluorescein isothiocyanate (FITC, Miltenyi Biotech) at 1:100 ratio for 30 min. After washing twice with PBSF, the yeast cells at different concentrations were sorted individually via FACS and regrown for 2 days. Next the cells from each subpool were lysated and their sequences were determined next-generation sequencing or MiSeq. FACS data were collected with the Sony SH800 software suite.
酵母表面显示筛选按所述进行15,17。简而言之,编码 minibinder 序列的 DNA 被转化到 EBY-100 酵母菌株中。酵母细胞在 CTUG 培养基中生长,并在 SGCAA 培养基中诱导。用 PBSF (PBS + 1% BSA) 洗涤后,将细胞与 1 μM 生物素化靶蛋白(IGF2R、ASGPR 或 sortilin)以及链霉亲和素-藻红蛋白(SAPE,Thermo Fisher,1:100)和抗 Myc 异硫氰酸荧光素(FITC,Miltenyi Biotech,6.8:100)一起孵育 30 分钟。用 PBSF 洗涤两次后,将酵母细胞重悬于 PBSF 中并通过 FACS 筛选。仅对具有 PE 和 FITC 双阳性信号的细胞进行下一轮筛选。另一轮富集后,用不同浓度的生物素化靶蛋白滴定细胞 30 分钟,洗涤,并进一步用链霉亲和素-藻红蛋白(SAPE,Thermo Fisher)和抗 Myc 异硫氰酸荧光素(FITC,Miltenyi Biotech)以 1:100 的比例染色 30 分钟。用 PBSF 洗涤两次后,通过 FACS 单独分选不同浓度的酵母细胞并重新生长 2 天。接下来,裂解来自每个子池的细胞,并使用下一代测序或 MiSeq 确定其序列。FACS 数据是使用 Sony SH800 软件套件收集的。
For N-linked glycan verification, yeast cells displaying Sort_EndoTag were incubated with 100nM N-glycan variants of sortilin (NN-0975, NN-0979, NN-0981 and NN-0977), separately. The percentage of yeast cells located within the pre-set gate was calculated for each N-glycan variants group and compared with the wild-type sortilin group.
对于 N-连接聚糖验证,显示 Sort_EndoTag 的酵母细胞分别与 sortilin 的 100 nM N-糖变体(NN-0975、NN-0979、NN-0981 和 NN-0977)一起孵育。计算每个 N-糖变体组位于预设门内的酵母细胞的百分比,并与野生型 sortilin 组进行比较。
Biolayer interferometry 生物膜干涉测量法
The binding affinity for the minibinders were determined using an Octet RED96 (ForteBio). To measure the binding affinity, Streptavidin-coated biosensors (ForteBio) were first loaded with biotinylated target proteins at 50–100 nM concentration, washed with Octet buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.05% surfactant P20 and 1% BSA), and incubated with titrated concentrations of corresponding binders. To measure the off rate (Koff), the biosensors were then dipped back into the Octet buffer. The on rate (Kon), Koff and Kd were further estimated with the Octet Analysis software.
使用 Octet RED96 (ForteBio) 测定微结合剂的结合亲和力。为了测量结合亲和力,首先用 50–100 nM 浓度的生物素化靶蛋白加载链霉亲和素包被的生物传感器 (ForteBio),用八位字节缓冲液(10 mM HEPES、150 mM NaCl、3 mM EDTA、0.05% 表面活性剂 P20 和 1% BSA)洗涤,并与滴定浓度的相应结合剂一起孵育。为了测量关闭速率 (Koff),然后将生物传感器浸回 Octet 缓冲液中。使用 Octet 分析软件进一步估计导通率 (Kon)、Koff 和 Kd。
Protein production and purification
蛋白质生产和纯化
Minibinders and minibinder fusions were expressed in E. coli BL21 as previously described1. In brief, the DNA fragments encoding the design sequences were assembled into PET-29 vectors via Gibson assembly and further transformed into BL21 strain with heat-shock. Protein expression was induced by the autoinduction system and proteins were purified with Immobilized metal affinity chromatography (IMAC) approach. Next the elutions were purified by FPLC SEC using Superdex 75 10/300 GL column (GE Healthcare). Protein concentrations were determined by NanoDrop (Thermo Scientific) and normalized by extinction coefficients.
如前所述,Minibinders 和 Minibinder 融合物在大肠杆菌 BL21 中表达1.简而言之,编码设计序列的 DNA 片段通过 Gibson 组装组装成 PET-29 载体,并通过热休克进一步转化为 BL21 菌株。通过自动诱导系统诱导蛋白质表达,并使用固定化金属亲和层析 (IMAC) 方法纯化蛋白质。接下来,使用 Superdex 75 10/300 GL 色谱柱 (GE Healthcare) 通过 FPLC SEC 纯化洗脱液。蛋白质浓度通过 NanoDrop (Thermo Scientific) 测定,并通过消光系数归一化。
Antibody–EndoTag fusions were produced with a mammalian expression system. Light chain of CTX/ATZ antibody and heavy chain fused with EndoTag at C-terminal constructs were ordered in CMVR from Genscript. Antibody–EndoTag fusions were then expressed via transient co-transfection of the EndoTag-heavy and light chains into Expi293F cells (Life Technologies) via PEI-MAX (Polyscience). In brief, 800 ml cultures of Expi293F cells were transfected at a density of 3 × 106 cells per millilitre of culture using 1 μg plasmid DNA and 3 μg PEI per millilitre of culture. These cultures were grown in Expi293F expression medium (Life Technologies) at 37 °C in a humidified, 8% CO2 incubator rotating at 125 rpm.
抗体-EndoTag 融合物是使用哺乳动物表达系统产生的。CTX/ATZ 抗体的轻链和在 C 端构建体处与 EndoTag 融合的重链从金斯瑞订购。然后通过 PEI-MAX (Polyscience) 将 EndoTag 重链和轻链瞬时共转染到 Expi293F 细胞 (Life Technologies) 中,表达抗体-EndoTag 融合。简而言之,使用 1 μg 质粒 DNA 和每毫升培养物 3 μg PEI,以每毫升培养物 3 × 10 6个细胞的密度转染 800 ml Expi293F 细胞培养物。这些培养物在 Expi293F 表达培养基 (Life Technologies) 中于 37 °C 下在加湿的 8% CO2 培养箱中以 125 rpm 旋转培养。
After 6 days of expression, culture supernatants were harvested via 5 min of centrifugation at 4,000g, 5 min of incubation with PDADMAC solution (Sigma Aldrich) added to a final concentration of 0.0375%, followed by an additional 5 min of centrifugation at 4,000g. Supernatants were clarified via 0.22-μm vacuum filtration and then treated to a final concentration of 50 mM Tris-HCl (pH 8) and 350 mM NaCl for IMAC. Gravity IMAC was performed by batch binding the clarified supernatants with 10 ml of Ni Sepharose Excel resin (GE Healthcare). After 20–30 min of incubation, the resin bed was washed with 10 column volumes of 20 mM Tris-HCl (pH 8), 300 mM NaCl solution. The proteins were then eluted with 3 column volumes of 20 mM Tris-HCl (pH 8), 300 mM NaCl, 300 mM imidazole solution. The batch bind process was then repeated with half the amount of resin (5 ml) and the eluates from both batch binds were combined. SDS–PAGE was performed on the IMAC eluates to assess purity.
表达 6 天后,通过 4,000g 离心 5 分钟,与 PDADMAC 溶液 (Sigma Aldrich) 孵育 5 分钟至终浓度为 0.0375%,然后再以 4,000g 离心 5 分钟收获培养上清液。通过 0.22 μm 真空过滤澄清上清液,然后处理至终浓度为 50 mM Tris-HCl (pH 8) 和 350 mM NaCl 的 IMAC。通过将澄清的上清液与 10 ml Ni Sepharose Excel 树脂 (GE Healthcare) 批量结合来进行重力 IMAC。孵育 20–30 分钟后,用 10 倍柱体积的 20 mM Tris-HCl (pH 8) 和 300 mM NaCl 溶液洗涤树脂床。然后用 3 倍柱体积的 20 mM Tris-HCl (pH 8)、300 mM NaCl、300 mM 咪唑溶液洗脱蛋白质。然后用一半量的填料 (5 mL) 重复批量结合过程,并将来自两个批次结合的洗脱液混合。对 IMAC 洗脱液进行 SDS-PAGE 以评估纯度。
The purified antibody–EndoTag fusions were subsequently concentrated in a 10 K MWCO Amicon Ultra centrifugal filter unit (Millipore) and polished via SEC using a Hiload 26/600 Superose 200 column (GE Healthcare) in DPBS (Gibco). The SEC fractions were re-concentrated in the same manner as before to a final concentration of 5 mg ml−1. Endotoxin levels were assayed via Endosafe LAL Endotoxin tests (Charles River) and analytical SEC was performed using a Superdex 200 Increase 5/150 column (GE Healthcare) to obtain a high-resolution size profile. Pre- and post-freeze stability was assessed via UV-vis spectrophotometry as well as SDS–PAGE.
