Abstract 摘要
Neural stem cells present in the subventricular zone (SVZ), the largest neurogenic niche of the mammalian brain, are able to self-renew as well as generate neural progenitor cells (NPCs). NPCs are highly migratory and traverse the rostral migratory stream (RMS) to the olfactory bulb, where they terminally differentiate into mature interneurons. NPCs from the SVZ are some of the few cells in the CNS that migrate long distances during adulthood. The migratory process of NPCs is highly regulated by intracellular pathway activation and signaling from the surrounding microenvironment. It involves modulation of cell volume, cytoskeletal rearrangement, and isolation from compact extracellular matrix. In malignant brain tumors including high-grade gliomas, there are cells called brain tumor stem cells (BTSCs) with similar stem cell characteristics to NPCs but with uncontrolled cell proliferation and contribute to tumor initiation capacity, tumor progression, invasion, and tumor maintenance. These BTSCs are resistant to chemotherapy and radiotherapy, and their presence is believed to lead to tumor recurrence at distal sites from the original tumor location, principally due to their high migratory capacity. BTSCs are able to invade the brain parenchyma by utilizing many of the migratory mechanisms used by NPCs. However, they have an increased ability to infiltrate the tight brain parenchyma and utilize brain structures such as myelin tracts and blood vessels as migratory paths. In this article, we summarize recent findings on the mechanisms of cellular migration that overlap between NPCs and BTSCs. A better understanding of the intersection between NPCs and BTSCs will to provide a better comprehension of the BTSCs’ invasive capacity and the molecular mechanisms that govern their migration and eventually lead to the development of new therapies to improve the prognosis of patients with malignant gliomas.
在哺乳动物大脑中最大的神经发生位点——室管膜区(SVZ)中存在的神经干细胞能够自我更新并产生神经前体细胞(NPCs)。NPCs 具有高度迁移性,穿越前向迁移束(RMS)到达嗅球,在那里它们最终分化为成熟的抑制性神经元。SVZ 的 NPC 在成年后能够长距离迁移的细胞中是少数几种细胞之一。NPC 的迁移过程受到细胞内信号通路激活和周围微环境信号的严格调控。这涉及细胞体积的调节、细胞骨架重排以及与致密的细胞外基质的隔离。在包括高级别胶质瘤在内的恶性脑肿瘤中,存在具有类似 NPC 干细胞特征的脑肿瘤干细胞(BTSCs),但具有不受控制的细胞增殖,并且能够促进肿瘤起始能力、肿瘤进展、侵袭和肿瘤维持。 这些 BTSC 对化疗和放疗具有抗性,它们的存在被认为会导致原发肿瘤位置远处的肿瘤复发,主要原因是它们具有很高的迁移能力。BTSC 能够利用 NPC 使用的许多迁移机制侵入脑实质。然而,它们具有更强的侵入紧密脑实质的能力,并利用脑结构如髓鞘束和血管作为迁移路径。在本文中,我们总结了 NPC 和 BTSC 之间重叠的细胞迁移机制的最新发现。更好地理解 NPC 和 BTSC 之间的交集将有助于更全面地理解 BTSC 的侵袭能力以及调控它们迁移的分子机制,最终为开发新的治疗方法以改善恶性胶质瘤患者的预后提供帮助。
Keywords: Subventricular zone, Neurogenesis, Neural progenitor cells, Brain tumors, Brain tumor stem cells, Migratory mechanisms
关键词: 亚 ventricular 区, 神经发生, 神经前体细胞, 脑肿瘤, 脑肿瘤干细胞, 迁移机制
Neurogenesis and neurogenic niches
神经发生和神经发生微环境
Neurogenesis is a complex process involving the proliferation, migration, and differentiation of neural stem cells (NSCs) into neural progenitor cells (NPCs) followed by terminal differentiation into mature neural cells to establish cellular patterns and connections integral to normal brain function. Although the majority of neurogenesis occurs during embryonic and early postnatal brain development, NSCs and NPCs persist into the adult brain and have the potential to generate new neurons and glia throughout life [1]. Postnatally, NSCs reside within specialized neurogenic niches that support proper production, maintenance, development, and movement of these cells to their sites of terminal differentiation [2].
神经发生是一个复杂的过程,涉及神经干细胞(NSCs)的增殖、迁移和分化为神经前体细胞(NPCs),随后进一步分化为成熟的神经细胞,以建立对正常脑功能至关重要的细胞模式和连接。尽管大多数神经发生发生在胚胎和早期后 natal 脑发育期间,但 NSCs 和 NPCs 在成年脑中仍然存在,并且具有在整个生命过程中生成新神经元和胶质细胞的潜力[ 1 ]。后 natal 期,NSCs 位于专门的神经发生位点内,支持这些细胞的正确产生、维持、发育和移动到其最终分化的位置[ 2 ]。
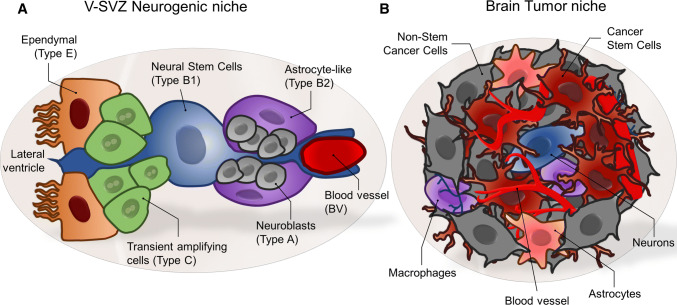
The largest of these niches is the subventricular zone (SVZ), located along the lateral walls of the lateral ventricles. The SVZ niche is primarily composed of four cell types: ependymal cells (type E cells), astrocytic cells (type B cells), transient amplifying progenitors (type C cells), and neuroblasts (type A cells; Fig. 1a) [3]. Type E cells are multiciliated and form a monolayer lining the lateral ventricle that is responsible for moving cerebrospinal fluid (CSF) through the ventricles by coordinating beating of their cilia on the apical surface [4]. The ependymal layer forms a tightly regulated barrier and bidirectional transport system between the CSF and interstitial fluid that regulates the communication between these two compartments [5, 6]. Behind the ependymal layer, B, C, and A cells form a dynamic niche that maintains its stem cell population and generates migratory neuroblasts that travel to target regions of the brain [2].
这些小窝中最大的是室管膜区(SVZ),位于侧脑室侧壁上。SVZ 小窝主要由四种细胞类型组成:室管膜细胞(E 型细胞)、星形胶质细胞(B 型细胞)、暂态扩增祖细胞(C 型细胞)和神经前体细胞(A 型细胞;图 1 a) [ 3 ]。E 型细胞是多纤毛细胞,形成一层围绕侧脑室的单层细胞,负责通过协调其顶端表面纤毛的搏动来移动脑脊液(CSF) [ 4 ]。室管膜层形成一个严格调控的屏障和双向运输系统,介导脑脊液(CSF)和间质液之间的交流 [ 5 , 6 ]。在室管膜层之后,B、C 和 A 细胞形成一个动态小窝,维持其干细胞群体,并生成迁移到大脑目标区域的神经前体细胞 [ 2 ]。
Fig. 1. 图 1.
Neural stem cells and brain tumor-initiating cell niches. a In the subventricular zone, cells at different stages of differentiation have a well-defined organization in which a monolayer of ependymal cells (type E) lines the ventricular wall, behind the ependymal cells, neural stem cells (type B1) extend a basal process that touches the ventricular wall, while an apical extension is in contact with blood vessels (BV), rapidly dividing transient amplifying cells (type C), non-stem GFAP-positive cells (type B2) and migratory neuroblasts (type A) complete the niche. b In glioblastoma, cells present a disorganized hierarchical distribution in which cancer stem cells are mainly localized near blood vessels
神经干细胞和脑肿瘤起始细胞龛。a 在室管膜区,不同分化阶段的细胞具有明确的组织结构,在室管膜区,一层室管膜细胞(类型 E)排列在室管膜壁上,室管膜细胞后面,神经干细胞(类型 B1)伸出基底过程接触室管膜壁,而顶延伸与血管(BV)接触,快速分裂的过渡扩增细胞(类型 C)、非干细胞 GFAP 阳性细胞(类型 B2)和迁移的神经母细胞(类型 A)共同构成龛。b 在胶质母细胞瘤中,细胞表现出无序的层次分布,在其中,癌干细胞主要定位在血管附近。
Type B cells are astrocyte-like cells that can be classified into two subtypes (B1 and B2). B1 cells are the true NSCs in the region, and are highly polarized with a short apical process that crosses the ependymal layer and allows them to directly interact with the CSF [2]. This small process surrounded by ependymal cells forms characteristic “pinwheel” structures on the ventricle lining [7]. B1 cells also have long basal processes with specialized end feet that contact surrounding vasculature [7, 8]. Type B2 cells are located further beneath the ventricle wall and have a multipolar morphology, closely resembling astrocytes of the brain parenchyma [7]. B2 cells help shaping the niche by isolating neuroblast chains from other cell types [3]. It is not currently known if there is a turnover between B1 and B2 cells. Type C cells are highly proliferative intermediate progenitor cells, also known as transit-amplifying cells, which are derived from type B1 cells [9]. These cells are located deeper within the niche close to the vascular network and produce neuronal precursors called neuroblasts (type A cells) after a series of divisions [3, 10]. Type A cells are highly migratory and organize into chains to leave the SVZ. Neuroblast chains travel through cellular tunnels formed by astrocytic cells in the rostral migratory stream (RMS) to reach the olfactory bulb [11]. Once they reach the olfactory bulb, Type A cells change their migration pattern from tangential to radial through the cellular layers of the olfactory bulb [2, 9]. After reaching their target layer they terminally differentiate into mature interneurons important for fine odor discrimination [11]. This transit through the RMS is partially mediated by the migration of neuroblasts along extracellular matrix (ECM) and vascular scaffolds [12, 13]. Characteristic markers for neurogenic cell types change as these cells divide, mature, and differentiate, and are summarized in Table 1. Furthermore, there is shared expression of the pluripotency markers Oct4 [14, 15], Nanog [16, 17], c-myc [18, 19], and Klf-4 [18, 20] between NSCs and BTSCs. However, the expression of these markers in intermediate progenitors and neuroblasts remains to be elucidated. These factors were introduced in 2006 as capable of inducing pluripotency in postmitotic epithelial cells [21]. This discovery gave rise to the highly exciting field of induced pluripotent stem cells (iPSCs) [22].
