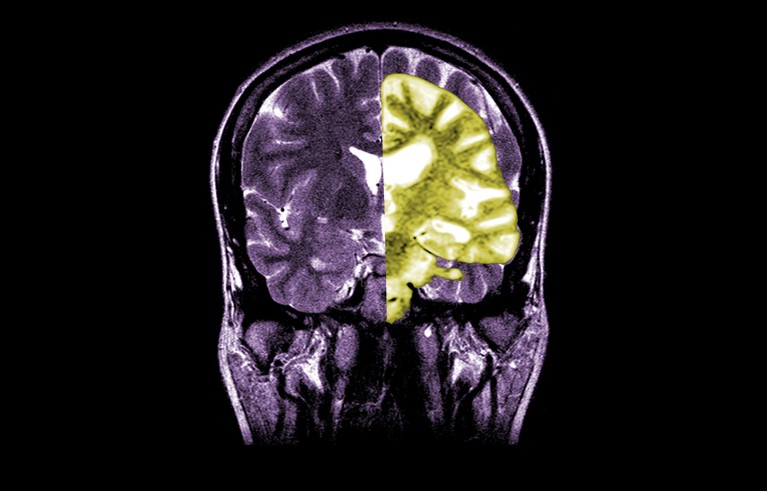
A composite MRI image showing a healthy brain (left) and one with advanced Alzheimer’s disease (right).Credit: Jessica Wilson/Medical Body Scans/Science History Images via Alamy
核磁共振成像复合图像显示了一个健康的大脑(左)和一个患有晚期阿尔茨海默氏症的大脑(右)。Credit: Jessica Wilson/Medical Body Scans/Science History Images via Alamy.
Debate over the Alzheimer’s disease drug lecanemab — one of the first to slow cognitive decline in people — is intensifying among researchers and clinicians over whether the potential benefits of treatment outweigh the risks of harm.
关于阿尔茨海默病药物莱卡奈单抗(lecanemab)--首款减缓人类认知能力衰退的药物之一--的争论正在研究人员和临床医生之间加剧,争论的焦点是治疗的潜在益处是否大于危害风险。
On 22 August, the UK Medicines and Healthcare products Regulatory Agency greenlit the drug. But at the same time, the UK healthcare regulator NICE, which determines whether drugs will be offered to patients on the government-funded UK National Health Service (NHS), said in draft guidance that lecanemab will not be made available on the NHS because the benefits are too small to justify the high cost.
8 月 22 日,英国药品和保健品管理局批准了该药物的上市。但与此同时,负责决定是否在政府资助的英国国民医疗服务体系(NHS)中向患者提供药物的英国医疗监管机构 NICE 却在指导草案中表示,不会在 NHS 中提供 lecanemab,因为该药的收益太小,不足以证明其高昂的成本是合理的。
“The unusually long time that they have spent considering the drug suggests that this has not been an easy or straightforward decision,” said psychiatrist Robert Howard at University College London in a statement to the UK Science Media Centre.
"伦敦大学学院的精神病学家罗伯特-霍华德(Robert Howard)在给英国科学媒体中心的一份声明中说:"他们花了异常长的时间来考虑这种药物,这表明这不是一个简单或直接的决定。
US regulators were the first to authorize the drug in 2023, and the European Medicines Agency (EMA) is now reassessing its decision following an appeal by the drugmaker.
美国监管机构于 2023 年率先批准了这种药物,而欧洲药品管理局(EMA)在该制药商提出上诉后,目前正在重新评估其决定。
Amyloid target 淀粉样蛋白目标
The EMA’s decision was also met with mixed responses from the Alzheimer’s community. “Emotions are really high here,” says Christian Haass, a biochemist at the Ludwig Maximilian University in Munich, Germany, who disagrees with the decision. “It’s the first disease-modifying drug we have in more than 30 years.” Denying patients the ability to access lecanemab means many will lose out on the opportunity to gain valuable time, he adds.
对于欧洲医学管理局的决定,阿尔茨海默氏症研究界的反应也是褒贬不一。"德国慕尼黑路德维希-马克西米利安大学(Ludwig Maximilian University)的生物化学家克里斯蒂安-哈斯(Christian Haass)不同意这一决定。"这是我们 30 多年来拥有的第一种改变疾病的药物。他补充说,拒绝让患者使用莱卡奈单抗意味着许多患者将失去获得宝贵时间的机会。
Lecanemab, or Leqembi, is a monoclonal antibody that works by clearing amyloid, a substance that builds up into toxic clumps in the brains of people with Alzheimer’s disease. The drug, which is made by Eisai in Tokyo and Biogen in Cambridge, Massachusetts, is also approved in China, Japan, South Korea and the United Arab Emirates.