纯化的抗体-EndoTag 融合物随后在 10 K MWCO Amicon Ultra 离心过滤装置 (Millipore) 中浓缩,并在 DPBS (Gibco) 中使用 Hiload 26/600 Superose 200 色谱柱 (GE Healthcare) 通过 SEC 精纯。以与之前相同的方式将 SEC 馏分重新浓缩至终浓度为 5 mg ml-1。通过 Endosafe 鲎试剂内毒素检测 (Charles River) 测定内毒素水平,并使用 Superdex 200 Increase 5/150 色谱柱 (GE Healthcare) 进行分析 SEC,以获得高分辨率的尺寸分布。通过 UV-vis 分光光度法和 SDS-PAGE 评估冷冻前和冷冻后的稳定性。
Cellular uptake evaluation and receptor degradation via flow cytometry
通过流式细胞术进行细胞摄取评估和受体降解
For cellular uptake assays using suspension cell lines (K562, Jurkat), the cells were incubated with corresponding fluorescence-labelled protein constructs at 37 °C for indicated time, then spun down at 500g for 5 min, resuspended and washed with cold PBS. After three washes, the cells were resuspended and transferred to a 96-well plate. For cellular uptake assays using adherent cell lines (U-251MG, Hep3B, HeLa and H1975), the cells were incubated with corresponding fluorescence-labelled protein constructs at 37 °C for indicated time, then washed with cold PBS for three times. The cells were then treated with 50 μl trypsin and incubated at 37 °C for 10 min followed by adding 50 μl DMEM. The resuspended cells were then transferred to a 96-well plate followed by 2 PBS washes. Flow cytometry was then performed in Attune NxT flow cytometer (Thermo Fisher). The data were analysed in FlowJo v9 software.
对于使用悬浮细胞系(K562、Jurkat)的细胞摄取测定,将细胞与相应的荧光标记的蛋白质构建体在 37 °C 下孵育指定时间,然后以 500g 离心 5 分钟,重悬并用冷 PBS 洗涤。洗涤 3 次后,将细胞重悬并转移至 96 孔板中。对于使用贴壁细胞系(U-251MG、Hep3B、HeLa 和 H1975)的细胞摄取测定,将细胞与相应的荧光标记的蛋白质构建体在 37 °C 下孵育指定时间,然后用冷 PBS 洗涤 3 次。然后用 50 μl 胰蛋白酶处理细胞,并在 37 °C 下孵育 10 分钟,然后加入 50 μl DMEM。然后将重悬的细胞转移到 96 孔板中,然后进行 2 次 PBS 洗涤。然后在 Attune NxT 流式细胞仪 (Thermo Fisher) 中进行流式细胞术。在 FlowJo v9 软件中分析数据。
For cell surface receptor-degradation experiments, the cells were first incubated with corresponding protein reagents for indicated time at 37 °C, then washed with cold PBS 3 times. For suspension cell lines, the cells were resuspended and transferred to the 96-well plate; for adherent cell lines, the cells were first treated with trypsin for 10 min then transferred to the 96-well plate. The cells were then stained with corresponding fluorescence-labelled antibodies against the corresponding receptor for 1 h at room temperature. After washing three times with cold PBS for flow cytometry, flow cytometry was performed in Attune NxT flow cytometer (Thermo Fisher). The data were analysed in FlowJo v9 software. Representative gating strategy for flow cytometry is provided in Supplementary Figs. 1–17.
对于细胞表面受体降解实验,首先将细胞与相应的蛋白质试剂在 37 °C 下孵育指定时间,然后用冷 PBS 洗涤 3 次。对于悬浮细胞系,将细胞重悬并转移至 96 孔板中;对于贴壁细胞系,首先用胰蛋白酶处理细胞 10 分钟,然后转移至 96 孔板中。然后在室温下用针对相应受体的相应荧光标记抗体对细胞染色 1 小时。用冷 PBS 洗涤 3 次用于流式细胞术后,在 Attune NxT 流式细胞仪 (Thermo Fisher) 中进行流式细胞术。在 FlowJo v9 软件中分析数据。流式细胞术的代表性设门策略在补充图中提供。1-17.
Monitoring protein degradation via western blot
通过 western blot 监测蛋白质降解
Cells were cultured in T75 flasks at 37 °C in a 5% CO2 atmosphere. HEP3B (ATCC), HeLa (ATCC), and MDA-MB-231 were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin. Jurkat-CTLA4 (Promega, JA3001) and H1975 were cultured in RPMI supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin. Adherent cells were plated (100,000 cells per well in a 24-well plate) one day before the experiment, whereas suspension cells were plated on the day of the treatment. Cells were incubated with 250 µl of complete growth media with pLYTAC or untreated controls for indicated time. Cells were then washed with PBS 3 times and lysed with RIPA buffer supplemented with protease inhibitor cocktail (Roche), 0.1% Benzonase (Millipore-Sigma), and phosphatase inhibitor cocktail (Roche) on ice for 30 min. The cells were scraped, transferred to Eppendorf tubes, and spun down at 21,000g for 15 min at 4 °C. The supernatant was collected and the protein concentration was determined by BCA assay (Pierce). Equal amounts of lysates were loaded onto 4–12% Bis-Tris gel and separated by SDS–PAGE. Then, the gel was transferred onto a nitrocellulose membrane and stained with REVERT Total Protein Stain (LI-COR), then blocked with Odyssey Blocking Buffer (TBS) (LI-COR) for 1 h at room temperature. The membrane was incubated with primary antibodies (rabbit anti-EGFR D38B1 Cell Signaling Technologies, rabbit anti-HER2 2242 Cell Signaling Technologies, rabbit anti-PD-L1 E1L3N Cell Signaling Technologies, rabbit anti-CTAL4 E1V6T Cell Signaling Technologies, mouse anti-vinculin V284 Bio-Rad) overnight at 4 °C, washed 3 times with TBST. Subsequently, the membrane was incubated with secondary antibody (800CW goat anti-mouse or goat anti-rabbit LI-COR 926-32211) for 1 h at room temperature, and washed 3 times with TBST for visualization with an Odyssey CLx Imager (LI-COR). Image Studio (LI-COR) was used to quantify band intensities. Full scans of western blot gels are provided in Supplementary Figs. 1–16.
将细胞置于 T75 培养瓶中,在 37 °C 和 5% CO2 气氛中培养。HEP3B (ATCC) 、 HeLa (ATCC) 和 MDA-MB-231 在补充有 10% 热灭活胎牛血清 (FBS) 和 1% 青霉素/链霉素的 DMEM 中培养。Jurkat-CTLA4 (Promega, JA3001) 和 H1975 在补充有 10% 热灭活胎牛血清 (FBS) 和 1% 青霉素/链霉素的 RPMI 中培养。在实验前一天接种贴壁细胞(在 24 孔板中每孔 100,000 个细胞),而悬浮细胞在处理当天接种。将细胞与 250 μl 含 pLYTAC 或未处理对照的完全生长培养基一起孵育指定时间。然后用 PBS 洗涤细胞 3 次,并用补充有蛋白酶抑制剂混合物 (Roche)、0.1% Benzonase (Millipore-Sigma) 和磷酸酶抑制剂混合物 (Roche) 的 RIPA 缓冲液在冰上裂解 30 分钟。刮取细胞,转移至Eppendorf管中,并在4°C下以21,000g离心15分钟。 收集上清液,通过 BCA 测定法 (Pierce) 测定蛋白质浓度。将等量的裂解物上样到 4–12% Bis-Tris 凝胶上,并通过 SDS-PAGE 分离。然后,将凝胶转印到硝酸纤维素膜上,用 REVERT 总蛋白染色剂 (LI-COR) 染色,然后在室温下用 Odyssey 封闭缓冲液 (TBS) (LI-COR) 封闭 1 小时。将膜与一抗(兔抗 EGFR D38B1 Cell Signaling Technologies、兔抗 HER2 2242 Cell Signaling Technologies、兔抗 PD-L1 E1L3N Cell Signaling Technologies、兔抗 CTAL4 E1V6T Cell Signaling Technologies、小鼠抗 Vinculin V284 Bio-Rad)在 4 °C 下孵育过夜,用 TBST 洗涤 3 次。 随后,将膜与二抗 (800CW 山羊抗小鼠或山羊抗兔 LI-COR 926-32211) 在室温下孵育 1 小时,并用 TBST 洗涤 3 次,以便用 Odyssey CLx 成像仪 (LI-COR) 进行可视化。Image Studio (LI-COR) 用于量化条带强度。Supplementary Figs 中提供了 western blot 凝胶的完整扫描。1-16.
Fluorescence imaging 荧光成像
Wild-type HeLa (ATCC CCL-2) were cultured at 37 °C with 5% CO2 in flasks with Dulbecco’s modified Eagle medium (DMEM) (Gibco) supplemented with 1 mM l-glutamine (Gibco), 4.5 g l−1 d-glucose (Gibco), 10% fetal bovine serum (FBS) (Hyclone) and 1% penicillin-streptomycin (PenStrep) (Gibco). To passage, cells were dissociated using 0.05% trypsin EDTA (Gibco) and split 1:5 or 1:10 into a new tissue culture-treated T75 flask (Thermo Scientific ref 156499).