Type B 细胞是星形胶质细胞样的细胞,可以分为两种亚型(B1 和 B2)。B1 细胞是该区域真正的 NSC,高度极化,具有短的顶端突起,穿过室管膜层,允许它们直接与脑脊液(CSF)相互作用[ 2 ]。这个小突起被室管膜细胞包围,形成了室壁上特有的“风车”结构[ 7 ]。B1 细胞还具有长的基底突起,带有特化的终足,与周围血管接触[ 7 , 8 ]。B2 细胞位于室壁下方更深处,具有多极形态,类似于大脑皮质的星形胶质细胞[ 7 ]。B2 细胞通过隔离神经原胚链与其他细胞类型来塑造微环境[ 3 ]。目前尚不清楚 B1 和 B2 细胞之间是否存在转换。Type C 细胞是高度增殖的中间祖细胞,也称为过渡扩增细胞,来源于 B1 细胞[ 9 ]。这些细胞位于微环境的更深处,靠近血管网络,在一系列分裂后产生神经原胚(A 型细胞)作为神经前体细胞[ 3 , 10 ]。 Type A 细胞具有高度的迁移性,并组织成链状离开 SVZ。神经原胚链通过由星形胶质细胞形成的细胞隧道,在前向迁移流(RMS)中移动,到达嗅球[ 11 ]。一旦到达嗅球,Type A 细胞的迁移模式从径向转变为嗅球的细胞层中的放射状,[ 2 , 9 ]。到达目标层后,它们最终分化为成熟的间神经元,对于精细的气味辨别非常重要[ 11 ]。这一通过 RMS 的迁移部分由神经原胚沿着细胞外基质(ECM)和血管支架的迁移介导[ 12 , 13 ]。神经发生细胞类型的特征性标记物在这些细胞分裂、成熟和分化时会发生变化,并总结在表 1 中。此外,多能性标记物 Oct4[ 14 , 15 ]、Nanog[ 16 , 17 ]、c-myc[ 18 , 19 ]和 Klf-4[ 18 , 20 ]在 NSCs 和 BTSCs 之间共享表达。然而,这些标记物在中间祖细胞和神经原胚中的表达仍需阐明。 这些因素于 2006 年被引入,被认为能够诱导后分裂上皮细胞的多能性[ 21 ]。这一发现催生了诱导多能干细胞(iPSCs)这一极其令人兴奋的研究领域[ 22 ]。
Table 1. 表 1.
Summary of markers for SVZ cell types and brain tumor stem cells
SVZ 细胞类型和脑肿瘤干细胞的标志物总结
| Marker 标记 | Neural stem cell (type B cell) 神经干细胞(B 型细胞) |
Intermediate progenitor cell (type C cell) 间质祖细胞 (类型 C 细胞) |
Neuroblast (type A Cell) 神经母细胞 (类型 A 细胞) |
Brain tumor stem cell (BTSC) 脑肿瘤干细胞 (BTSC) |
References 参考文献 |
|---|---|---|---|---|---|
| Ascl1/Mash1 | – | + | – | – | [17] |
| CD15/LeX/SSEA-1 | + | – | – | + | [23, 24] |
| CD44 | – | – | – | + | [25] |
| CD133 | + | – | – | + | [26, 27] |
| Doublecortin (DCX) 双皮质蛋白 (DCX) | – | – | + | + | [28] |
| Dlx2 | – | + | + | – | [29] [ 29 ] |
| Epidermal growth factor receptor (EGFR) 表皮生长因子受体(EGFR) |
– | + | – | + | [29, 30] |
| Glial fibrillary acidic protein (GFAP) 神经纤维酸蛋白(GFAP) |
+ | – | – | + | [3, 31] |
| Glutamate aspartate transporter (GLAST) 谷氨酸天冬氨酸转运蛋白(GLAST) |
+ | – | – | – | [32] |
| Musashi 1 马萨希 1 | + | – | – | + | [33, 34] |
| Nestin | + | + | + | + | [3, 35] [ 3 , 35 ] |
| Polysialylated neural stem cell adhesion molecule (PSA-NCAM) 多聚唾液酸化神经干细胞黏附分子(PSA-NCAM) |
– | – | + | + | [3, 36] |
| Sox2 | + | – | – | + | [37, 38] |
| Vimentin 细胞质纤维蛋白 | + | – | – | + | [3, 39] |
The human SVZ follows a slightly different structure than its murine counterpart. During fetal development and soon after birth, doublecortin (DCX)-positive cells can be observed behind the ependymal layer and follow a pattern towards the olfactory tract [40]. By 6 months after birth, the migration target switches from the olfactory tract to the prefrontal cortex [41]. In the adult human brain, the existence of a migration process from the SVZ has been subject of debate [42, 43]. However, the cytoarchitecture of the human adult SVZ has been described with a similar ependymal layer but a very different organization underneath it. Behind the ependymal cell layer lining the lateral ventricles is a hypocellular gap, where there are GFAP-positive extensions from underlying astrocytes but few cell bodies [44]. Under this hypocellular layer lays the astrocytic ribbon, a unique structure where human NSCs similar to type B1 cells reside [45]. Several of the astrocytes extend processes across the hypocellular gap to maintain contact with the surface of the lateral ventricle as well as another process contacting blood vessels [40].
人类的 SVZ 在结构上与鼠类的对应物略有不同。在胎儿发育过程中以及出生后不久,可以在室管膜层后面观察到双皮质蛋白(DCX)阳性细胞,并且这些细胞沿着向嗅觉通路的模式迁移[ 40 ]。出生后 6 个月,迁移目标从嗅觉通路切换到前额叶皮层[ 41 ]。在成年的人类大脑中,SVZ 的迁移过程是否存在一直是一个有争议的话题[ 42 , 43 ]。然而,人类成年 SVZ 的细胞结构描述显示,有一个类似的室管膜层,但其下方的组织结构非常不同。室管膜细胞层围绕侧脑室排列后面是一个低细胞间隙,在这个低细胞间隙中,有来自下方星形胶质细胞的 GFAP 阳性延伸,但细胞体很少[ 44 ]。在这一低细胞间隙之下是星形胶质细胞带,这是一种独特的结构,在其中人类的 NSC 类似于 B1 型细胞居住[ 45 ]。许多星形胶质细胞延伸过程穿过低细胞间隙,以保持与侧脑室表面的接触,以及另一个过程接触血管[ 40 ]。
The process of neurogenesis and the controlled migration of neuroblasts is absolutely essential for proper brain development and response to injury. Although much of our understanding of NPC biology comes from rodent models, several basic mechanisms of cellular migration allowing these cells to respond to extracellular cues and interact with the extracellular environment have been preserved across species [46, 47]. Despite the importance of these processes during development and injury, it is possible that NPC machinery can be hijacked and spiral out of control, leading to disease. Recent evidence suggests that these cells may be transformed by mutations, leading to abnormal migration into the parenchyma and the formation of brain tumors [48]. We describe a subset of brain tumor cells that shares several characteristics with NPCs, including many migratory mechanisms.
神经发生过程和神经原祖细胞的受控迁移对于大脑的正常发育和对损伤的反应是绝对必要的。尽管我们对 NPC 生物学的理解主要来自啮齿动物模型,但这些细胞响应细胞外信号并与其他细胞外环境相互作用的基本迁移机制在不同物种中得到了保留 [ 46 , 47 ]。尽管这些过程在发育和损伤期间非常重要,但有可能 NPC 机制被劫持并失去控制,导致疾病。最近的证据表明,这些细胞可能因突变而发生转化,导致异常迁移至脑实质并形成脑肿瘤 [ 48 ]。我们描述了一类脑肿瘤细胞,它们与 NPCs 共享多种特征,包括许多迁移机制。
Brain tumor stem cell niche and migration
脑肿瘤干细胞 niche 和迁移
Primary brain tumors occur when neural cells divide uncontrollably in the brain parenchyma. Several factors affect the development of this pathology including age, gender, genetics, and environment. Primary brain tumors, those that start in the brain, are classified depending on the location of the tumor, the histological characteristics, and presence of molecular markers like mutations. Among primary brain tumors, gliomas are the most common type [49]. The World Health Organization classifies them into four grades (I to IV) [50]. Glioblastoma (GBM), or grade IV glioma, is overall the most common and aggressive type of primary tumor in adults [51]. Despite surgical intervention and treatment with chemotherapy and radiation, the average survival expectancy for patients with GBM is only 14 months [52]. Multiple aspects contribute to the unsatisfactory response of GBM to current standard of care. The highly invasive nature of its tumor cells makes complete surgical resection virtually impossible, resulting in an almost absolute recurrence rate [53]. In addition, a low proliferation rate and specific genetic mutations provide a subpopulation of GBM cells with the ability to evade therapeutic strategies. These GBM cells have the ability to re-enter the cell cycle and proliferate, resulting in recurrent brain tumor [54]. These stem-like cells are called brain tumor stem cells (BTSCs). NSCs and BTSCs are quite similar; they are both quiescent cell types able to self-renew to maintain their population, and produce the rest of the cells of the brain or brain tumor, respectively [55, 56]. Similar to NSCs, BTSCs organize and form a niche that consists of blood vessels, parenchymal cells, microglia, proliferating cells and tumor cells (Fig. 1b) [57, 58]. Niches of BTSCs are found in four specific structures in the brain: (1) the subarachnoid space, (2) perivascular region, (3) myelinated tracts, and (4) perinecrotic zones [58]. Cell membrane-molecules such as CD109, toll-like receptors, as well as transcription factors from the HIF family participate in the regulation of BTSCs within their niche [58–60]. The interaction of BTSCs with the microenvironment is not only limited to non-cancer cells. Differentiated and non-differentiated cancer cells continuously interact with each other to maintain tumor homeostasis [61, 62].
主要脑肿瘤发生在脑实质中神经细胞无控制地分裂。多种因素会影响这种病理的发展,包括年龄、性别、遗传和环境。主要脑肿瘤,即起源于脑的肿瘤,根据肿瘤的位置、组织学特征以及分子标志物如突变进行分类。在主要脑肿瘤中,胶质瘤是最常见的类型 [ 49 ]。世界卫生组织将它们分为四个等级(I 到 IV) [ 50 ]。胶质母细胞瘤(GBM),或四级胶质瘤,是成人中最常见和最具侵略性的原发性肿瘤 [ 51 ]。尽管进行了手术干预以及化疗和放疗治疗,GBM 患者的平均生存期仅为 14 个月 [ 52 ]。GBM 对当前标准治疗的不满意反应有多方面的原因。其肿瘤细胞的高度侵袭性使得完全手术切除几乎不可能,导致几乎绝对的复发率 [ 53 ]。 此外,低增殖率和特定的遗传突变赋予 GBM 细胞逃避治疗策略的能力。这些 GBM 细胞具有重新进入细胞周期并增殖的能力,导致脑肿瘤复发 [ 54 ]。这些类似干细胞的细胞被称为脑肿瘤干细胞(BTSCs)。NSCs 和 BTSCs 非常相似;它们都是静止的细胞类型,能够自我更新以维持其种群,并分别产生大脑或脑肿瘤的其他细胞 [ 55 , 56 ]。类似于 NSCs,BTSCs 组织并形成一个包括血管、实质细胞、小胶质细胞、增殖细胞和肿瘤细胞的利基(图 1 b)[ 57 , 58 ]。BTSCs 的利基存在于大脑的四个特定结构中:(1)蛛网膜下腔,(2)血管周围区域,(3)髓鞘化的纤维束,和(4)坏死区 [ 58 ]。细胞膜分子如 CD109、TLR 以及 HIF 家族的转录因子参与调节 BTSCs 在利基中的作用 [ 58 – 60 ]。BTSCs 与微环境的相互作用不仅限于非癌细胞。 分化和未分化癌细胞不断相互作用以维持肿瘤稳态 [ 61 , 62 ]。
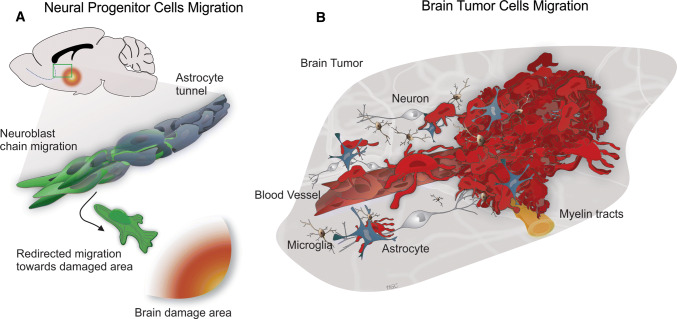
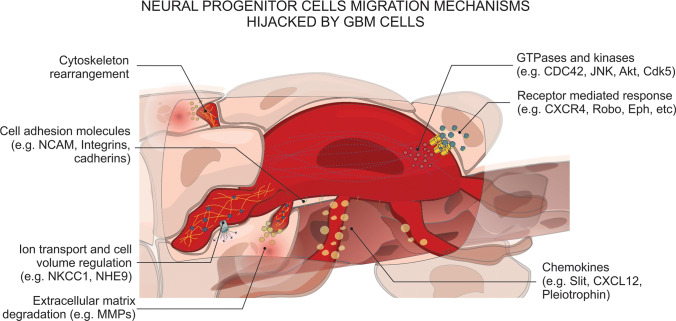
BTSCs also have the capacity to migrate to distal regions from the original tumor site, and are thus responsible for the invasion and recurrence properties in brain tumors [63]. The migratory behavior of glioma cells in vitro provides insight into tumor recurrence and tumor location in patients [64]. The migration of NPCs through the brain to targeted areas is highly regulated by several molecules and pathways [46]. However, many of these pathways are exploited by BTSCs to increase cell invasiveness, allowing for these cells to persist and for tumor recurrence despite treatment. SVZ-derived neuroblasts utilize glial tunnels in the RMS that isolate them from the rest of the brain cells and allow them to migrate towards the olfactory bulb [11, 65]. Brain tumor cells migrate individually and do not use protective tunnels; in contrast, they migrate as either groups or single cells and commonly utilize Scherer structures (myelin tracts, blood vessels, and the subarachnoid space) to invade the brain parenchyma (Fig. 2) [58–60]. Here, we discuss the mechanisms of migration that are shared between NPCs and BTSCs and contribute to brain tumor severity and recurrence. These mechanisms include (i) intracellular modifications to allow cell movement like cytoskeleton proteins and kinases, (ii) proteins that receive information from the microenvironment including receptor-mediated signals and adhesion molecules, and (iii) molecules that directly modify the cells’ surrounding like metalloproteinases (Fig. 3).