Lecanemab或Leqembi是一种单克隆抗体,通过清除淀粉样蛋白发挥作用,淀粉样蛋白是一种在阿尔茨海默病患者大脑中堆积成有毒团块的物质。这种药物由位于东京的卫材公司和位于马萨诸塞州剑桥的百健公司生产,在中国、日本、韩国和阿拉伯联合酋长国也获得了批准。
Others applaud the EMA and say that while the drug did effectively lower amyloid levels in the brain, whether the reduction in cognitive decline it led to will result in clinically meaningful benefits for patients remains unclear. They say that the possibility of serious complications such as bleeding or swelling in the brain caused by a side effect known as amyloid-related imaging abnormalities (ARIA), although small, is a big concern. “Any reasonable assessment of the risks versus the benefit of this drug should lead people to be very sceptical of it,” says Matthew Schrag , a neurologist at Vanderbilt University in Nashville, Tennessee.
另一些人则对EMA表示赞赏,并说虽然药物确实有效降低了大脑中的淀粉样蛋白水平,但它导致的认知能力下降是否会给患者带来有临床意义的益处仍不清楚。他们说,由一种被称为淀粉样蛋白相关成像异常(ARIA)的副作用引起的严重并发症(如脑出血或脑肿)的可能性虽然很小,但却令人十分担忧。"田纳西州纳什维尔范德比尔特大学(Vanderbilt University)的神经学家马修-施拉格(Matthew Schrag)说:"对这种药物的风险与益处进行任何合理的评估,都会让人们对它持非常怀疑的态度。
Modest effects 影响不大
Whether lecanemab, which is administered by infusion, offers people a clinically meaningful reduction in cognitive decline has long been debated.
长期以来,人们一直在争论通过输液给药的莱卡奈单抗是否能减少认知能力下降。
A phase III clinical trial of the drug, which was published1 in 2022, included 1,795 people in the early stages of Alzheimer’s disease and found that after 18 months, those who received the drug showed a 27% reduction in cognitive decline compared with those who received a placebo. Some researchers celebrated the news as a win for the field. But others argued that the effects are too small to have a meaningful effect on patients.
2022 年发表的一项关于这种药物的 III 期临床试验1 包括了 1795 名阿尔茨海默氏症早期患者,结果发现,18 个月后,与服用安慰剂的患者相比,服用这种药物的患者认知能力下降了 27%。一些研究人员庆祝了这一消息,认为这是该领域的一次胜利。但也有研究人员认为,效果太小,不足以对患者产生有意义的影响。
One reason for this difference in perspective lies in how people look at the data, says Sebastian Walsh, a public-health researcher at the University of Cambridge, UK. The 27% reduction represents the relative difference in the amount of cognitive decline experienced in the drug group versus the placebo group. The absolute difference in cognitive function is much smaller: 0.45 points on an 18-point scale. “People can extract from the effect size what they want,” says Walsh. “If they want to sell the drug, you could stick to the relative changes — and if you’re very sceptical, you could talk about the absolute differences.”
英国剑桥大学公共卫生研究员塞巴斯蒂安-沃尔什说,造成这种观点差异的原因之一在于人们如何看待数据。27%的降幅是药物组与安慰剂组认知能力下降的相对差异。认知功能的绝对差异则要小得多:在18分的量表上为0.45分。"沃尔什说:"人们可以从效应大小中提取他们想要的东西。沃尔什说:"如果他们想推销药物,你可以坚持使用相对变化--如果你非常怀疑,你可以谈论绝对差异"。
But even small effects could become meaningful if maintained over time, particularly in the later stages of the disease when decline is quicker, Walsh says. “It ultimately comes down to what you think the long-term effect is going to be, and we don’t have an answer to that.”
但沃尔什说,即使是很小的影响,如果长期保持下去,也会变得很有意义,尤其是在疾病晚期,衰退更快的时候。"最终要看你认为长期效果会如何,我们对此还没有答案"。
Some longer-term data is now available. At the Alzheimer’s Association International Conference (AAIC) in Philadelphia last month, Eisai and Biogen presented findings from an open-label extension study, which continued to monitor the patients who received lecanemab following the completion of the phase III trial. After three years of continuous treatment, more than half of patients showed improvement, and most cases of ARIA occurred in the first six months of treatment. They also reported that the rate of cognitive decline returned to placebo levels when people stopped taking the drug, even if amyloid plaques had been removed before ceasing treatment.
现在已经有了一些长期数据。在上个月于费城举行的阿尔茨海默病协会国际会议(AAIC)上,卫材和百健公布了一项开放标签扩展研究的结果。经过三年的持续治疗,半数以上的患者病情有所改善,大多数 ARIA 病例发生在治疗的前六个月。他们还报告说,即使淀粉样蛋白斑块在停止治疗前已被清除,当患者停止服药后,认知能力下降的速度也会恢复到安慰剂的水平。
Lecanemab, which is sold as Leqembi, is infused into patients in a hospital every few weeks.Credit: Kota Kiriyama/Yomiuri Shimbun via AP/Alamy
莱卡奈单抗(Lecanemab)以 "Leqembi "的名称出售,每隔几周就会被注入医院的病人体内。Credit: Kota Kiriyama/Yomiuri Shimbun via AP/Alamy
Some are optimistic about these findings — Haass says that it’s exciting to see that the drug not only clears amyloid but also slows the spread of tau, another protein that accumulates into clumps in the brain of people with Alzheimer’s. Others are more cautious. Paresh Malhotra, a neurologist at Imperial College London, points out that the positive findings presented at AAIC were not compared to a placebo, so more data is needed to determine the drug’s long term effectiveness.