野生型 HeLa (ATCC CCL-2) 在 37 °C 和 5% CO2 的培养瓶中与补充有 1 mM l-谷氨酰胺 (Gibco)、4.5 g l-1d-葡萄糖 (Gibco)、10% 胎牛血清 (FBS) (Hyclone) 和 1% 青霉素-链霉素 (PenStrep) (Gibco) 的 Dulbecco 改良 Eagle 培养基 (DMEM) (Gibco) 一起培养。传代时,使用 0.05% 胰蛋白酶 EDTA (Gibco) 解离细胞,并以 1:5 或 1:10 的比例分流到经组织培养处理的新 T75 培养瓶中(Thermo Scientific 参考文献 156499)。
For imaging 35-mm glass bottom dishes were seeded at a density of 20,000 cells per dish. A final monomeric concentration of 100 nM of ligands were incubated with cultured cells. Cells were fixed 4% paraformaldehyde, permeabilized with 100% methanol, and blocked with PBS + 1% BSA. Cells were immunostained with LAMP2A antibody (Abcam ab18528) followed by goat anti-rabbit IgG Alexa Fluor 488 secondary antibody (Thermo Fisher A-11034) and 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher D1306) and stored in the dark at 4 °C until imaging.
为了成像,以每个培养皿 20,000 个细胞的密度接种 35 mm 玻璃底培养皿。将最终单体浓度为 100 nM 的配体与培养的细胞一起孵育。将细胞固定 4% 多聚甲醛,用 100% 甲醇透化,并用 PBS + 1% BSA 封闭。用 LAMP2A 抗体 (Abcam ab18528) 对细胞进行免疫染色,然后用山羊抗兔 IgG Alexa Fluor 488 二抗 (Thermo Fisher A-11034) 和 4′,6-二脒基-2-苯基吲哚 (DAPI) (Thermo Fisher D1306) 对细胞进行免疫染色,并在 4 °C 避光下储存直至成像。
Cells were washed twice with HBSS and subsequently imaged in HBSS in the dark at 37 °C. Right before imaging, cells were incubated with 25 µM DTZ. Epifluorescence imaging was conducted on a Yokogawa CSU-X1 microscope equipped with a Hamamatsu ORCA-Fusion scientific CMOS camera and Lumencor Celesta light engine. Objectives used were: 10×, NA 0.45, WD 4.0 mm, 20×, NA 1.4, WD 0.13 mm, and 40×, NA 0.95, WD 0.17–0.25 mm with correction collar for cover glass thickness (0.11 mm to 0.23 mm) (Plan Apochromat Lambda). All epifluorescence experiments were subsequently analysed using NIS Elements software.
用 HBSS 洗涤细胞两次,然后在 37 °C 的黑暗中在 HBSS 中成像。 在成像之前,将细胞与 25 μM DTZ 一起孵育。落射荧光成像是在配备滨松 ORCA-Fusion scientific CMOS 相机和 Lumencor Celesta 光引擎的横河电机 CSU-X1 显微镜上进行的。使用的物镜为:10×、NA 0.45、WD 4.0 mm、20×、NA 1.4、WD 0.13 mm 和 40×、NA 0.95、WD 0.17–0.25 mm,带盖玻片厚度(0.11 mm 至 0.23 mm)校正环(平场复消色差 lambda)。随后使用 NIS Elements 软件分析所有落射荧光实验。
Generation of knockout lines
生成敲落线
IGF2R-knockout HeLa cells were a generous gift form S. Banik. SORT1 and TfR KO cells were generated using Gene Knockout Kit v2 (Synthego) using the manufacturer’s protocols.
IGF2R 敲除的 HeLa 细胞是 S. Banik 的慷慨礼物。使用基因敲除试剂盒 v2 (Synthego) 按照制造商的方案生成 SORT1 和 TfR 敲除细胞。
Confocal microscopy 共聚焦显微镜
Indicated cells were seeded in 18-well glass bottom µ-Slides (Ibidi, 81817) at a density of 15,000 cells per well. Fluorescently labelled ligands were incubated with the cultured cells for 0.25, 3, 6 or 24 h. Thirty minutes before image acquisition, cells were additionally incubated with LysoTracker (Thermo Fisher Scientific, L7528, L7526, L12492) was added for 30 min. Fluorescently labelled anti-IGF2R (Novus Biological, NB300-514AF647) was added for 30 min. Cells were washed 3× in PBS and immediately proceeded to imaging.
将指示的细胞以每孔 15,000 个细胞的密度接种在 18 孔玻璃底 μ 玻片 (Ibidi, 81817) 中。将荧光标记的配体与培养的细胞一起孵育 0.25 、 3 、 6 或 24 小时。在图像采集前 30 分钟,将细胞与 LysoTracker(Thermo Fisher Scientific、L7528、L7526、L12492)一起孵育 30 分钟。加入荧光标记的抗 IGF2R (Novus Biological, NB300-514AF647) 30 分钟。在 PBS 中洗涤细胞 3× 并立即进行成像。
Confocal laser scanning microscopy was performed on a Nikon A1R HD25 system equipped with a LU-N4 laser unit (Lasers used: 488 nm, 561 nm, 640 nm). Data were acquired using a 20×, NA 0.75, WD 1.00 mm air objective (Plan Apochromat Lambda) in combination with 1 multialkaline (EM 650 LP) and 2 GaAsP detectors (DM 560 LP EM 524/42 (503-545) and DM 652 EM 600/45 (578-623)). Acquisition was controlled via NIS Elements software and data were analysed via Fiji and custom-written Python scripts.
在配备 LU-N4 激光单元的尼康 A1R HD25 系统上进行共聚焦激光扫描显微镜(使用的激光器:488 nm、561 nm、640 nm)。使用 20×、NA 0.75、WD 1.00 mm 空气物镜 (Plan Apochromat Lambda) 结合 1 个多碱性 (EM 650 LP) 和 2 个 GaAsP 检测器(DM 560 LP、EM 524/42 (503-545) 和 DM 652 EM 600/45 (578-623))获取数据。通过 NIS Elements 软件控制采集,并通过 Fiji 和定制编写的 Python 脚本分析数据。
Mass spectrometry and proteomics
质谱和蛋白质组学
Cell pellets were thawed on ice and lysed in a lysis buffer (400 μl, 1 tablet of Pierce EDTA-free Protease Inhibitor Tablets dissolved in 50 ml of PBS) using a probe sonicator (3× 3 pulses). Protein concentration was adjusted to 2.0 mg ml−1 and the samples (100 μl, 200 μg protein) were transferred to new Eppendorf tubes (1.5 ml) containing urea (48 mg per tube, final urea concentration: 8 M). DTT (5 μl, 200 mM fresh stock in H2O, final DTT concentration: 10 mM) was then added to the tubes and the samples were incubated at 65 °C for 15 min. Following this incubation, iodoacetamide (5 μl, 400 mM fresh stock in H2O, final iodoacetamide concentration: 20 mM) was added and the samples were incubated in the dark at 37 °C with shaking for 30 min. Ice-cold methanol (600 μl), CHCl3 (200 μl), and H2O (500 μl) were then added, and the mixture was vortexed and centrifuged (10,000g, 10 min, 4 °C) to afford a protein disc at the interface between CHCl3 and aqueous layers. The top layer was aspirated without perturbing the disk, additional methanol (600 μl) was added, and the proteins were pelleted (10,000g, 10 min, 4 °C) and used in the next step or stored at −80 °C overnight.
将细胞沉淀在冰上解冻,并使用探针超声仪(3× 3 个脉冲)在裂解缓冲液(400 μl,1 片溶于 50 ml PBS 中的 Pierce 不含 EDTA 的蛋白酶抑制剂片剂)中裂解。将蛋白质浓度调节至 2.0 mg ml-1,并将样品(100 μl,200 μg 蛋白质)转移到含有尿素(每管 48 mg,最终尿素浓度:8 M)的新 Eppendorf 管 (1.5 ml) 中。然后将 DTT(5 μl,200 mM 新鲜储备液,溶于 H2O,最终 DTT 浓度:10 mM)加入试管中,并将样品在 65 °C 下孵育 15 分钟。孵育后,加入碘乙酰胺(5 μl,400 mM 新鲜储备液,溶于 H2O,最终碘乙酰胺浓度:20 mM),并将样品在 37 °C 避光中振荡孵育 30 分钟。然后加入冰冷的甲醇 (600 μl)、CHCl 3 (200 μl) 和 H2O (500 μl),涡旋混合并离心(10,000g,10 分钟,4 °C),以在 CHCl3 和水层之间的界面处形成蛋白质盘。吸出顶层而不扰动转盘,加入额外的甲醇 (600 μl),并将蛋白质沉淀(10,000 g,10 分钟,4 °C)并在下一步中使用或在 -80 °C 下储存过夜。
The resulting protein pellets were resuspended in EPPS buffer (160 μl, 200 mM, pH 8) using probe sonicator (3× 3 pulses). Trypsin (10 μl, 0.5 μg μl−1 in trypsin reconstitute buffer) and CaCl2 (1.8 μl, 100 mM in H2O) were added and the samples were incubated at 37 °C with shaking overnight.