BTSCs 也具有从原发肿瘤部位迁移到远端区域的能力,因此它们负责脑肿瘤的侵袭和复发特性 [ 63 ]。体外胶质瘤细胞的迁移行为为肿瘤复发和肿瘤位置提供了见解 [ 64 ]。神经前体细胞通过大脑迁移到靶向区域的高度受到多种分子和途径的调控 [ 46 ]。然而,许多这些途径被 BTSCs 利用以增加细胞侵袭性,使这些细胞得以存活并在治疗后复发。SVZ 导出的神经前体细胞利用 RMS 中的胶质隧道,将它们与大脑其他细胞隔离,并允许它们迁移到嗅球 [ 11 , 65 ]。脑肿瘤细胞单独迁移且不使用保护性隧道;相反,它们以群体或单个细胞的形式迁移,并且通常利用 Scherer 结构(髓鞘束、血管和蛛网膜下腔)侵入脑实质(图 2 )[ 58 – 60 ]。在这里,我们讨论了神经前体细胞和 BTSCs 共享的迁移机制,这些机制有助于脑肿瘤的严重性和复发。 这些机制包括(i)细胞内修饰以允许细胞移动,如细胞骨架蛋白和激酶,(ii)从微环境中接收信息的蛋白质,包括受体介导的信号和黏附分子,以及(iii)直接修饰细胞周围环境的分子,如金属蛋白酶(图 3 )。
Fig. 2. 图 2.
Cell migration of neural progenitor and brain tumor cells. a Neuroblasts, originated in the SVZ migrate forming chains that are isolated from the rest of the parenchyma by a tunnel of astrocytes in the rostral migratory stream (RMS). Neuroblasts can leave the RMS and migrate individually in response to brain damage. b Brain tumor cell migration follows structural features like blood vessels and myelin tracts to invade the brain parenchyma
神经前体细胞和脑肿瘤细胞的迁移。a 神经原细胞起源于 SVZ,迁移形成链状结构,这些链状结构由前向迁移束(RMS)中的星形胶质细胞隧道隔离,其余的实质组织与之分离。神经原细胞可以离开 RMS,响应脑损伤单独迁移。b 脑肿瘤细胞的迁移遵循如血管和髓鞘纤维等结构特征,侵入脑实质。
Fig. 3. 图 3.
Glioblastoma cells exploit mechanisms that neural progenitor cells utilize to migrate through the brain parenchyma. Commonly these mechanisms have increased activity due to overexpression or mutations
胶质母细胞瘤细胞利用神经前体细胞用于在脑实质中迁移的机制。通常这些机制由于过表达或突变而活性增加。
Intracellular regulation of cell migration
细胞内调节细胞迁移
The migratory processes of NPCs are largely mediated through the activation and regulation of factors inside the cell in response to a variety of cues. The modification of cytoskeletal proteins and cell volume allows for these cells to physically move themselves through the brain. By changing shape and size, cells fit through small spaces and extend their bodies towards the intended destination. These mechanisms are essential for the proper migration of NPCs, whether it be down the RMS or in response to brain damage or disease. Given the high biological similarity between NPCs and BTSCs, it is not surprising that these two cell populations share several of these intracellular regulators of migration. However, these processes are often dysregulated in BTSCs leading to aberrant migration and invasion into distal parenchymal regions. Ultimately, the dysregulated activation of these shared regulators contributes to BTSC invasion and tumor recurrence.
NPCs 的迁移过程主要通过细胞内对各种信号的激活和调节来实现。细胞内骨架蛋白的修饰和细胞体积的变化使这些细胞能够通过大脑物理移动自己。通过改变形状和大小,细胞可以穿过小空间并向目标位置延伸其身体。这些机制对于 NPCs 的正确迁移至关重要,无论是沿着 RMS 迁移,还是对脑损伤或疾病的响应。由于 NPCs 和 BTSCs 在生物学上高度相似,这两种细胞群体共享一些调节迁移的细胞内调节因子并不令人惊讶。然而,这些过程在 BTSCs 中常常失调,导致异常迁移和侵入远端脑实质区域。最终,这些共享调节因子的异常激活促进了 BTSC 侵袭和肿瘤复发。
Doublecortin (DCX) 双皮质蛋白 (DCX)
Doublecortin (DCX) is a microtubule-associated protein (MAP) expressed predominantly in immature migrating neurons [66]. When bound, it stabilizes and promotes the bundling of microtubules, regulating cytoskeletal organization [67, 68]. DCX has been linked extensively to neuronal migration and is one of the primarily used markers for migrating neuroblasts [69]. It is mutated in human X-linked lissencephaly and subcortical band heterotopia, where developing cortical neurons are unable to effectively migrate to their final destinations, ultimately resulting in seizures, developmental defects, and severe neurological impairment [70, 71]. In vitro study of neuronal progenitors derived from induced pluripotent stem cells of lissencephaly patients has revealed that along with decreased migratory capacity and neurite outgrowth, DCX-deficiency leads to decreased neuronal differentiation and deregulation of other important genes related to migration and differentiation [72]. Conversely, the overexpression of DCX in embryonic NPCs leads to increased migration, further highlighting its role in NPC migration during neurodevelopment [73]. In addition to being a key player in proper embryonic development, DCX is expressed in postnatal migrating NPCs and is necessary for the migration of neuroblasts out of adult SVZ-derived neurospheres, highlighting DCX as an important part of progenitor migration throughout life [70, 74]. Activity of DCX is regulated through the phosphorylation of different sites controlled by several intracellular signaling cascades, including the JNK and PKA/Akt pathways. JNK phosphorylates DCX at multiple sites, contributing significantly to neurite outgrowth and migration [75, 76]. PKA similarly regulates DCX at the leading edge of migrating NPCs through phosphorylation of Ser47 [77, 78]. DCX is expressed in both low- and high-grade invasive brain tumors, and its expression is higher in the leading edge than in the core of the tumor [79]. Subsequent studies have shown that DCX is expressed in infiltrating, migratory glioma cells, especially infiltrating low-grade glioma cells that are difficult to label with markers of cell division [79, 80]. Interestingly, it was reported that the synthesis of DCX reduces the self-renewal capacity of BTSCs in human primary and commercial glioma cell lines, and its activity is dependent on JNK signaling pathway [81]. The DCX modification of the cytoskeleton shared between NPCs and brain tumor cells across different grades highlights the importance of this molecule in regulating cell migration within the brain.
Doublecortin (DCX) 是一种微管相关蛋白(MAP),主要在未成熟迁移神经元中表达 [ 66 ]。结合时,它稳定并促进微管的束化,调节细胞骨架组织 [ 67 , 68 ]。DCX 广泛与神经元迁移相关,并且是迁移神经前体细胞的主要标记之一 [ 69 ]。在人类 X 连锁光滑皮质发育不良和亚皮质带异位症中,DCX 突变导致发育中的皮质神经元无法有效迁移到最终目的地,最终导致癫痫、发育缺陷和严重的神经功能障碍 [ 70 , 71 ]。从光滑皮质发育不良患者的诱导多能干细胞衍生的神经元前体细胞的体外研究显示,除了迁移能力和神经突生长能力下降外,DCX 缺陷还导致神经元分化能力下降,并且与迁移和分化相关的其他重要基因的调控失常 [ 72 ]。相反,在胚胎神经前体细胞中过表达 DCX 会导致迁移增加,进一步突显其在神经发育过程中神经前体细胞迁移中的作用 [ 73 ]。 除了在正常的胚胎发育中扮演关键角色外,DCX 在出生后迁移的 NPCs 中也表达,并且对于神经芽从成年 SVZ 衍生的神经球迁出是必要的,突显了 DCX 在整个生命过程中作为祖细胞迁移的重要组成部分 [ 70 , 74 ]。DCX 的活性通过不同的位点被多种细胞内信号传导途径调节,包括 JNK 和 PKA/Akt 途径。JNK 在多个位点磷酸化 DCX,显著促进了神经突的生长和迁移 [ 75 , 76 ]。PKA 通过磷酸化 Ser47 在迁移的 NPCs 的前缘调节 DCX [ 77 , 78 ]。DCX 在低度和高度侵袭性脑肿瘤中表达,并且在肿瘤的前缘比在核心中的表达更高 [ 79 ]。后续的研究表明,DCX 在浸润性、迁移性胶质瘤细胞中表达,尤其是难以用细胞分裂标记物标记的低度侵袭性胶质瘤细胞 [ 79 , 80 ]。 有趣的是,有报道指出,DCX 的合成会减少人原代和商业型胶质瘤细胞系中的 BTSCs 自我更新能力,且其活性依赖于 JNK 信号通路 [ 81 ]。NPCs 和不同级别脑肿瘤细胞共有的 DCX 对细胞骨架的修饰突显了该分子在调节脑内细胞迁移中的重要性。
GTPases and Cdc42 GTPases 和 Cdc42
Postnatal brain development and NPC migration rely heavily on the activation and regulation of small GTPases. These small GTPases are proteins which are activated when bound to guanosine triphosphate (GTP). There are several different small GTPases, such as Rac, Rho, and Cdc42 [82]. The activation of GTPases such as Rac1 is important for neurite length, lamellipodia formation, and migration of NPCs [83, 84]. Similarly, the Rho family of GTPases is important in the postnatal migration of NPCs. RhoE knockout leads to the widening of the SVZ and caudal RMS, as well as disorganized migration of NPCs with impaired cell–cell interactions [85]. Additionally, the inhibition of RhoA controls the distribution of newly formed neurons in the olfactory bulb and the speed at which NPCs migrate [86]. Cdc42 also regulates the outgrowth of neurites [83] and is involved in the regulation of cell migration through the Slit–Robo interaction [87]. Ultimately, the activation of several GTPases is essential for the proper migration of NPCs. In BTSCs, the Rho family of GTPases appears to be particularly important for their invasion into distal areas of the brain. RhoG overexpression in GBM results in an increased formation of lamellipodia and invadopodia in response to HGF and EGF [88]. In addition, RHPN2 (Rhophilin Rho GTPase Binding Protein 2) induces mesenchymal transformation in glioma cells through the activation of RhoA, and its overexpression in NSCs and normal astrocytes promotes an invasive phenotype by increasing the expression of mesenchymal related genes [89]. In animal models, higher activity of Rac1 and Cdc42 is present in the perivascular regions [90]. While the inhibition of Cdc42 in glioblastomas decreases GBM cell migration and invasion trough TWEAK inactivation [91]. In summary, the migratory capacity of NPCs and BTSCs depends on the activity of small GTPases and the inhibition of these proteins results in a decreased invasive capacity of GBM cells.