一些人对这些研究结果持乐观态度--哈斯说,看到这种药物不仅能清除淀粉样蛋白,还能减缓tau蛋白的扩散,这令人兴奋,tau蛋白是另一种在阿尔茨海默氏症患者大脑中积聚成块的蛋白质。其他人则更为谨慎。伦敦帝国学院的神经学家帕雷什-马尔霍特拉(Paresh Malhotra)指出,在AAIC上展示的积极研究结果并没有与安慰剂进行比较,因此需要更多的数据来确定这种药物的长期有效性。
Cost is also a concern. Walsh says that, given the drug’s modest effects, it is difficult to justify the expense of administering the drug (which costs more the US$20,000 yearly in the United States) and the procedures, such as neuoimaging and genetic testing, that are required to identify people eligible to receive it.
成本也是一个令人担忧的问题。沃尔什说,考虑到这种药物的效果并不明显,很难证明使用这种药物的费用(在美国每年的费用超过 2 万美元)以及为确定有资格接受这种药物的人而需要进行的神经成像和基因检测等程序是合理的。
Safety worries 安全问题
The biggest concern about lecanemab is ARIA, which the US Food and Drug Administration (FDA) warned about in its approval. Although most cases are asymptomatic — and none were reported during the initial 18-month clinical study — there have been a handful of ARIA-linked deaths in the extended phase of the trial.
莱卡奈单抗最令人担忧的是ARIA,美国食品和药物管理局(FDA)在批准时曾对此提出警告。虽然大多数病例都没有症状,而且在最初的 18 个月临床研究中也没有任何病例报告,但在试验的延长阶段,已经出现了少数与 ARIA 相关的死亡病例。
Some experts say that although the risk of severe ARIA is small, it’s also important to consider that the drug is administered during the earliest stages of Alzheimer’s. “This is the time period where people have the most to lose,” says Schrag. “We are often encouraging patients in this window to travel, to think about their bucket list, to get done the things that they want to accomplish in life.”
一些专家说,虽然出现严重 ARIA 的风险很小,但考虑到在阿尔茨海默氏症的早期阶段用药也很重要。"施拉格说:"这是人们损失最大的时期。"我们经常鼓励处于这个窗口期的患者去旅行,去思考他们的遗愿清单,去完成他们在生活中想要完成的事情"。
Ellis van Etten, a neurologist at Leiden University Medical Center in the Netherlands, says that there are still many open questions about ARIA, and how clinicians should respond when they see patients develop these abnormalities during treatment. For example: who will develop severe or life-threatening ARIA? At what point does ARIA go from being benign to harmful, and when should treatment with lecanemab be stopped?
荷兰莱顿大学医学中心(Leiden University Medical Center)的神经学家埃利斯-范-埃特滕(Ellis van Etten)说,关于ARIA,以及临床医生在治疗过程中发现患者出现这些异常时应该如何应对,还有很多问题没有解决。例如:哪些人会出现严重或危及生命的 ARIA?ARIA在什么情况下会从良性转变为有害?
Many of the same questions about benefits and risks apply to another amyloid-clearing antibody, donanemab — made by Eli Lilly in Indianapolis, Indiana — which received FDA approval in July. Donanemab seems to offer roughly the same degree of reduction in cognitive decline as lecanamab — and it has been associated with ARIA-related deaths.
另一种清除淀粉样蛋白的抗体多那尼单抗(由位于印第安纳州印第安纳波利斯的礼来公司生产)也面临着许多关于益处和风险的问题,该抗体已于今年 7 月获得美国食品药品管理局的批准。多奈单抗对认知能力下降的缓解程度似乎与莱卡纳单抗大致相同,但它也与 ARIA 相关死亡有关。
“We know from biomarker-related work that these antibodies clear amyloid, so we know that they’re addressing a fundamental mechanism of the disease,” Malhotra says. But these drugs alone will likely not be enough, and addressing other aspects of the disease will be important, he adds. “It’s very likely that in ten years’ time, we’ll be talking about combination therapies and that amyloid-clearing will be part of this approach.”
"马尔霍特拉说:"我们从生物标志物相关的工作中得知,这些抗体可以清除淀粉样蛋白,因此我们知道它们正在解决疾病的一个基本机制。他补充说,但仅靠这些药物可能还不够,解决疾病的其他方面问题也很重要。"他补充说:"很有可能在十年后,我们将讨论综合疗法,而清除淀粉样蛋白将是这种方法的一部分。