使用探针超声仪(3× 3 个脉冲)将所得蛋白质沉淀重悬于 EPPS 缓冲液(160 μl,200 mM,pH 8)中。加入胰蛋白酶(10 μl,0.5 μg μl-1 胰蛋白酶重构缓冲液中)和 CaCl2(1.8 μl,100 mM 在 H2O 中),并将样品在 37 °C 下振荡孵育过夜。
Peptide concentration was determined using the microBCA assay (Thermo Scientific) according to the manufacturer’s instructions. For each sample, a volume corresponding to 25 μg of peptides was transferred to a new Eppendorf tube and the total volume was brought up to 35 μl with EPPS buffer (200 mM, pH 8). The samples were diluted with CH3CN (9 μl) and incubated with the corresponding TMT tags (3 μl per channel, 20 μg μl−1) at room temperature for 30 min. An additional TMT tag (3 μl per channel, 20 μg μl−1, 30 min) was added and the samples were incubated for another 30 min. Labeling was quenched by the addition of hydroxylamine (6 μl, 5% in H2O). Following a 15 min incubation at room temperature, formic acid was added (2.5 μl, final formic acid concentration: 5%). Twenty microlitres of labelled peptides for each channel were combined into a 2.0 ml low-binding Eppendorf tube, and 25 μl of 20% formic acid was added. The resulting mixture was lyophilized to remove the solvents before high pH fractionation.
根据制造商的说明,使用 microBCA 测定法 (Thermo Scientific) 测定肽浓度。对于每个样品,将相当于 25 μg 肽的体积转移到新的 Eppendorf 管中,并使用 EPPS 缓冲液(200 mM,pH 值 8)将总体积调至 35 μl。用 CH3CN (9 μl) 稀释样品,并与相应的 TMT 标签(每通道 3 μl,20 μg μl-1)在室温下孵育 30 分钟。添加额外的 TMT 标签(每通道 3 μl,20 μg μl-1,30 分钟),并将样品再孵育 30 分钟。通过添加羟胺 (6 μl,5% 的 H2O 溶液) 来淬灭标记。在室温下孵育 15 分钟后,加入甲酸(2.5 μl,最终甲酸浓度:5%)。将每个通道的 20 μL 标记肽合并到 2.0 ml 低结合 Eppendorf 管中,并加入 25 μl 20% 甲酸。将所得混合物冻干以去除溶剂,然后再进行高 pH 分馏。
The spin columns from Pierce High pH Reversed-Phase Peptide Fractionation Kit were pre-equilibrated prior to use. In brief, the columns were placed in Eppendorf tubes (2 ml), spun down to remove the storage solution (5,000g, 2 min), and washed with CH3CN (2× 300 μl, 5,000g, 2 min) and buffer A (2× 300 μl, 95% H2O, 5% CH3CN, 0.1% formic acid, 5,000g, 2 min). TMT-labelled peptides were re-dissolved in buffer A (300 μl, 95% H2O, 5% CH3CN, 0.1% formic acid) and loaded onto pre-equilibrated spin columns for high pH fractionation. The columns were spun down (2,000g, 2 min) and the flow through was used to wash the original Eppendorf tube and passed through the spin column again (2,000g, 2 min). The column was then washed with buffer A (300 μl, 2,000g, 2 min) and 10 mM aqueous NH4HCO3 containing 5% CH3CN (300 μl, 2,000g, 2 min), and the flow through was discarded. The peptides were eluted from the spin column into fresh Eppendorf tubes (2.0 ml) with a series of 10 mM NH4HCO3/CH3CN buffers (2,000g, 2 min). The following buffers were used for peptide elution (CH3CN (%)): 7.5, 10, 12.5, 15, 17.5, 20, 22.5, 25, 27.5, 30, 32.5, 35, 37.5, 40, 42.5, 45, 47.5, 50, 52.5, 55, 57.5, 60, 62.5, 65, 67.5, 70, 72.5, 75, 80 and 95. Every tenth fraction was combined into a new clean Eppendorf tube (2 ml) and the solvent was removed using a benchtop lyophilizer and stored at −20 °C before analysis.
使用前对 Pierce 高 pH 值反相肽分离试剂盒的离心柱进行预平衡。简而言之,将色谱柱置于 Eppendorf 管 (2 ml) 中,离心以除去储存溶液(5,000g,2 分钟),并用 CH3CN(2× 300 μl,5,000g,2 分钟)和缓冲液 A(2× 300 μl,95% H2O,5% CH3CN,0.1% 甲酸,5,000g,2 分钟)。将 TMT 标记的肽重新溶解在缓冲液 A(300 μl、95% H2O、5% CH3CN、0.1% 甲酸)中,并上样到预平衡的离心柱上进行高 pH 分馏。离心色谱柱(2,000g,2 分钟),流出液用于洗涤原始 Eppendorf 管,然后再次通过离心柱(2,000g,2 分钟)。然后用缓冲液 A(300 μl,2,000g,2 分钟)和含有 5% CH3CN(300 μl,2,000g,2 分钟)的 10 mM NH4HCO3 水溶液洗涤色谱柱,弃去流出液。将肽从离心柱洗脱到新鲜的 Eppendorf 管 (2.0 ml) 中,加入一系列 10 mM NH4HCO3/CH3CN 缓冲液(2,000g,2 分钟)。使用以下缓冲液进行肽洗脱 (CH3CN (%)):7.5、10、12.5、15、17.5、20、22.5、25、27.5、30、32.5、35、37.5、40、42.5、45、47.5、50、52.5、55、57.5、60、62.5、65、67.5、70、72.5、75、80 和 95。将每 10 分混合到新的干净 Eppendorf 管 (2 mL) 中,使用台式冻干机除去溶剂,并在分析前储存在 −20 °C 下。
The resulting 10 combined fractions were resuspended in buffer A (25 μl) and analysed on the Orbitrap Fusion mass-spectrometer (4 μl injection volume) coupled to a Thermo Scientific EASY-nLC 1200 LC system and autosampler. The peptides were eluted onto a capillary column (75 μm inner diameter fused silica, packed with C18) and separated at a flow rate of 0.3 μl/min−1 using the following gradient: 5% buffer B in buffer A from 0–10 min, 5%–35% buffer B from 10–129 min, 35%–100% buffer B from 129–130 min, 100% buffer B from 130–139 min, 100%–5% buffer B from 139–140 min, and 5% buffer B from 140–150 min (buffer A: 100% H2O, 0.1% formic acid; buffer B: 20% H2O, 80% CH3CN, 0.1% formic acid). Data were acquired using an MS3-based TMT method. In brief, the scan sequence began with an MS1 master scan (Orbitrap analysis, resolution 120,000, 375 − 1,600 m/z, cycle time 3 s) with dynamic exclusion enabled (repeat count 1, duration 30 s). The top precursors were then selected for MS2/MS3 analysis. MS2 analysis consisted of: quadrupole isolation (isolation window was set to 1.2 for charge state z = 2; 0.7 for charge state z = 3; 0.5 for charge states z = 4–6) of precursor ion followed by collision-induced dissociation (CID) in the ion trap (normalized collision energy 35%, maximum injection time 50 ms, MS2 resolution was set to turbo). Following the acquisition of each MS2 spectrum, synchronous precursor selection (SPS) enabled the selection of MS2 fragment ions for MS3 analysis (SPS isolation window was set to 1.3 for charge state z = 2; 0.7 for charge state z = 3; 0.5 for charge states z = 4–6). MS3 precursors were fragmented by HCD and analysed using the Orbitrap (collision energy 65%, maximum injection time 120 ms). The raw files were converted to mzML files using the MSConvert tool from ProteoWizard (version 3.0.22088). A reverse concatenated, non-redundant variant of the Human UniProt database (29 November 2022) was searched using FragPipe (version 18.0) with the built-in TMT10-MS3 workflow. The virtual references were used for the data sets due to the lack of a pooled sample. The quantified proteins were filtered with false discovery rate < 1% with median centreing normalization. Data are presented as the mean fold change to DMSO-treated controls. n = 3 per group. P values were calculated by a two-tailed unpaired t-test with Welch’s correction.