产后大脑发育和 NPC 迁移很大程度上依赖于小 GTP 酶的激活和调节。这些小 GTP 酶是当与鸟苷三磷酸(GTP)结合时被激活的蛋白质。有几种不同类型的小 GTP 酶,如 Rac、Rho 和 Cdc42 [ 82 ]。Rac1 等 GTP 酶的激活对于神经突长度、伪足形成和 NPC 迁移非常重要 [ 83 , 84 ]。同样,Rho 家族的 GTP 酶对于产后 NPC 的迁移也很重要。RhoE 敲除会导致 SVZ 和尾部 RMS 加宽,NPC 迁移组织混乱且细胞-细胞相互作用受损 [ 85 ]。此外,RhoA 的抑制控制新形成的神经元在嗅球中的分布以及 NPC 迁移的速度 [ 86 ]。Cdc42 还调节神经突的生长 [ 83 ],并通过 Slit-Robo 相互作用参与细胞迁移的调节 [ 87 ]。最终,几种 GTP 酶的激活对于 NPC 的正确迁移至关重要。在 BTSCs 中,Rho 家族的 GTP 酶似乎特别重要,对于它们向大脑远端区域的侵入至关重要。 RhoG 在 GBM 中的过表达会导致在 HGF 和 EGF 响应下增加伪足和侵袭足的形成[ 88 ]。此外,RHPN2(Rhophilin Rho GTP 酶结合蛋白 2)通过激活 RhoA 诱导胶质瘤细胞的间质转化,并且在 NSCs 和正常星形胶质细胞中的过表达通过增加间质相关基因的表达促进侵袭表型[ 89 ]。在动物模型中,血管周围区域存在更高的 Rac1 和 Cdc42 活性[ 90 ]。而在胶质母细胞瘤中抑制 Cdc42 通过 TWEAK 失活减少 GBM 细胞的迁移和侵袭[ 91 ]。总之,NPCs 和 BTSCs 的迁移能力取决于小 GTP 酶的活性,这些蛋白质的抑制会导致 GBM 细胞侵袭能力的降低。
PI3K/Akt
In response to a variety of extracellular stimuli, intracellular phosphoinositide 3-kinase (PI3K) becomes activated and catalyzes the phosphorylation of a variety of downstream targets, including the protein kinase Akt [92]. Activated Akt then regulates several secondary proteins affecting translation, proliferation, and metabolism. Phosphatase and tensin homolog (PTEN) acts as a major regulator of the PI3K/Akt pathway by dephosphorylating a pathway intermediate and thereby reversing the activity of PI3K [93]. This kinase pathway plays an important role in regulating several processes of NPCs including self-renewal [94–96], proliferation [95, 97, 98], survival [99, 100], differentiation [97, 101], and migration to maintain the cellular balance of the neurogenic niche. The migration of NPCs in response to electrical fields, a process required for normal nervous system development, is dependent on PI3K/Akt signaling [102, 103]. Signaling through this pathway also regulates neuroblast migration from the RMS to compromised tissue following stroke [104]. Additionally, stimulation of the PI3K/Akt pathway through growth factors [98] and alkaloids [105] results in increased proliferation and migration of NPCs. The knockdown of PTEN leads to significantly higher migratory and invasive behavior of NPCs independent of proliferation, further implicating the importance of activated PI3K activity in NPC migration [100]. The PI3K signaling pathway also plays an important role in migrating glioma cells. The activation of PI3K signaling is essential for the invasion of glioma cells into the parenchyma and is linked to the previous phosphorylation of Akt [106]. PTEN is an important regulator of this pathway and plays a significant role in the development of glioblastoma [107]. Decreases in PTEN activity modulate the migration, invasion, and survival of BTSCs, principally by regulating the phosphorylation of Akt [108–110]. This shared signaling pathway contributing to migratory regulation in NPCs and BTSCs further emphasizes the similarities between these two cell types and the mechanisms they use to move throughout the brain.
在多种细胞外刺激的响应下,细胞内磷脂酰肌醇 3 激酶(PI3K)被激活并催化多种下游靶标的磷酸化,包括蛋白激酶 Akt [ 92 ]。激活的 Akt 随后调节多个次级蛋白,影响翻译、增殖和代谢。磷酸酶和 tensin 同源蛋白(PTEN)通过去磷酸化途径中间体并从而逆转 PI3K 的活性,作为 PI3K/Akt 途径的主要调节器 [ 93 ]。该激酶途径在调节 NPCs 的多种过程中起重要作用,包括自我更新 [ 94 – 96 ]、增殖 [ 95 、 97 、 98 ]、存活 [ 99 、 100 ]、分化 [ 97 、 101 ] 和迁移,以维持神经发生龛内的细胞平衡。NPCs 在电场响应下的迁移,一个对于正常神经系统发育所需的进程,依赖于 PI3K/Akt 信号 [ 102 、 103 ]。通过该途径的信号传导也调节中风后从室管膜迁移到受损组织的神经元前体细胞的迁移 [ 104 ]。 此外,通过生长因子[ 98 ]和生物碱[ 105 ]刺激 PI3K/Akt 途径会导致 NPCs 的增殖和迁移增加。PTEN 的敲低会导致 NPCs 显著更高的迁移和侵袭行为,这与增殖无关,进一步表明激活的 PI3K 活性在 NPC 迁移中的重要性[ 100 ]。PI3K 信号通路在迁移的胶质母细胞瘤细胞中也发挥重要作用。PI3K 信号通路的激活对于胶质母细胞瘤细胞侵入脑实质至关重要,并与 Akt 的先前磷酸化有关[ 106 ]。PTEN 是这一途径的重要调节因子,在胶质母细胞瘤的发展中发挥重要作用[ 107 ]。PTEN 活性的降低会通过调节 Akt 的磷酸化来调节 BTSCs 的迁移、侵袭和存活[ 108 – 110 ]。这一参与 NPC 和 BTSCs 迁移调节的共同信号通路进一步强调了这两种细胞类型之间的相似性以及它们在大脑中移动所使用机制的相似性。
JNK
The c-Jun N-terminal kinases (JNKs) are members of the mitogen activated protein kinase (MAPK) family and comprise of several isoforms derived from three related genes—Jnk1, Jnk2, and Jnk3—all of which are expressed in the developing brain [111]. The JNK signaling cascade regulates levels of self-renewal, proliferation, and differentiation in NPCs [112] and contributes to cell migration during cortical development. When JNK levels are depleted through in-utero electroporation or pharmacological inhibitors there is markedly decreased NPC migration to outer cortical layers from the ventricular zone [113, 114]. Targeted knockouts of upstream activators of JNK delay radial migration in the cortex, leading to significant defects in brain development and premature death in vivo [115, 116]. Both JNK1 and JNK2, but not JNK3, are involved in these processes [112, 117]. The effects of JNK on NPC migration are likely due to the phosphorylation of MAPs such as DCX described above, ultimately regulating microtubule stability and dynamics [114, 118]. Through some of these mechanisms, the activation of the JNK signaling pathway contributes strongly to the tumor initiation potential of BTSCs. The phosphorylation levels of JNK and c-Jun play a role in the proliferative and migratory capacity of human glioma cells, as reducing phosphorylation through Fyn-related kinase (FRK) inhibits these abilities [119]. The inhibition of JNK also reduces O6-methylguanine DNA methyltransferase (MGMT) expression and decreases temozolomide resistance in glioblastoma BTSCs [120], implicating JNK activity in BTSC chemotherapeutic evasion and recurrence. Others proteins such as the PI3K p110β isoform synergize with JNK in the regulation of glioblastoma cell proliferation and migration through Akt and FAK inhibition [121]. Taken together, these results suggest that JNK is required for the migration and invasion capacity in brain cancer, as well as a key player in the normal migration of NPCs during brain development.
The c-Jun N-末端激酶(JNKs)是丝裂原活化蛋白激酶(MAPK)家族的成员,并且由三个相关基因——Jnk1、Jnk2 和 Jnk3——衍生出几种异构体——所有这些都在发育中的大脑中表达[ 111 ]。JNK 信号级联调节 NPC 的自我更新、增殖和分化水平[ 112 ],并且在皮层发育过程中促进细胞迁移。当通过宫内电穿孔或药理学抑制剂降低 JNK 水平时,NPC 迁移到外皮层区的皮质区明显减少[ 113 , 114 ]。上游激活 JNK 的靶向敲除在皮层中延迟了径向迁移,导致大脑发育中的显著缺陷和体内过早死亡[ 115 , 116 ]。JNK1 和 JNK2,但不是 JNK3,参与了这些过程[ 112 , 117 ]。JNK 对 NPC 迁移的影响可能是由于上述描述的 DCX 等微管蛋白的磷酸化,最终调节微管的稳定性和动态性[ 114 , 118 ]。 通过这些机制,JNK 信号通路的激活强烈地促进了 BTSCs 的肿瘤起始潜能。JNK 和 c-Jun 的磷酸化水平在人类胶质瘤细胞的增殖和迁移能力中起作用,通过 Fyn 相关激酶(FRK)降低磷酸化水平会抑制这些能力[ 119 ]。抑制 JNK 也会减少胶质母细胞瘤 BTSCs 中的 O6-甲基鸟嘌呤 DNA 甲基转移酶(MGMT)表达并降低对替莫唑胺的耐药性[ 120 ],这表明 JNK 活性与 BTSC 化疗逃逸和复发有关。其他蛋白质如 PI3K p110β异构体通过 Akt 和 FAK 抑制协同调节胶质母细胞瘤细胞的增殖和迁移[ 121 ]。综上所述,这些结果表明 JNK 对于脑癌的迁移和侵袭能力是必需的,同时也是脑发育期间 NPCs 正常迁移的关键参与者。
Ion cotransporters 离子共转运蛋白
Ion cotransporters are transmembrane proteins that allow the movement of solutes and water from the intra to the extracellular space and vice versa. These cotransporters play an important role in cell volume regulation and signal transduction. The Na–K–Cl cotransporter 1 (NKCC1) is an ion symporter responsible for the active transport of sodium, potassium, and two chloride ions across the cell membrane. In the developing central nervous system, it has a significant role in switching GABAergic transmission from excitatory to inhibitory in developing neurons [122]. NKCC1 also has a prominent role in regulating cell volume through ion co-transport and a following osmotic force [123]. In NPCs, the relation of NKCC1 to GABAA signaling contributes to the proliferation of new neuroblasts in the murine SVZ as well as changes in the dendritic complexity of immature neurons [124]. In terms of migration, NKCC1 regulates the movement of NPCs along the RMS to the OB by controlling the speed of migration down this pathway without contributing to cell directionality [125]. The regulation of cell volume has significant importance for cancer cell infiltration as well. During cellular migration, infiltrative glioma cells modulate their intracellular water content through ionic transport to squeeze through narrow openings when infiltrating into the brain parenchyma [126]. NKCC1 and its regulator, with-no-lysine kinase 3 (WNK3), are well studied in terms of glioma cell migration. Blockade of the NKCC1 axis decreases glioma cell invasion by preventing the regulation of cell volume in vitro and in vivo [127, 128]. In cancer cells, NKCC1 localizes to the leading edge and modulates migration in additional ways including regulation of focal adhesion proteins and actin dynamics [127, 129]. Other ion cotransporters have also been implicated in BTSC migration. Na+(K+)/H+ Exchanger 9 (NHE9/SLC9A9) is a member of the solute carrier 9 protein family that codifies a protein with endosomal location and also modulates pH homeostasis by cation exchange [130]. Glioblastoma BTSCs express higher levels of NHE9 compared to normal NPCs, and its upregulation in human glioblastoma tissue is related with a shorter survival rate. NHE9 limits endosome luminal acidification and regulates EGFR turnover, thus maintaining pathways which contribute to tumor growth and migration [131]. Furthermore, downregulating NHE9 in U87 glioblastoma cells through the use of miR-135a reduces the proliferative and migratory capacity [132]. Due to the high importance of ion cotransporters in BTSC migration, they may be ideal therapeutic targets. However, more research on the importance of ion cotransporters in NPC migration, particularly in NHE9, should be conducted to confirm the safety of such treatments.