将所得 10 种混合馏分重悬于缓冲液 A (25 μl) 中,并在 Orbitrap Fusion 质谱仪(进样体积 4 μl)上与 Thermo Scientific EASY-nLC 1200 液相色谱系统和自动进样器联用进行分析。将肽洗脱到毛细管柱(内径为 75 μm 的熔融石英,填充有 C18)上,并使用以下梯度以 0.3 μl/min-1 的流速分离:缓冲液 B 中 5% 缓冲液 B 0-10 min,缓冲液 B 5%-35% 10-129 min,缓冲液 B 35%-100% 129 min, 100% 缓冲液 B 130-139 分钟,100%-5% 缓冲液 B 139-140 分钟,5% 缓冲液 B 140-150 分钟(缓冲液 A:100% H2O,0.1% 甲酸;缓冲液 B:20% H2O,80% CH3CN,0.1% 甲酸)。使用基于 MS3 的 TMT 方法采集数据。简而言之,扫描序列从 MS1 主扫描(Orbitrap 分析,分辨率 120,000,分辨率 375 − 1,600 m/z,循环时间 3 s)开始,启用动态排除(重复计数 1,持续时间 30 s)。然后选择顶部母离子进行 MS2/MS3 分析。MS2分析包括:母离子的四极杆隔离(电荷态z = 2时隔离窗口设置为1.2;电荷态z = 3时隔离窗口设置为0.7;电荷态z = 4–6时设置为0.5),然后在离子阱中进行碰撞诱导解离(CID)(归一化碰撞能量35%,最大进样时间50 ms,MS2分辨率设置为turbo)。采集每个 MS2 谱图后,同步母离子选择 (SPS) 能够选择用于 MS3 分析的 MS2 碎片离子(电荷态 z = 2 时,SPS 隔离窗口设置为 1.3;电荷态 z = 3 时设置为 0.7;电荷态 z = 4–6 时设置为 0.5)。 MS3 前离子体通过 HCD 碎裂,并使用 Orbitrap 进行分析(碰撞能量 65%,最大进样时间 120 ms)。使用 ProteoWizard(版本 3.0.22088)的 MSConvert 工具将原始文件转换为 mzML 文件。使用带有内置 TMT10-MS3 工作流程的 FragPipe(18.0 版)检索了人类 UniProt 数据库的反向连接、非冗余变体(2022 年 11 月 29 日)。由于缺少合并样本,虚拟参考被用于数据集。定量蛋白质过滤,错误发现率< 1%,中位居中归一化。数据表示为 DMSO 处理对照的平均倍数变化。n = 每组 3。P 值通过双尾未配对 t 检验和 Welch 校正计算。
IgG and LHDB supernatant clearance assays
IgG 和 LHDB 上清液清除率测定
Jurkat or K562 cells seeded in 96-well culture plates in 300 μl medium were incubated with AF647-conjugated IgG (Novusbio) or LHDB alone or together with protein G-EndoTag reagents. At various timepoints, the cells were pelleted down and 30 μl of supernatants were extracted and further diluted to 45 μl by using a PBS buffer. After shaking in an orbital shaker for 5 min, the fluorescence intensity was measured using a Neo2 plate reader (BioTek) at wavelength 647 nm. The percentage clearance was measured by normalizing the control group without adding protein G-EndoTag reagent.
将接种在 300 μl 培养基中的 96 孔培养板中的 Jurkat 或 K562 细胞与 AF647 偶联的 IgG (Novusbio) 或 LHDB 单独孵育或与蛋白质 G-EndoTag 试剂一起孵育。在不同时间点,将细胞沉淀下来,提取 30 μl 上清液,并使用 PBS 缓冲液进一步稀释至 45 μl。在轨道摇床中摇动 5 分钟后,使用 Neo2 读板器 (BioTek) 测量波长为 647 nm 的荧光强度。通过对对照组进行归一化而不添加蛋白质 G-EndoTag 试剂来测量清除率百分比。
SEC binding assay SEC 结合测定
ASGPR protein (28.4 kDa) at 1 μM (diluted in PBS) was incubated with 3 μM AS_EndoTag-3C for 30 min and run through an ÄKTA SEC protein purification system using a S200 16/90 column. The absorbance at 230 nm was used as a readout for binding. The SEC traces of the complex was compared to the traces of individual ASGPR or AS_EndoTag-3C at same concentration.
将 1 μM(用 PBS 稀释)的 ASGPR 蛋白 (28.4 kDa) 与 3 μM AS_EndoTag-3C 一起孵育 30 分钟,并使用 S200 16/90 色谱柱通过 ÄKTA SEC 蛋白纯化系统。230 nm 处的吸光度用作结合的读数。将复合物的 SEC 痕量与相同浓度的单个 ASGPR 或 AS_EndoTag-3C 的痕量进行比较。
In vivo mouse study 体内小鼠研究
Mouse lymphoma cell line A20 cell was purchased from American Type Culture Collection (ATCC, TIB-208). The cell was cultured in RPMI-1640 medium (Gibco, Thermo Scientific, 31870074), supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Thermo Scientific), 1× GlutaMAX (Gibco, Thermo Scientific), 1× penicillin/streptomycin solution (Gibco, Thermo Scientific). The cell was cultured at 37 °C in humidified condition with 5% CO2.
小鼠淋巴瘤细胞系 A20 细胞购自美国典型培养物保藏中心 (ATCC, TIB-208)。细胞在 RPMI-1640 培养基(Gibco,Thermo Scientific,31870074),补充有 10% 热灭活胎牛血清 (FBS)(Gibco,Thermo Scientific)、1× GlutaMAX(Gibco,Thermo Scientific)、1× 青霉素/链霉素溶液(Gibco,Thermo Scientific)中培养。将细胞在 37 °C 下加湿条件下,含 5% CO2 培养。
All animal experiments were conducted at the Instituto de Medicina Molecular João Lobo Antunes (IMM), Lisbon. Animal work was performed in strict accordance with Portuguese Law (Portaria 1005/92) and the European Guideline 86/609/EEC and follow the Federation of European Laboratory Animal Science Associations guidelines and recommendations concerning laboratory animal welfare. All animal experiments were approved by the Portuguese official veterinary department for welfare licensing (Direção Geral de Alimentação e Veterinária) and the IMM Animal Ethics Committee (authorization AWB_2021_03_GB_Targ CancerDrugs). Eight-week-old female BALB/c mice (purchased from Charles River) were used in this study, with 5 × 106 A20 cells inoculated subcutaneously in the flank. Tumour growth was monitored over time, by performing bilateral vernier caliper measurements every day and mean tumour volumes were calculated using the formula (length × width2)/2. Treatments were initiated when tumours reached approximately 100 mm3 (approximately 10 days after tumour induction), with the mice been randomly assigned to receive ATZ, ATZ–IGF_EndoTag1 or ATZ–IGF_EndoTag4 and isotype as controls (n = 6 mice per group). Treatments were administered intratumourally in a total of three injections for every three days. Animals were monitored every day; tumours were measured as described before and mouse weight was evaluated throughout the study. Animals were killed whenever reaching humane endpoints: loss of 20% of body weight, breathing impairment, or poor reaction to external stimuli. No signs of animal suffering or discomfort were observed during the experiment. For efficacy study, once control (isotype-treated mice) tumours reached 1,000 mm3, all mice were killed (by isoflurane overdose), and the tumours were removed for western blot analysis. For survival monitor, each mouse was killed respectively when tumours reached 1,000 mm3. The light/dark cycle was 14 h light/10 h dark (lights on at 07:00; lights off at 21:00). The temperature was 20–24 °C and the relative humidity was 55 ± 10%, with controlled supply of HEPA-filtered air provided to individually ventilated cages. Maximum number of animals per cage was five. Social isolation was avoided whenever possible. The type of food was autoclaved diet pellets RM3A (P), from SDS Special Diets Services (801030). Food was placed in a grid inside the cage and provided ad libitum to animals. The type of water was sterile water treated by reverse osmosis. Water was provided ad libitum to animals through bottles with a capillary hole. The data collected was analysed using GraphPad Prism9.
所有动物实验均在里斯本 João Lobo Antunes 分子医学研究所 (IMM) 进行。动物工作严格按照葡萄牙法律 (Portaria 1005/92) 和欧洲准则 86/609/EEC 进行,并遵循欧洲实验动物科学协会联合会关于实验动物福利的准则和建议。所有动物实验均已获得葡萄牙官方兽医部门的福利许可 (Direção Geral de Alimentação e Veterinária) 和 IMM 动物伦理委员会(CancerDrugs AWB_2021_03_GB_Targ授权)的批准。本研究使用 8 周龄雌性 BALB/c 小鼠 (购自 Charles River),在侧腹皮下接种 5 × 10个 6 A20 细胞。通过每天进行双侧游标卡尺测量,监测肿瘤随时间的生长,并使用公式 (长度×宽度2)/2 计算平均肿瘤体积。当肿瘤达到约 100 mm3 时(肿瘤诱导后约 10 天)开始治疗,小鼠被随机分配接受 ATZ、ATZ-IGF_EndoTag1 或 ATZ-IGF_EndoTag4 和同种型作为对照(n = 每组 6 只小鼠)。治疗在瘤内给药,每 3 天总共注射 3 次。每天监测动物;如前所述测量肿瘤,并在整个研究过程中评估小鼠体重。每当达到人道终点时,动物就会被杀死:体重减轻 20%、呼吸障碍或对外部刺激反应不佳。在实验过程中没有观察到动物痛苦或不适的迹象。 对于疗效研究,一旦对照(同种型处理的小鼠)肿瘤达到 1,000 mm3,所有小鼠都被杀死(通过异氟醚过量),并切除肿瘤进行蛋白质印迹分析。对于生存监测器,当肿瘤达到 1,000 毫米3 时,分别杀死每只小鼠。光照/黑暗周期为 14 小时光照/10 小时黑暗(07:00 开灯;21:00 关灯)。温度为 20–24 °C,相对湿度为 55 ± 10%,受控供应 HEPA 过滤空气,提供给单独通风的笼子。每个笼子的最大动物数量为 5 只。尽可能避免社交孤立。该食物类型是来自 SDS Special Diets Services (801030) 的高压灭菌减肥颗粒 RM3A (P)。食物被放置在笼子内的网格中,并随意提供给动物。水类型为反渗透处理的无菌水。通过带有毛细管孔的瓶子随意向动物提供水。使用 GraphPad Prism9 分析收集的数据。
In vivo PD-L1 degradation of tumour samples by western blot
Western blot 对肿瘤样本的体内 PD-L1 降解
Tumour samples isolated from the mice were homogenized, lysed in RIPA buffer containing protease inhibitor (Roche), phosphatase inhibitors (Sigma) and 0.1% Benzonase (Sigma) on ice for 30 min. The lysates were spun at 21,000g for 15 min at 4 °C. The supernatant was collected, and the protein concentrations were quantified using BCA assay (Sigma). Fifty micrograms of protein were loaded per lane and separated on 12% SDS–PAGE gels, and then transferred onto polyvinylidene difluoride (PVDF) membranes (GE Healthcare). Membranes were then blocked with 5% BSA in TBS supplemented with 0.1% Tween-20 (TBST) for 1 h at room temperature, and then probed with following specific primary antibodies at 4 °C overnight. After three times of washing with TBST, secondary antibodies were added to the membrane for 1 h at room temperature. All membranes were washed three times and exposed using ECL substrate (Bio-Rad, 170–5060) and Amersham 800 Imaging System (Cytiva). The primary antibodies used included PD-L1 (sc-518027) and beta-actin (sc-47778), the secondary antibody was goat anti-mouse IgG H&L (HRP) (Abcam, ab205719). The intensities of the bands were quantified by ImageJ.
将从小鼠中分离的肿瘤样品匀浆,在含有蛋白酶抑制剂 (Roche) 、磷酸酶抑制剂 (Sigma) 和 0.1% 苯佐酶 (Sigma) 的 RIPA 缓冲液中在冰上裂解 30 分钟。将裂解物在 4 °C 下以 21,000g 离心 15 分钟。 收集上清液,并使用 BCA 测定 (Sigma) 定量蛋白质浓度。每个泳道上样 50 μg 蛋白质,并在 12% SDS-PAGE 凝胶上分离,然后转移到聚偏二氟乙烯 (PVDF) 膜 (GE Healthcare) 上。然后在室温下用补充有 0.1% Tween-20 (TBST) 的 TBS 中的 5% BSA 封闭膜 1 小时,然后在 4 °C 下使用以下特异性一抗探测过夜。用 TBST 洗涤 3 次后,在室温下将二抗加入膜 1 小时。所有膜洗涤 3 次,并使用 ECL 底物 (Bio-Rad, 170–5060) 和 Amersham 800 成像系统 (Cytiva) 曝光。使用的一抗包括 PD-L1 (sc-518027) 和 β-肌动蛋白 (sc-47778),二抗是山羊抗小鼠 IgG H&L (HRP) (Abcam, ab205719)。条带的强度由 ImageJ 量化。
Statistical analysis 统计分析
No statistical analysis was used to determine the sample size. The sample size was determined by our ability to detect meaningful differences between treatments. Western blot experiments in vitro and BLI binding assays were conducted with sample size of one based on low variance from previous experience. Western blot experiments in mice were performed with sample size of 3 to reduce the variance of protein level across animals from our previous best practice. The data were collected as biological replicates as indicated in the figure legend. All cell experiments were done multiple times to ensure reproducibility. All images were representative of three independently replicated samples. Statistical analyses are specified in figure legends.
未使用统计分析来确定样本量。样本量取决于我们检测治疗之间有意义差异的能力。体外 western blot 实验和 BLI 结合测定以 1 的样本量进行,这是基于与以前经验的低方差。在小鼠中进行蛋白质印迹实验,样本量为 3,以减少动物之间蛋白质水平与我们之前的最佳实践的差异。数据以生物学重复的形式收集,如图例所示。所有细胞实验均进行多次以确保可重复性。所有图像均代表三个独立复制的样本。统计分析在图例中指定。
Reporting summary 报告摘要
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
有关研究设计的更多信息,请参阅本文链接的 Nature Portfolio Reporting Summary。
Data availability 数据可用性
The raw data for the flow cytometry, next-generation sequencing, designed binder models and sequences are available at https://doi.org/10.5281/zenodo.11002950 (ref. 52). Source data are provided with this paper.
流式细胞术、下一代测序、设计的结合物模型和序列的原始数据可在 https://doi.org/10.5281/zenodo.11002950 上获得(参考文献 52)。源数据随本文提供。
Code availability 代码可用性
The Rosetta modelling suite (https://www.rosettacommons.org) is available to academic and non-commercial users for free. Commercial licences for the suite are also available through the University of Washington Technology Transfer Office. The source code for RIF docking is available at https://github.com/rifdock/rifdock. The source code for protein inpainting is available at https://github.com/RosettaCommons/RFDesign. The source code for ProteinMPNN is available at https://github.com/dauparas/ProteinMPNN. The scripts used in this paper for binder design applying the above codes, customized image processing and data processing are available at https://doi.org/10.5281/zenodo.11002950 (ref. 52).
Rosetta 建模套件 (https://www.rosettacommons.org) 免费提供给学术和非商业用户。该套件的商业许可证也可通过华盛顿大学技术转让办公室获得。RIF 停靠的源代码可在 https://github.com/rifdock/rifdock 获取。蛋白质修复的源代码可在 https://github.com/RosettaCommons/RFDesign 获得。ProteinMPNN 的源代码可在 https://github.com/dauparas/ProteinMPNN 获取。本文用于应用上述代码的活页夹设计、自定义图像处理和数据处理的脚本可在 https://doi.org/10.5281/zenodo.11002950 中找到(参考文献 52)。
References 引用
Banik, S. M. et al. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 584, 291–297 (2020).
Banik, SM 等人。用于降解细胞外蛋白的溶酶体靶向嵌合体。自然584, 291–297 (2020)。Ahn, G. et al. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat. Chem. Biol. 17, 937–946 (2021).
Ahn, G. et al. 参与唾液酸糖蛋白受体进行靶向蛋白质降解的 LYTAC。Nat. Chem. Biol.17, 937–946 (2021 年)。Pance, K. et al. Modular cytokine receptor-targeting chimeras for targeted degradation of cell surface and extracellular proteins. Nat. Biotechnol. 41, 273–281 (2023).
Pance, K. 等人。模块化细胞因子受体靶向嵌合体,用于细胞表面和细胞外蛋白的靶向降解。Nat. 生物技术。41, 273–281 (2023 年)。Sorkin, A. & von Zastrow, M. Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622 (2009).
Sorkin, A. & von Zastrow, M. 内吞作用和信号传导:交织在一起的分子网络。Nat. Rev. Mol. 细胞生物学10, 609–622 (2009 年)。Gao, H., Shi, W. & Freund, L. B. Mechanics of receptor-mediated endocytosis. Proc. Natl Acad. Sci. USA 102, 9469–9474 (2005).
Kaksonen, M. & Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 19, 313–326 (2018).
Harris, L. K. & Westwood, M. Biology and significance of signalling pathways activated by IGF-II. Growth Factors 30, 1–12 (2012).
Ahn, G. et al. Elucidating the cellular determinants of targeted membrane protein degradation by lysosome-targeting chimeras. Science 382, eadf6249 (2023).
Leader, B., Baca, Q. J. & Golan, D. E. Protein therapeutics: a summary and pharmacological classification. Nat. Rev. Drug Discov. 7, 21–39 (2008).
Marei, H. et al. Antibody targeting of E3 ubiquitin ligases for receptor degradation. Nature 610, 182–189 (2022).
Cotton, A. D., Nguyen, D. P., Gramespacher, J. A., Seiple, I. B. & Wells, J. A. Development of antibody-based PROTACs for the degradation of the cell-surface immune checkpoint protein PD-L1. J. Am. Chem. Soc. 143, 593–598 (2021).
Nykjaer, A. & Willnow, T. E. Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci. 35, 261–270 (2012).
Gammella, E., Buratti, P., Cairo, G. & Recalcati, S. The transferrin receptor: the cellular iron gate. Metallomics 9, 1367–1375 (2017).
Gustafsen, C. et al. Sortilin and SorLA display distinct roles in processing and trafficking of amyloid precursor protein. J. Neurosci. 33, 64–71 (2013).
Cao, L. et al. Design of protein binding proteins fromthe target structure alone. Nature 605, 551–560 (2022).
Cao, L. et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science 370, 426–431 (2020).
Chevalier, A. et al. Massively parallel de novo protein design for targeted therapeutics. Nature 550, 74–79 (2017).
Leloup, N. et al. Low pH-induced conformational change and dimerization of sortilin triggers endocytosed ligand release. Nat. Commun. 8, 1708 (2017).
Gatter, K. C., Brown, G., Trowbridge, I. S., Woolston, R. E. & Mason, D. Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J. Clin. Pathol. 36, 539–545 (1983).
Sahtoe, D. D. et al. Transferrin receptor targeting by de novo sheet extension. Proc. Natl Acad. Sci. USA 118, e2021569118 (2021).
Dauparas, J. et al. Robust deep learning–based protein sequence design using ProteinMPNN. Science 378, 49–56 (2022).
Sahtoe, D. D. et al. Reconfigurable asymmetric protein assemblies through implicit negative design. Science 375, eabj7662 (2022).
Wang, R., Qi, X., Schmiege, P., Coutavas, E. & Li, X. Marked structural rearrangement of mannose 6-phosphate/IGF2 receptor at different pH environments. Sci. Adv. 6, eaaz1466 (2020).