离子共转运蛋白是跨膜蛋白,允许溶质和水从细胞内向细胞外空间或反之移动。这些共转运蛋白在细胞体积调节和信号传导中起着重要作用。Na-K-Cl 共转运蛋白 1(NKCC1)是一种离子共转运体,负责在细胞膜上主动运输钠、钾和两个氯离子。在发育中的中枢神经系统中,它在将 GABA 能传递从兴奋性转变为抑制性方面在发育神经元中起着重要作用[ 122 ]。NKCC1 还通过离子共转运和随后的渗透压力量在调节细胞体积方面起着重要作用[ 123 ]。在 NPCs 中,NKCC1 与 GABA A 信号的关系有助于在小鼠 SVZ 中新神经元的增殖以及幼稚神经元树突复杂性的变化[ 124 ]。在迁移方面,NKCC1 通过控制沿 RMS 向 OB 迁移的速度来调节 NPCs 的移动,而不影响细胞的方向性[ 125 ]。细胞体积的调节对癌细胞侵袭具有重要意义。 在细胞迁移过程中,侵袭性胶质瘤细胞通过离子转运调节其细胞内水分含量,以在侵入脑实质时通过狭窄开口[ 126 ]。NKCC1 及其调节因子无赖氨酸激酶 3(WNK3)在胶质瘤细胞迁移方面已有充分研究。阻断 NKCC1 轴通过防止体内外细胞体积的调节来减少胶质瘤细胞的侵袭[ 127 , 128 ]。在癌细胞中,NKCC1 定位在细胞前端,并通过调节焦黏附蛋白和肌动蛋白动力学等方式进一步调节迁移[ 127 , 129 ]。其他离子共转运体也被发现参与 BTSC 迁移。Na + (K + )/H + 交换器 9(NHE9/SLC9A9)是溶质载体 9 蛋白家族的一员,编码具有内体定位的蛋白质,并通过阳离子交换调节 pH 稳态[ 130 ]。胶质母细胞瘤 BTSCs 中 NHE9 的表达水平高于正常 NPCs,其在人类胶质母细胞瘤组织中的上调与较短的生存率相关。 NHE9 限制内体腔室酸化并调节 EGFR 降解,从而维持促进肿瘤生长和迁移的途径 [ 131 ]。此外,通过 miR-135a 下调 U87 胶质母细胞瘤细胞中的 NHE9 可减少其增殖和迁移能力 [ 132 ]。由于离子共转运蛋白在 BTSC 迁移中的高度重要性,它们可能是理想的治疗靶点。然而,为了确认此类治疗的安全性,特别是在 NPC 迁移中对 NHE9 的重要性方面,还需要进行更多的研究。
Cdk5
Cyclin-dependent kinase 5 (Cdk5) is a serine/threonine kinase with high levels of expression in postmitotic neurons in the developing and adult brain [133]. Cdk5 has a strong role in migration of NPCs and immature neurons. Unlike other members of the Cdk family, Cdk5 is not activated by cyclins, but rather by brain-specific subunits p35 and p39 [133]. Although Cdk5 knockout mice develop severe CNS defects and die perinatally [134], the lack of the p35 coactivator in mice results in severe disruption of the patterning of the cerebral cortex, inverted neuronal lamination, and unusual fiber fascicles running through the neocortex [135], implicating this kinase in brain development. Perinatal targeting of Cdk5 results in migration defects in later-born cortical neurons as well as fewer granule cells integrating into the OB [136]. When Cdk5 is knocked out in adult mice, the SVZ and RMS become severely thickened, implicating this protein in the migration of neuroblasts to the OB [137]. Furthermore, this results in irregular morphology and orientation of neuroblast chains along with a decrease in neuroblast migration in vitro [137]. Therefore, the expression of this kinase and its subunits is important for the activation and organization of NPC migration both in perinatal brain development and through the adult RMS. In brain cancer, Cdk5 expression is increased in gliomas compared to normal brain tissue and increases in correlation with glioma grade [138], perhaps implicating it in the increased migratory capacity of BTSCs present in high-grade gliomas. Additionally, the activation of Cdk5 by annexin A2, a protein shown to be overexpressed in human gliomas, is involved in the chemotaxis of both commercial NPCs and glioma cell lines [139], pointing to a shared mechanism between the two cell types. Although there is little information on Cdk5 specifically within the BTSC population, it seems as though this kinase important for NPC migration is also involved in the invasive behavior of high-grade glioma cells.
Cyclin 依赖的丝苏氨酸激酶 5(Cdk5)是一种在发育和成年大脑的后有丝分裂神经元中高度表达的丝苏氨酸激酶[ 133 ]。Cdk5 在 NPC 和未成熟神经元的迁移中起着重要作用。与其他 Cdk 家族成员不同,Cdk5 不是由 cyclin 激活的,而是由脑特异性亚基 p35 和 p39 激活[ 133 ]。虽然 Cdk5 敲除小鼠会出现严重的中枢神经系统缺陷并在围产期死亡[ 134 ],但在小鼠中缺乏 p35 共激活子会导致大脑皮层的模式严重破坏、神经元倒置排列以及通过新皮层的异常纤维束[ 135 ],这表明该激酶参与了大脑发育。在围产期靶向 Cdk5 会导致晚出生的皮层神经元迁移缺陷以及进入 OB 的颗粒细胞减少[ 136 ]。当在成年小鼠中敲除 Cdk5 时,SVZ 和 RMS 会变得严重增厚,表明该蛋白质参与了神经前体细胞向 OB 的迁移[ 137 ]。此外,这还会导致神经前体细胞链的不规则形态和排列,并减少体外神经前体细胞的迁移[ 137 ]。 因此,这种激酶及其亚单位的表达对于 NPC 迁移在围产期大脑发育过程中的激活和组织以及通过成年室管膜细胞系都非常重要。在脑癌中,与正常脑组织相比,胶质瘤中的 Cdk5 表达增加,并且与胶质瘤分级呈正相关[ 138 ],这可能意味着它参与了高分级胶质瘤中 BTSCs 的增强迁移能力。此外,由 annexin A2 激活的 Cdk5,一种在人类胶质瘤中过表达的蛋白质,参与了商业 NPC 和胶质瘤细胞系的趋化性[ 139 ],表明这两种细胞类型之间存在共同机制。尽管关于 BTSC 群体中 Cdk5 的具体信息很少,但似乎这种对于 NPC 迁移很重要的激酶也参与了高分级胶质瘤细胞的侵袭性行为。
Receptor-mediated interactions
受体介导的相互作用
The migratory processes of NPCs are largely mediated through the activation and regulation of factors inside the cell in response to a variety of cues. The modification of cytoskeletal proteins and cell volume allows for these cells to physically move themselves through the brain. By changing shape and size, cells fit through small spaces and extend their bodies towards the intended destination. These mechanisms are essential for the proper migration of NPCs, whether it be down the RMS or in response to brain damage or disease. Given the high biological similarity between NPCs and BTSCs, it is not surprising that these two cell populations share several of these intracellular regulators of migration. However, these processes are often dysregulated in BTSCs leading to aberrant migration and invasion into distal parenchymal regions. Ultimately, the dysregulated activation of these shared regulators contributes to BTSC invasion and tumor recurrence.
NPCs 的迁移过程主要通过细胞内对各种信号的激活和调节来实现。细胞内骨架蛋白的修饰和细胞体积的变化使这些细胞能够通过大脑物理移动自己。通过改变形状和大小,细胞可以穿过小空间并向目标位置延伸其身体。这些机制对于 NPCs 的正确迁移至关重要,无论是沿着 RMS 迁移,还是对脑损伤或疾病的响应。由于 NPCs 和 BTSCs 在生物学上高度相似,这两种细胞群体共享一些调节迁移的细胞内调节因子并不令人惊讶。然而,这些过程在 BTSCs 中常常失调,导致异常迁移和侵入远端脑实质区域。最终,这些共享调节因子的异常激活促进了 BTSC 侵袭和肿瘤复发。
Eph/ephrin
Eph receptors are the largest family of surface-bound tyrosine kinase receptors. These receptors, along with their corresponding ephrin ligands, are designated as either the A or B subtype depending on surface attachment and binding affinities [140]. The Eph–ephrin interaction elicits bidirectional signaling; classical forward signaling occurs in one cell through the tyrosine kinase activity of the Eph receptor, while reverse signaling occurs in the other through ephrin activation [140]. Both Eph and ephrins are enriched in the adult SVZ, RMS, and OB [141–143]. The B class of Ephs and ephrins is heavily involved in neuroblast chain formation and regulating migratory capacity in SVZ precursor cells. A decrease in normal EphB2-ephrinB signaling through the addition of clustered EphB2 molecules significantly increases the proliferation of neuroblasts and reduces the migration of SVZ precursor cells in vitro [144]. In vivo, the intraventricular infusion of either EphB2 or ephrinB2 severely disrupts neuroblast transit out of the SVZ, resulting in clumps and aggregates of cells instead of the characteristic parallel chains [141]. More recently, signaling through the most promiscuous Eph receptor, EphA4, has also been tied to NPC migration from the SVZ. EphA4-induced reverse signaling through the ephrinA2 ligand promotes the migration of cortical interneurons from the medial ganglion eminence in vitro and in vivo [145]. EphA4 forward signaling is important in the regulation of neuroblasts and has effects on chain formation, migration into the RMS, and final integration into the correct layers of the olfactory bulb [143]. These signals also contribute to the overall structure of the RMS by regulating compactness and the arrangement of the surrounding astrocytic meshwork [143]. In brain tumors, members of the ephrin family of receptors and ligands are also related to cell migration. Particularly ephrin B1 and B2 expression is higher in brain tumors compared to non-cancer tissue and their expression shows a negative correlation with patient survival [146]. In addition, ephrin-B3 ligand promotes glioma invasion through the activation of Rac1 [147, 148]. Similar results were obtained on the EphB2 activity in pediatric medulloblastoma cell adhesion and invasion, and in the perivascular invasion and proliferation of glioblastoma stem-like cells [149, 150]. Conversely, it was found that ligand-dependent EphB1 signaling suppresses glioma invasion and correlates with patient survival [151]. These findings demonstrate the important role these factors play in the migration of cancer cells, as well as controlling proper cell organization and migration in the SVZ and RMS.