Wang, J. et al. Scaffolding protein functional sites using deep learning. Science 377, 387–394 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Cullen, P. J. & Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696 (2018).
Zhang, H. et al. Covalently engineered nanobody chimeras for targeted membrane protein degradation. J. Am. Chem. Soc. 143, 16377–16382 (2021).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Brown, J., Jones, E. Y. & Forbes, B. E. Keeping IGF-II under control: Lessons from the IGF-II–IGF2R crystal structure. Trends Biochem. Sci 34, 612–619 (2009).
Sharma, S. V., Bell, D. W., Settleman, J. & Haber, D. A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 7, 169–181 (2007).
Steiner, P. et al. Tumor growth inhibition with cetuximab and chemotherapy in non–small cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor. Clin. Cancer Res. 13, 1540–1551 (2007).
Doroshow, D. B. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 18, 345–362 (2021).
Ryu, R. & Ward, K. E. Atezolizumab for the first-line treatment of non-small cell lung cancer (NSCLC): current status and future prospects. Front. Oncol. 8, 277 (2018).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Walker, L. S. K. & Sansom, D. M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 11, 852–863 (2011).
Yang, W. et al. Design of high affinity binders to convex protein target sites. Preprint at bioRxiv https://doi.org/10.1101/2024.05.01.592114 (2024).
Piraner, D. I. et al. Engineered receptors for soluble cell-to-cell communication. Preprint at bioRxiv https://doi.org/10.1101/2024.09.17.613377 (2024).
Elkon, K. & Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 4, 491–498 (2008).
Akerström, B., Brodin, T., Reis, K. & Björck, L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J. Immunol. 135, 2589–2592 (1985).
Lajoie, M. J. et al. Designed protein logic to target cells with precise combinations of surface antigens. Science 369, 1637–1643 (2020).
Langan, R. A. et al. De novo design of bioactive protein switches. Nature 572, 205–210 (2019).
Yeh, A. H.-W. et al. De novo design of luciferases using deep learning. Nature 614, 774–780 (2023).
Silva, D.-A. et al. De novo design of potent and selective mimics of IL-2 and IL-15. Nature 565, 186–191 (2019).
Murphy, J. E., Padilla, B. E., Hasdemir, B., Cottrell, G. S. & Bunnett, N. W. Endosomes: a legitimate platform for the signaling train. Proc. Natl Acad. Sci. USA 106, 17615–17622 (2009).
Debacker, A. J., Voutila, J., Catley, M., Blakey, D. & Habib, N. Delivery of oligonucleotides to the liver with GalNAc: from research to registered therapeutic drug. Mol. Ther. 28, 1759–1771 (2020).
Neoleukin Therapeutics. Neoleukin Therapeutics Announces Third Quarter 2022 Financial Results and Corporate Update. GlobeNewswire https://www.globenewswire.com/en/news-release/2022/11/14/2555436/0/en/Neoleukin-Therapeutics-Announces-Third-Quarter-2022-Financial-Results-and-Corporate-Update.html (2022).
Quistgaard, E. M. et al. Revisiting the structure of the Vps10 domain of human sortilin and its interaction with neurotensin. Protein Sci. 23, 1291–1300 (2014).
Cheng, Y., Zak, O., Aisen, P., Harrison, S. C. & Walz, T. Structure of the human transferrin receptor–transferrin complex. Cell 116, 565–576 (2004).
Greisen, P. Jr et al. Computational design of N-linked glycans for high throughput epitope profiling. Protein Sci. 32, e4726 (2023).
Zanghellini, A. et al. New algorithms and an in silico benchmark for computational enzyme design. Protein Sci. 15, 2785–2794 (2006).
Huang, B. Designed endocytosis inducing proteins degrade targets and amplify signals. Zenodo https://doi.org/10.5281/zenodo.11002950 (2024).
Acknowledgements
The project or effort depicted was or is sponsored by the Department of Defense, Defense Threat Reduction Agency grant HDTRA1-21-1-0007 (B.H. and L.S.); DARPA Synergistic Discovery and Design (SD2) HR0011835403 contract FA8750-17-C-0219 (W.Y.) and Defense Threat Reduction Agency Grant HDTRA1-21-1-0038 (I.G. and W.Y.). This research was supported by the National Institutes of Health’s National Institute on Aging, grant R01AG063845 (B.H., B.C., I.G. and W.Y.); the National Institutes of Health’s National Cancer Institute, grant R01CA240339 (I.G.); the Audacious Project at the Institute for Protein Design (L.S., X.W., T.S., I.S. and S.W.); the Nordstrom Barrier Institute for Protein Design Directors Fund (B.H., I.G. and M. Abedi); AMGEN Donation to the Institute for Protein Design (S.W. and X.W.); The Open Philanthropy Project Improving Protein Design Fund (B.C. and I.G.); E. Schmidt, W. Schmidt and Schmidt Futures funding from E. Schmidt and W. Schmidt by recommendation of the Schmidt Futures programme (I.G.); the European Molecular Biology Organization via ALTF191-2021 (T.S.); NIH grant GM058867 (C.R.B.); and the Jane Coffin Childs Memorial Fund for Medical Research (M. Abedi). This research was also funded by National Science Foundation Graduate Research Fellowship and Stanford Center for Molecular Analysis and Design (G.A.). We acknowledge the excellent support from the Biology Imaging Facility at the University of Washington during the confocal imaging experiments. The authors thank M. Gloegl for help with the SPR experiment setup; M. Exposit for support with the OT-2 liquid handler; X. Li for help with mass spectrometry analysis of proteins; B. Wicky, L. Milles and R. Ragotte for optimizing the golden gate assembly protocol; A.Coubet for the analysis of preliminary cryo-electron microscopy (cryo-EM) data; D. Lee for transfection of secretable EGFR-IGF_EndoTag; S. Thompson for bridging the connection of the main collaborators in this project; the Banik laboratory for providing the IGF2R-knockout cell line; and I. Haydon for help with graphics. Figures 2b–g and 4a,c include elements created with BioRender.com.
Author information
Authors and Affiliations
Contributions
B.H., M. Abedi, G.A. and D.B. designed the research. B.H. designed, screened and optimized the binders for IGF2R and ASGPR. B.H. and I.S. designed and screened the rigid IGF_EndoTags. P.V. ran partial diffusion for ASGPR binders. B.C. designed and optimized the binders for sortilin. I.G. screened and optimized the binders for sortilin. J.O., Y.L., R.Y., Y.L., A. Misquith, A.W. and P.G. modelled, characterized and produced the N-glycan variants for sortilin. B.C. and L. Cao developed the Rifdock binder design pipeline. N.R.B. set up the PPI MPNN and AlphaFold2 pipeline for computational binder design. B.H. and M. Abedi studied the endocytosis enhancement effect in vitro. B.H., M. Abedi and G.A. evaluated the protein-degradation function of all EndoTags. M. Abedi, J.Z.Z. and T.S. performed the imaging experiments. M. Abedi, D.I.P., A.C.-G., M.J.D.G. and K.T.R. developed the SNIPR platform. W.Y. developed the CTLA4mb. W.Y. and X.W. contributed to binder design, library preparation and assay development. M. Abedi evaluated the application of EndoTag for signalling activation. M. Abedi evaluated the co-LOCKR AND gate degrader and secretable degrader. R.W. prepared the IGF2R target protein with advice from X.L. R.W. and S.W. performed the cryo-EM experiment and collected the cryo-EM density map. S.S. wrote the image analysis script used for imaging. Y.W. performed the proteomics analysis for whole-cell protein degradation. L.M.T. and J.B.S. characterized the antibody fusion of pLYTACs. D.D.S. generated the design basis of the TfR_EndoTag. C.T. and G.J.L.B. designed the in vivo anti-tumour experiments. C.T. conducted the in vivo experiments and data collection. B.H., C.W.C, S.C. and S.G produced and purified the protein used in the research. D.D.S. optimized the TfR_EndoTag. G.A., M. Abedi, M. Ahlrichs and C.D. prepared the cells used for this study. L. Carter and L.S. coordinated the resources and funding required for the research. C.R.B. and D.B. supervised this research. B.H., M. Abedi, G.A. and D.B. wrote the manuscript with the input from the other authors. All authors analysed data and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.H., M. Abedi, G.A., I.S., L.S. and D.B. are co-inventors on a provisional patent application that incorporates discoveries described in this manuscript. B.C., I.G., J.O., P.G., L.S. and D.B. are co-inventors on a provisional patent application that incorporates discoveries described in this manuscript.
Peer review
Peer review information
Nature thanks Felipe de Sousa e Melo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Binder design strategy and epitope selection.
a, Rifdock-based binder design pipeline. b, Selected target region for IGF2R D6, yellow region highlighted the selected residues for rifdock. c, Design model for D6mb in complex with IGF2R D6. d, Selected target region for IGF2R D11, yellow region highlighted the selected residues for rifdock. e, Design model for D11mb in complex with IGF2R D11. f, Selected target region for Sortilin, yellow region highlighted the selected residues for rifdock. g, Design model for Sort_EndoTag (green) in complex with Sortilin. h, Selected target region for ASGPR orthogonal binding sites, yellow region highlighted the selected residues for rifdock. i, Design model for ASmb1 in complex with ASGPR.