Eph 受体是表面结合酪氨酸激酶受体中最大的家族。这些受体与其相应的 ephrin 配体,根据表面连接和结合亲和力,被指定为 A 或 B 亚型[ 140 ]。Eph–ephrin 相互作用引发双向信号传导;经典的前向信号传导通过 Eph 受体的酪氨酸激酶活性在一个细胞中发生,而反向信号传导通过 ephrin 激活在另一个细胞中发生[ 140 ]。Eph 和 ephrin 在成年 SVZ、RMS 和 OB 中富集[ 141 – 143 ]。Eph B 类和 ephrin B 类在神经元前体细胞中神经元前体细胞迁移能力的形成和调节中发挥重要作用。通过添加簇集的 EphB2 分子降低正常的 EphB2-ephrinB 信号传导显著增加了神经元前体细胞中神经元前体细胞的增殖并减少了 SVZ 前体细胞的迁移[ 144 ]。体内实验中,通过室管膜内输注 EphB2 或 ephrinB2 严重干扰了神经元前体细胞从 SVZ 的迁移,导致细胞团块和聚集而不是特征性的平行链[ 141 ]。 More recently, 通过最泛滥的 Eph 受体 EphA4 信号传导也与 NPC 从 SVZ 迁移有关。EphA4 诱导的通过 ephrinA2 配体的反向信号传导促进来自内侧柱状核胚隆起的皮层中间神经元的体外和体内迁移 [ 145 ]。EphA4 前向信号传导在神经前体细胞的调节中很重要,并对链形成、迁移到 RMS 和最终整合到嗅球的正确层有影响 [ 143 ]。这些信号还通过调节紧凑性和周围星形胶质细胞网状结构的排列来影响 RMS 的整体结构 [ 143 ]。在脑肿瘤中,Ephrin 家族的受体和配体也与细胞迁移有关。特别是 ephrin B1 和 B2 在脑肿瘤中的表达高于非癌组织,其表达与患者生存率呈负相关 [ 146 ]。此外,Ephrin-B3 配体通过激活 Rac1 促进胶质瘤侵袭 [ 147 , 148 ]。 相似的结果在儿童髓母细胞瘤细胞的黏附和侵袭以及胶质母细胞瘤干细胞的血管周侵袭和增殖中的 EphB2 活性中得到,且在胶质母细胞瘤干细胞的血管周侵袭和增殖中的 EphB2 活性中也得到 [ 149 , 150 ]。相反,发现配体依赖性的 EphB1 信号通路抑制胶质瘤侵袭并与患者生存率相关 [ 151 ]。这些发现表明这些因素在癌细胞迁移中发挥着重要作用,同时也控制着 SVZ 和 RMS 中正确的细胞组织和迁移。
CXCL12/CXCR4
CXCL12, also known as SDF-1, is a small chemokine secreted by a variety of cell types and tissues. This chemokine signals to cells through interaction with its receptor, CXCR4, and produces a significant chemoattractive response [152]. In postnatal development CXCR4 is highly expressed on NPCs and has been shown to mediate their migration to the surrounding vasculature via the release of CXCL12 from endothelial cells [153]. The chemoattractive properties of the CXCL12/CXCR4 interaction are also involved in the migratory response of NPCs to brain injury. When the brain is damaged, neuroblasts are able to exit the SVZ and RMS and migrate to the tissue surrounding the injury [154, 155]. The activation of the CXCL12/CXCR4 axis participates in this response, contributing to neuroblast proliferation and migration to the sites of stroke [156, 157], traumatic brain injury [158, 159], and cortical lesion [160]. Furthermore, exposure of NPCs to human CSF increases the expression of CXCR4, leading to increased migratory capabilities in these cells [161]. In GBM cell lines, CXCL12/CXCR4 promotes motility, proliferation, and survival [162]. Blocking this pathway induces apoptosis and reduces viability of human glioblastoma stem-like cells, affecting self-renewal activity [163]. One downstream molecular mechanism of CXCL12/CXCR4 signaling is stimulating the overexpression of FOXM1 through the PI3K/Akt pathway, which in turn induces GBM cell invasion [164]. Interestingly, glioblastoma stem cells invade the SVZ through stimulation of the CXCL12/CXCR4 axis in an orthotopic model of GBM in mice. The inhibition of this signaling pathway using AMD3100, a specific CXCR4 antagonist, prevents the migration of glioma cells towards SVZ [165]. Recently, similar results were found using a different CXCR4 antagonist, PRX177561 [166], demonstrating the therapeutic potential of the inhibition of this signaling pathway in future adjuvant treatments of glioblastoma.
CXCL12,也称为 SDF-1,是一种由多种细胞类型和组织分泌的小型趋化因子。这种趋化因子通过与其受体 CXCR4 的相互作用向细胞发出信号,并产生显著的趋化吸引反应[ 152 ]。在出生后发育过程中,CXCR4 在 NPCs 上高度表达,并已被证明通过内皮细胞释放 CXCL12 介导 NPCs 向周围血管的迁移[ 153 ]。CXCL12/CXCR4 交互作用的趋化特性也参与了 NPCs 对脑损伤的迁移反应。当大脑受损时,神经前体细胞能够从 SVZ 和 RMS 中迁出并迁移到损伤周围组织[ 154 , 155 ]。CXCL12/CXCR4 轴的激活参与了这一反应,促进了神经前体细胞在中风[ 156 , 157 ]、创伤性脑损伤[ 158 , 159 ]和皮层损伤[ 160 ]部位的增殖和迁移。此外,NPCs 接触人脑脊液会增加 CXCR4 的表达,从而提高这些细胞的迁移能力[ 161 ]。在 GBM 细胞系中,CXCL12/CXCR4 促进迁移、增殖和存活[ 162 ]。 阻断这一通路会诱导凋亡并减少人胶质母细胞瘤干细胞样细胞的存活率,影响自我更新活性 [ 163 ]。CXCL12/CXCR4 信号通路的下游分子机制之一是通过 PI3K/Akt 途径刺激 FOXM1 的过表达,进而诱导胶质母细胞瘤细胞侵袭 [ 164 ]。有趣的是,在小鼠原位胶质母细胞瘤模型中,通过 CXCL12/CXCR4 轴的刺激,胶质母细胞瘤干细胞侵入 SVZ。使用 AMD3100 这种特异性 CXCR4 阻断剂抑制这一信号通路可以防止胶质瘤细胞向 SVZ 迁移 [ 165 ]。最近,使用另一种 CXCR4 阻断剂 PRX177561 也得到了类似的结果 [ 166 ],这表明抑制这一信号通路在未来胶质母细胞瘤辅助治疗中的治疗潜力。
Slit/Robo
The Slit/Robo signaling pathway has a wide range of functions, including axon guidance, angiogenesis, and cell migration [167]. In vertebrates, there are three different Slit proteins (Slit1, Slit2, and Slit3) that interact preferentially through four Robo receptors (Robo1, Robo2, Robo3/Rig-1, and Robo4). Slit proteins are produced in the choroid plexus and septum and are released into the cerebrospinal fluid where they form a gradient across the ependymal layer [4]. The chemorepulsive effect of Slit ligands interacting with Robo receptors contributes to the regulated guidance of neuroblasts from the SVZ to the olfactory bulb via the RMS in postnatal development [168]. The correct orientation of cells into the pathway, as well as the formation of neuroblast chains, relies on the interaction of Slit1 with the Robo-expressing cells of the RMS [169]. Rodent neuroblasts release Slit1 to regulate the organization of Robo2-expressing RMS astrocytes, allowing for neuroblasts to open the pathway and effectively migrate to the olfactory bulb [65]. In this way the neuroblasts repel the surrounding RMS structure and are able to direct their own migration. Additionally, our group has recently shown that Robo1/2 is expressed in human fetal neural progenitor cells and that the interaction of Robo1 with Slit2 is a key player in the transit of these cells through the RMS [170]. Conversely, it was shown that Slit2 is downregulated in primary gliomas and glioma cell lines, and that this correlates with high hypermethylation levels in CpG island of Slit2 transcriptional promoter [171]. The same phenomenon was also demonstrated in neuroblastoma, Wilms’ tumor, and renal cell carcinoma [172]. Furthermore, in CNS tumor, the stimulation of GBM cells with Slit2 decreases the number of cells crossing a transwell membrane [173]. This effect was later demonstrated to be a gradient-dependent and dependent on CDC42 inhibition by Robo activation [174, 175]. All of these studies demonstrate the emergent role of Slit/Robo signaling pathway contributing to the invasion of cancer cells, implicating it as a possible therapeutic target.
Slit/Robo 信号通路具有广泛的功能,包括轴突导向、血管生成和细胞迁移 [ 167 ]。在脊椎动物中,存在三种不同的 Slit 蛋白(Slit1、Slit2 和 Slit3),它们通过四个 Robo 受体(Robo1、Robo2、Robo3/Rig-1 和 Robo4)进行优先相互作用。Slit 蛋白在脉络膜丛和室间隔中产生,并释放到脑脊液中,在室管膜层形成梯度 [ 4 ]。Slit 拮抗剂与 Robo 受体相互作用的化学排斥作用有助于在后 natal 发育过程中通过 RMS 从 SVZ 向嗅球引导神经前体细胞 [ 168 ]。细胞沿通路的正确排列以及神经前体细胞链的形成依赖于 Slit1 与 RMS 中 Robo 表达细胞的相互作用 [ 169 ]。啮齿动物神经前体细胞释放 Slit1 以调节 Robo2 表达的 RMS 星形胶质细胞的组织,从而使神经前体细胞能够打开通路并有效迁移到嗅球 [ 65 ]。通过这种方式,神经前体细胞排斥周围的 RMS 结构,并能够引导自身的迁移。 此外,我们小组最近表明,Robo1/2 在人类胎儿神经祖细胞中表达,并且 Robo1 与 Slit2 的相互作用是这些细胞通过 RMS 迁移的关键因素 [ 170 ]。相反,研究表明 Slit2 在原发性胶质母细胞瘤和胶质母细胞瘤细胞系中下调,并且这与 Slit2 转录启动子的 CpG 岛高甲基化水平相关 [ 171 ]。同样的现象也在神经母细胞瘤、Wilms’ 瘤和肾细胞癌中得到证实 [ 172 ]。此外,在中枢神经系统肿瘤中,用 Slit2 刺激 GBM 细胞会减少穿过转膜的细胞数量 [ 173 ]。这种效应后来被证明是依赖梯度的,并且依赖于 Robo 激活对 CDC42 的抑制 [ 174 , 175 ]。所有这些研究都表明,Slit/Robo 信号通路在癌细胞侵袭中发挥着新兴作用,将其作为可能的治疗靶点。
Semaphorins
Semaphorins are a class of proteins initially found to be involved in axon guidance during development. Since their discovery, they have been found to serve a variety of other functions such as cell adhesion, organ morphogenesis, angiogenesis, and cellular migration [176]. Semaphorins mediate these effects by interacting with cells through either neuropilin or plexin receptors [177, 178]. These interactions contribute to corticogenesis and embryonic development in the proliferation, migration, and positioning of immature neurons [179–182]. They also have several effects in postnatal and adult neurogenesis. The regulation of Sema3A through miR-30c has been linked to changes in NPCs proliferation and differentiation in the SVZ, where it promotes neurite formation and cell differentiation [183]. Sema3A and receptor neuropilin-1 are highly expressed in the endothelial cells forming the blood vessels of the RMS, pointing to a link between semaphorins, angiogenesis, and NPCs migration [177]. Additionally, the plexin-B2 receptor is expressed by migrating neuroblasts in the RMS and its activation is linked to NPCs proliferation, migration speed, the transition between tangential migration in the RMS to radial migration in the olfactory bulb, and the prevention of ectopic migration from the RMS to the corpus callosum and septum [184]. Semaphorins also play an important role in cancer cells. For example, Sema3C protein, produced and secreted by glioma stem cells, promotes survival and tumorigenicity in an autocrine and paracrine way [185]. Sema3A promotes glioblastoma dispersal through the neuropilin-1 receptor, thus the inhibition of this pathway may have an effect decreasing the migration of GBM cells [186]. Conversely, the overexpression of Sema3G inhibits the migratory and invasive capacity of commercial glioblastoma cell line by the inhibition of matrix metalloproteinase 2 (MMP2), a protein involved in the breakdown of ECM [187]. Ultimately, the interaction of semaphorins with plexin and neuropilin receptors modulates the migration of both NPCs and BTSCs in a similar manner.
Semaphorins 是一类最初发现参与发育过程中轴突导向的蛋白质。自发现以来,它们被发现具有其他多种功能,如细胞黏附、器官形态发生、血管生成和细胞迁移 [ 176 ]。Semaphorins 通过与神经节蛋白或 plexin 受体相互作用来介导这些效果 [ 177 , 178 ]。这些相互作用有助于皮层发生和胚胎发育中的增殖、迁移和未成熟神经元的定位 [ 179 – 182 ]。它们在出生后和成年神经发生中也有多种影响。miR-30c 调节 Sema3A 与 SVZ 中 NPCs 增殖和分化的变化有关,它促进神经突形成和细胞分化 [ 183 ]。Sema3A 和受体 neuropilin-1 在形成 RMS 血管的内皮细胞中高度表达,表明 semaphorins、血管生成和 NPCs 迁移之间存在联系 [ 177 ]。 此外,plexin-B2 受体在 RMS 迁移神经元中表达,其激活与 NPCs 的增殖、迁移速度、RMS 中的定向迁移向嗅球中的径向迁移的转换以及防止 RMS 向胼胝体和隔区的异常迁移有关[ 184 ]。Semaphorin 在癌细胞中也发挥重要作用。例如,由胶质瘤干细胞产生的并分泌的 Sema3C 蛋白以自分泌和旁分泌的方式促进存活和肿瘤发生[ 185 ]。Sema3A 通过神经纤丝蛋白-1 受体促进胶质母细胞瘤在神经纤维丛中的扩散,因此抑制这一途径可能减少 GBM 细胞的迁移[ 186 ]。相反,Sema3G 的过表达通过抑制基质金属蛋白酶 2(MMP2),一种参与 ECM 降解的蛋白质,抑制商业胶质母细胞瘤细胞系的迁移和侵袭能力[ 187 ]。最终,Semaphorin 与 plexin 和神经纤丝蛋白受体的相互作用以类似的方式调节 NPCs 和 BTSCs 的迁移。
Brain-derived neurotrophic factor (BDNF)
脑源性神经营养因子 (BDNF)
Brain-derived neurotrophic factor (BDNF) contributes to proper brain development during embryogenesis. Aside from effects on proliferation and survival, BDNF stimulates chemotaxis of embryonic cortical neurons in vitro, potentially through the TrkB receptor [188]. However, it appears as though the effect of neurotrophins on embryonic NPCs’ migration is specific to the stage of neurogenesis. Treatment with excess BDNF in vivo increases proliferation and affects laminar organization of the cortex, but only at particular developmental stages [189, 190]. Neurotrophins such as BDNF contribute to NPC migration in postnatal development and adulthood, although the exact mechanism for this process is unclear. BDNF has been identified as a chemoattractant for SVZ-derived progenitors in vitro, where activation of the TrkB receptor with BDNF leads to the activation of PI3K and MAPK pathways, ultimately increasing migration [191]. Despite the chemoattractant properties in vitro, there is no detectable BDNF gradient between the SVZ and olfactory bulb to guide neuroblasts along the RMS [192]. Interestingly, mature BDNF is overexpressed in glioma tissues and directly correlates with glioma grade, the expression of TrkB, and malignancy [193]. The p75NTR receptor for BDNF enhances the migration and invasion of genetically distinct human glioma cell lines in a neurotrophin-dependent manner and promotes cytoskeletal changes by reducing RhoA activity [194]. Similar results were found in C6 rat glioma cells, where mature BDNF inhibits apoptosis and increases cell growth and migration [195]. BDNF also activates Trk receptors, which in turn enhances BTSCs viability through activation of ERK and Akt pathways [196]. The overexpression of these proteins and their relationship to glioma cell viability, migration, and invasion shows the close relationship these cells have to NPCs originating in the SVZ.
脑源性神经营养因子(BDNF)在胚胎发育过程中对正常大脑发育有贡献。除了促进增殖和存活外,BDNF 还刺激体外胚胎皮层神经元的趋化性,可能通过 TrkB 受体 [ 188 ]。然而,神经生长因子对胚胎 NPCs 迁移的影响似乎仅限于神经发生阶段。体内使用过多 BDNF 增加了增殖并影响皮层的分层组织,但仅在特定的发育阶段 [ 189 , 190 ]。神经生长因子如 BDNF 在出生后发育和成年期对 NPCs 迁移有贡献,尽管这一过程的确切机制尚不清楚。BDNF 被识别为体外 SVZ 来源的祖细胞的趋化因子,BDNF 激活 TrkB 受体导致 PI3K 和 MAPK 途径的激活,最终增加迁移 [ 191 ]。尽管体外具有趋化性,但在 SVZ 和嗅球之间没有可检测到的 BDNF 梯度来引导神经元沿着 RMS 迁移 [ 192 ]。 有趣的是,成熟的 BDNF 在胶质瘤组织中过度表达,并直接与胶质瘤分级、TrkB 表达和恶性程度相关[ 193 ]。BDNF 的 p75NTR 受体以神经营养因子依赖的方式增强遗传上不同的人类胶质瘤细胞系的迁移和侵袭,并通过降低 RhoA 活性促进细胞骨架的变化[ 194 ]。类似的结果也在 C6 大鼠胶质瘤细胞中发现,其中成熟的 BDNF 抑制细胞凋亡并增加细胞生长和迁移[ 195 ]。BDNF 还激活 Trk 受体,进而通过激活 ERK 和 Akt 途径增强 BTSCs 的存活[ 196 ]。这些蛋白质的过度表达及其与胶质瘤细胞存活、迁移和侵袭的关系显示了这些细胞与 SVZ 起源的 NPCs 之间的密切关系。
Pleiotrophin
A more recently studied molecule involved in the migration of NPCs and BTSCs is pleiotrophin (PTN). PTN, or heparin-binding growth-associated molecule, plays a large role in neurite outgrowth and pathfinding during cortical development [197, 198]. It also regulates cell motility of neuroblasts in vitro [199]. Recently, PTN has been discovered to be highly enriched in the human and mouse postnatal SVZ, perhaps implicating it in the migration of neuroblasts during adult neurogenesis [200]. PTN is also overexpressed in human glioma, when compared to non-cancer brain tissue [201]. The presence of PTN impacts angiogenic and chemotactic processes [201, 202], both of which can contribute to glioma progression. PTN expression correlates with glioma grade [203] and is related to the prognosis of the patient, in part by promoting abnormal vascularization [204]. In addition, PTN regulates self-renewal and tumorigenicity in glioblastoma stem cells through the anaplastic lymphoma kinase (ALK) receptor [205] via the PI3K/AKT/MAPK signaling pathway [206]. PTN also induces a strong chemotactic and haptotactic response of glioblastoma cells through protein tyrosine phosphatase zeta/receptor-type protein tyrosine phosphatase beta (PTPzeta/RPTPbeta) acing as a substrate for migrating cancer cells [207]. Interestingly, NPCs in the SVZ secrete PTN which results in an increase of glioma cells’ invasion through the activation of Rho/ROCK signaling pathway. This key cell communication pathway promotes V-SVZ colonization by cancer cells revealing a close, shared migratory relationship between SVZ neurogenic niche and glioma [200].
最近研究的一种参与 NPC 和 BTSC 迁移的分子是多效蛋白(PTN)。PTN,或称肝素结合生长相关分子,在皮层发育过程中神经突起生长和路径寻找中起重要作用[ 197 , 198 ]。它还在体外神经原细胞迁移中调节细胞运动[ 199 ]。最近发现,PTN 在人类和小鼠出生后 SVZ 中高度富集,可能参与成人神经发生过程中神经原细胞的迁移[ 200 ]。PTN 在人类胶质瘤中也过度表达,与非癌脑组织相比[ 201 ]。PTN 的存在影响了血管生成和趋化过程[ 201 , 202 ],这两种过程都可能促进胶质瘤的进展。PTN 表达与胶质瘤分级相关[ 203 ],并与患者的预后有关,部分原因是通过促进异常血管生成[ 204 ]。此外,PTN 通过异位淋巴瘤激酶(ALK)受体调节胶质母细胞瘤干细胞的自我更新和致癌性[ 205 ],并通过 PI3K/AKT/MAPK 信号通路[ 206 ]。 PTN 通过蛋白酪氨酸磷酸酶ζ/受体型蛋白酪氨酸磷酸酶β(PTPzeta/RPTPbeta)作为迁移癌细胞的底物,诱导胶质母细胞瘤细胞产生强烈的趋化性和趋肤性反应 [ 207 ]。有趣的是,SVZ 中的 NPCs 分泌 PTN,这导致胶质瘤细胞的侵袭增加,通过激活 Rho/ROCK 信号通路。这一关键的细胞通讯通路促进了 V-SVZ 中癌细胞的定植,揭示了 SVZ 神经发生微环境与胶质瘤之间密切且共享的迁移关系 [ 200 ]。
Cellular junction and ECM-related proteins
细胞连接和 ECM 相关蛋白
An important aspect of a cell’s migration through tissue is direct interaction with other cell types and the ECM. For cells to migrate to new locations in the brain, they need to recognize, bind, and modify ECM to pull themselves through small spaces and utilize ECM scaffolds. Cells, including NPCs and BTSCs, can use these processes to facilitate and direct their migration. For NPCs, these processes primarily allow for them to traverse the RMS to terminally differentiate at the olfactory bulb. However, these processes can be regulated and allow the migration of NPCs out of their normal path to travel to sites of injury in the parenchyma. BTSCs take advantage of very similar pathways to migrate away from the tumor bulk, invade the brain parenchyma, and contribute to recurrence. Here, we describe cell junction and ECM-related processes shared by NPCs and BTSCs that enable the migration of these cells along tissue.