Extended Data Fig. 2 Binding affinity measurement for IGF2R and ASGPR minibinders.
a, BLI binding affinity measurement for D6mb against IGF2R D6. b, BLI binding affinity measurement for D11mb against IGF2R D11. c, BLI binding affinity measurement for EndoTag2 against IGF2R D6 (left) and D11 (right). d, BLI binding affinity measurement for EndoTag3 against IGF2R D6 (left) and D11 (right). e, BLI binding affinity measurement for EndoTag4 against IGF2R D6 (left) and D11 (right). f, BLI binding affinity measurement for ASmb1 against ASGPR. g, BLI binding affinity measurement for Sort_EndoTag against Sortilin. All affinity data was collected by Octet R8 and binding affinity is estimated by Octet ForteBio software package.
Extended Data Fig. 3 Computational design strategy to make rigid IGF_EndoTags (EndoTag3 and EndoTag4).
Starting from the structure of IGF-2 in complex with IGF2R, de novo minibinders were generated and screened against the IGF-2 binding sites at IGF2R domain 6 and domain 11, separately. Individual binders for each domain (D6mb and D11mb) were fused with flexible linkers or a rigid fusion interdomain connection. For flexible fusion, multiple linker lengths and fusion directions were sampled. For rigid fusion, the two major binding helices from D6mb and one major binding helix from D11mb were extracted as starting motifs. With protein Inpainting, geometries and fusion orders were sampled, and ranked based on Rosetta and alphafold2 metrics.
Extended Data Fig. 4 Cellular uptake evaluation IGF_EndoTags.
a, Cellular uptake comparison between homodimer and heterodimer fusion of D6mb and D11mb. b, Cellular uptake comparison of linker length of D11mb-D6mb fusion with various lengths of GS linkers in the middle. c, Cellular uptake of IGF_EndoTags. Jurkat cells were treated with biotinylated 100 nM IGF_EndoTags or IGF-2, and 33 nM Strapavidin-AF647 for 24 h. After washing 3 times, the cellular uptake was measured by flow cytometry. The data were normalized with the control group treated with 33 nM Strapavidin-AF647 alone. The data was collected as mean values ± SEM across n = 3 biologically independent samples. d, Fluorescence Microscopy imaging of IGF-2 co-localized with lysosomal. e, Fluorescence Microscopy imaging of EndoTag2 co-localized with lysosomal. f, Fluorescence Microscopy imaging of EndoTag4 co-localized with lysosomal. For a, and b, 200 nM biotinylated fusion proteins were incubated with 50 nM of Strapavidin-AF647 and incubated with Jurkat cells for 24 h. After wash 3 times with cold PBS, the cellular uptake was measured by flow cytometry. For d-f, HeLa cells were incubated with 100 nM of biotinylated IGF-2, EndoTag2 or EndoTag4 for various time length. After cells were washed twice and fixed, they were stained with anti-LAMP2A antibody followed by goat secondary anti-IgG Alexa Fluo 488 antibody and DAPI. Epifluorescence imaging was conducted on a Yokogawa CSU-X1 microscope. These images are representative of three independently replicated samples per time point.
Extended Data Fig. 5 Receptor degradation with EndoTags.
a, Levels of EGFR after treatment with 10 nM or 100 nM CTX-M6P or CTX-IGF_EndoTag1 in H1975 cells for 48 h. b, Levels of EGFR after treatment with 100 nM EGFRn or EGFRn-IGF_EndoTags in H1975 cells for 48 h. c, Levels of CTLA4 with 200 nM of CTLA4mb or CTLA4mb-IGF_EndoTag1 in Jurkat-CTLA4 cells after treatment for 3 h. d, Fold change in abundance of EGFR with treatment of EGFRn compared with control (untreated group). e, Fold change in abundance of EGFR with treatment of EGFRn-IGF_EndoTag1 compared with control (untreated group). f, Fold change in abundance of EGFR with treatment of CTX compared with control (untreated group). g, Fold change in abundance of EGFR with treatment of CTX-IGF_EndoTag1 compared with control (untreated group). h, Flow cytometry analysis of surface PD-L1 levels in MDA-MB-231 cells after treatment with 200 nM ATZ or ATZ-pLYTACs. MFI was normalized by the PD-L1 level of untreated groups. The data was collected as mean values ± SEM across n = 3 biologically independent samples. i, Functional EGF signaling assay. Hela WT cells were pre-treated with 100 nM of EGFRn, EGFRn-IGF_EndoTag2 or PBS control for 24 h, and then washed and stimulated with 100 nM of human EGF for 15 min followed by phosphorylation flow cytometry using anti-pERK AF-488. Data represents mean of biological triplicates and error bar indicates standard deviation. P values were determined by unpaired two-tailed t-test. For d-g, the proteomic data was collected in H1975 cells with the treatment 100 nM of corresponding reagents for 48 h and data collected is the replicated with sample size n = 2. P values were calculated by a two-tailed unpaired t-test with Welch’s correction.
Extended Data Fig. 6 Clearance of soluble proteins with EndoTags.
a, LHDB-AF647 cellular uptake ability comparison among flexible and rigid LHDA-IGF_EndoTags in K562 cells. b, LHDB-AF647 cellular uptake ability comparison among flexible and rigid LHDA-IGF_EndoTags in Jurkat cells. c, IgG-AF647 cellular uptake ability comparison between flexible and rigid designs. d, Quantitative clearance of IgG-AF647 in cell media comparison between flexible and rigid designs. e, IgG-AF647 cellular uptake ability comparison between pLYTACs and M6Pn. f, Quantitative clearance of IgG-AF647 in cell media comparison between pLYTACs and M6Pn. g, Quantitative clearance of IgG-AF647 in cell media with titrated proteinG-EndoTag3. h, Quantitative clearance of IgG-AF647 in cell media with titrated proteinG-EndoTag4. For a,b, cells were incubated with 100 nM LHDB-biotin + 33 nM Strapavidin-AF647 with/without 500 nM LHDB-pLYTACs for 48 h, washed twice with cold PBS and analyzed by flow cytometry. The fold change in MFI (mean fluorescence intensity) was calculated by normalizing the LHDB-AF647 alone group. For c,e, cells were incubated with 33 nM IgG-AF647 with/wihtout 1uM proteinG-IGF_EndoTags for 24 h, washed twice with cold PBS and analyzed by flow cytometry. The fold change in MFI (mean fluorescence intensity) was calculated by normalizing the IgG-AF647 alone group. For a,b,c,e, data are presented as mean values ± SEM with biologically replicates with n = 3. For d,f,g,h, cells were incubated with 33 nM IgG-AF647 with/without proteinG-IGF_EndoTags. At timepoints 24 h, 48 h, the cells were pelleted down, and supernatant IgG-AF647 levels were quantified by Neo2 plate reader. The percentage of IgG-AF647 level was normalized with the IgG-AF647 alone control group. For d,f,g,h, data are presented as mean values ± SEM with biologically replicates with n = 3. For a-c,e, p values were determined by unpaired two-tailed t-test.
Extended Data Fig. 7 Orthogonality of EndoTag.
a, Confocal imaging of biotinylated IGF-2 labeled with AF-555-Streptavidin (green). Hela cells were pre-incubated with 100 nM non-labelled IGF_EndoTags, PBS control for 24 h. After washing with PBS cells were incubated with AF-555 labeled Streptavidin together with biotinylated IGF-2 (the mix was preincubated for 10 min) for 4 h. Cells were washed and lysosomes were stained with AF488-labeled Lysotracker for 30 min. b, Averaged IGF-2 intensity in single cells based on a Lysotracker cell mask. Data represents mean and error bar indicates SEM (N = 6 images per condition with at least 10 cells per image). c, Binding of AF-647 labeled IGF_EndoTag2 on the cell surface of WT and GNPTAB KO UMRC2 cells on ice. d, Binding of AF647 labeled transferrin to HeLa cells with and without 100 nM of Tfr-EndoTag on ice. e, Internalization of transferrin-647 in HeLa cells with and without 100 nM TfR-EndoTag treatment. For c-e, data represents mean of 3 biological replicates and error bar indicates SEC. P values were determined by unpaired two-tailed t-test.
Extended Data Fig. 8 In vivo PD-L1 degradation by ATZ-pLYTAC.
Western Blot analysis of PD-L1 levels in tumor tissues colleted at sacrifice at day21 from different treatment groups of mice with β-actin as loading control.
Extended Data Fig. 9
a, Confocal imaging of lysosome co-localization of IgG-AF647 with lysosome in Hela WT cells with ProteinG-IGF_EndoTags treatment. b, Confocal imaging of lysosome co-localization of IgG-AF647 with lysosome in Hela IGF2R KO cells with ProteinG-IGF_EndoTags treatment. For a,b, the cells were incubated with 200 nM IgG-AF647 and 1uM of proteinG-IGF_EndoTag for 24 h, washed and stained with anti-LAMP2A antibody followed by AF488-labelled secondary antibody. The scale bar indicates 40 µm for a and 50 µm for b.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–23 and Supplementary Tables 1–5.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, B., Abedi, M., Ahn, G. et al. Designed endocytosis-inducing proteins degrade targets and amplify signals. Nature (2024). https://doi.org/10.1038/s41586-024-07948-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-024-07948-2