细胞通过组织迁移的一个重要方面是与其他细胞类型和细胞外基质(ECM)直接相互作用。为了使细胞迁移到大脑的新位置,它们需要识别、结合并修改 ECM 以穿过小空间并利用 ECM 支架。包括 NPCs 和 BTSCs 在内的细胞可以利用这些过程来促进和引导它们的迁移。对于 NPCs 而言,这些过程主要允许它们穿越室管膜下区(RMS)并在嗅球终末分化。然而,这些过程可以被调节,使 NPCs 从其正常路径迁移到脑实质的损伤部位。BTSCs 利用非常相似的途径迁移到肿瘤外,侵入脑实质并促进复发。在这里,我们描述了 NPCs 和 BTSCs 共享的细胞连接和 ECM 相关过程,这些过程使这些细胞能够沿着组织迁移。
Integrins 整合素
Integrins are receptor proteins which facilitate the attachment of cells to ECM and transduce signals from that matrix into the cell. They comprise of one alpha subunit and one beta subunit which form heterodimers to recognize various ECM molecules including laminin, collagen, and fibronectin [208]. Integrins play a large role in directed migration of neuroblasts down the RMS and into the proper layers of the olfactory bulb. β1 integrins are expressed by migratory neuroblasts and promote the formation of neuroblast chains [209, 210]. Targeting β1 integrin through the injection of antibodies or selective knockout in neural lineage cells results in a severely disrupted RMS without contributing to cell death [12, 209]. The function of the β1 integrins in these cells is likely tied to their role as receptors for laminin, an ECM molecule expressed within the RMS [209]. Integrins have been determined to contribute to laminin-dependent migration of rodent and human NPCs in vitro by neurosphere assay [210, 211]. Additionally, injection of laminin peptide agonists or a laminin tract draws neuroblasts out of the RMS, implicating the laminin–integrin interaction as a chemoattractive force for neuronal migration [12]. β1 integrins are also needed to maintain the glial meshwork structure of the RMS, further contributing to the maintenance and direction of postnatal neuronal migration [209]. The switch from tangential migration down the RMS to radial migration when immature neurons reach the olfactory bulb has also been related to the activity of β1 integrins in these cells [212]. Similarly, several integrins have a role regulating stemness and migratory processes in glioma cells. For example, integrin α6 is expressed in glioblastoma stem cells, where its inhibition decreases self-renewal, proliferation, and tumor formation capacity [213]. Master transcription factors such as KLF9 play an important role regulating GSC stemness and oncogenesis through multiple signaling pathways [214]. KLF9 acts as a transcriptional repressor on integrin α6 expression, which in turn decreases the stemness and tumorigenicity of GSCs [214]. In addition, integrin a5 regulates the cell–matrix and cell–cell interactions that drive glioma cell dispersion, implicating this as an important factor in glioma cell migration [215]. Integrin a7 is a major laminin receptor in GSCs and in primary high-grade glioma where it participates in tumor growth and invasiveness [216]. Interestingly, GBM tumors and GSCs exhibit high expression levels of CD151 which contribute to stemness and migration [217]. CD151 belongs to the cell surface tetraspanin family of proteins and is able to form a complex with integrins to form the CD151-α3β1 integrin which promotes cell motility and invasion of glioblastoma cells [217, 218]. CD151 interactions with integrins α3, α6, and β1 promote AKT phosphorylation and activation, and blocking these interactions decreases sphere formation and cell migration in GSCs, highlighting the importance of integrins as therapeutic targets [217]. Similarly, CD151-integrin ß3 can couple with EGFR and EGFRVIII and promote cell motility, which induces GBM progression and metastasis [218, 219]. The importance of integrin proteins in both adult neurogenesis and glioma cell migration points to interaction with the ECM as a significant regulator of cell migration in the brain.
Integrins 是细胞与 ECM 附着的受体蛋白,能够将来自基质的信号传递到细胞内。它们由一个 α 亚基和一个 β 亚基组成,形成异二聚体以识别各种 ECM 分子,包括 laminin、collagen 和 fibronectin [ 208 ]。Integrins 在神经前体细胞沿 RMS 有向迁移并进入嗅球的正确层中发挥重要作用。迁徙的神经前体细胞表达 β1 整联蛋白,促进神经前体细胞链的形成 [ 209 , 210 ]。通过注射抗体或在神经谱系细胞中进行选择性敲除 β1 整联蛋白,会导致严重的 RMS 破坏,但不导致细胞死亡 [ 12 , 209 ]。这些细胞中 β1 整联蛋白的功能很可能与其作为 laminin 受体的作用有关,laminin 是 RMS 内表达的 ECM 分子 [ 209 ]。研究表明,Integrins 在体外神经球试验中对依赖 laminin 的小鼠和人类 NPCs 迁移有贡献 [ 210 , 211 ]。 此外,注射 laminin 肽激动剂或 laminin 轨道可将神经前体细胞从 RMS 中吸引出来,表明 laminin-整合素相互作用是神经元迁移的趋化性力 [ 12 ]。β1 整合素还需要维持 RMS 的胶质网状结构,进一步有助于产后神经元迁移的维持和方向 [ 209 ]。当未成熟神经元到达嗅球时,RMS 中 β1 整合素的活动与从径向迁移转变为沿 RMS 的横向迁移也有关 [ 212 ]。同样,几种整合素在胶质母细胞瘤细胞的干性和迁移过程中也起着调节作用。例如,整合素 α6 在胶质母细胞瘤干细胞中表达,其抑制会降低自我更新、增殖和肿瘤形成的能力 [ 213 ]。主转录因子如 KLF9 通过多种信号通路在调节 GSC 干性和致癌作用中起重要作用 [ 214 ]。KLF9 作为整合素 α6 表达的转录抑制因子,进而降低 GSC 的干性和致癌性 [ 214 ]。 此外,整联蛋白α5 调节胶质瘤细胞分散的细胞-基质和细胞-细胞相互作用,表明这是胶质瘤细胞迁移的重要因素[ 215 ]。整联蛋白α7 是 GSCs 和原发性高级别胶质瘤的主要 laminin 受体,在肿瘤生长和侵袭性中发挥作用[ 216 ]。有趣的是,GBM 肿瘤和 GSCs 表现出高水平的 CD151,这有助于干细胞特性和迁移[ 217 ]。CD151 属于细胞表面四跨膜蛋白家族,能够与整联蛋白形成复合物,形成 CD151-α3β1 整联蛋白,促进胶质母细胞瘤细胞的迁移和侵袭[ 217 , 218 ]。CD151 与整联蛋白α3、α6 和β1 的相互作用促进 AKT 磷酸化和激活,阻断这些相互作用可以减少 GSCs 的球体形成和细胞迁移,突显整联蛋白作为治疗靶点的重要性[ 217 ]。同样,CD151-整联蛋白β3 可以与 EGFR 和 EGFRVIII 耦合,促进细胞迁移,这诱导胶质母细胞瘤进展和转移[ 218 , 219 ]。 成年神经发生和胶质细胞迁移中整联蛋白蛋白的重要性表明,与细胞外基质的相互作用是脑内细胞迁移的一个重要调节因素。
Cadherins
Cadherin proteins are molecules that allow for cell–cell adhesion through the formation of adherens junctions [220]. Cadherins regulate different facets of postnatal neurogenesis such as NSCs quiescence and NPCs differentiation [221, 222], but have a marked role in NPCs migration. N-cadherin is highly expressed in the SVZ and RMS, particularly in neuroblast chains, but is downregulated when NPCs exit these regions [223, 224]. Cadherins are implicated in maintaining chain formation while preventing additional differentiation of NPCs [222]. Furthermore, increased migration of NPCs stimulated by physiological electrical fields and growth factors is partially facilitated by N-cadherin upregulation [225, 226]. N-cadherin also promotes recruitment of neuroblasts from the SVZ and increases their migration in response to injury [224]. Other cadherins are involved in the regulation of NPC migration. E-cadherin maintains the metabolic activity of NSCs while decreasing their migration [227], while the mesenchymal cadherin-11 is related to cell migration during corticogenesis [228]. One of the most important processes in tumor infiltration and metastasis is the epithelial to mesenchymal transition (EMT), which involves a shift of E-cadherin to N-cadherin [229]. In gliomas, N-cadherin expression is more abundant than E-cadherin and positively correlates with the grade of astrocytomas [230, 231]. Another player in the same family is cadherin-11 which is expressed in GBM tissue and BTSCs [232]. Cadherin-11 is considered a mesenchymal marker with an important role in the migration and survival of these cells [232]. The shared significance of cell junction formation in migration regulation displays the close molecular relationship between NPCs and BTSCs.
Cadherin 蛋白是通过形成粘着连接允许细胞-细胞黏附的分子[ 220 ]。Cadherin 调节神经发生的不同方面,如 NSCs 的静止和 NPCs 的分化[ 221 , 222 ],但在 NPCs 迁移方面起着明显的作用。N-cadherin 在 SVZ 和 RMS 中高度表达,尤其是在神经祖细胞链中,但当 NPCs 退出这些区域时其表达下调[ 223 , 224 ]。Cadherin 在维持链形成的同时防止 NPCs 进一步分化[ 222 ]。此外,由生理电场和生长因子刺激的 NPCs 迁移增加部分是通过 N-cadherin 表达上调来促进的[ 225 , 226 ]。N-cadherin 还促进从 SVZ 招募神经祖细胞并增加其在损伤响应中的迁移[ 224 ]。其他 Cadherin 参与调节 NPC 迁移。E-cadherin 维持 NSCs 的代谢活性同时减少其迁移[ 227 ],而间充质 Cadherin-11 与皮质发生中的细胞迁移有关[ 228 ]。 肿瘤浸润和转移中最重要的一系列过程之一是上皮向间质转化(EMT),涉及 E-钙黏蛋白向 N-钙黏蛋白的转变 [ 229 ]。在胶质瘤中,N-钙黏蛋白的表达比 E-钙黏蛋白更为丰富,并且与星形胶质细胞瘤的分级呈正相关 [ 230 , 231 ]。同一家族的另一个参与者是钙黏蛋白-11,它在 GBM 组织和 BTSCs 中表达 [ 232 ]。钙黏蛋白-11 被认为是间质标记物,在这些细胞的迁移和存活中起着重要作用 [ 232 ]。细胞连接形成在迁移调节中的共同重要性显示了 NPCs 和 BTSCs 之间的密切分子关系。
Neural cell adhesion molecule (NCAM)
神经细胞黏附分子 (NCAM)
Neural cell adhesion molecule (NCAM; also known as CD56) is a cell surface receptor immunoglobulin broadly expressed across the central nervous system [233]. There are multiple splicing variants of NCAM which can regulate the specificity of the cell–cell and cell–substrate interactions it mediates [234]. In addition, NCAM can be posttranslationally modified through the addition of many polysialic acids (PSA), resulting in PSA-NCAM [235, 236]. PSA-NCAM is heavily involved in the migration of NPCs down the RMS and is used as one of the primary markers of migrating neuroblasts [237]. Additionally, NCAM mutation results in a decrease in the size of the olfactory bulb and an accumulation of neuroblasts in the caudal portion of the RMS, suggesting a migratory defect in NPCs [238, 239]. However, a similar phenotype also occurs when PSA expression is abolished, suggesting that PSA is the molecule responsible for the role PSA-NCAM plays in neuroblast migration [240, 241]. The loss of polysialtransferase also leads to an accumulation of precursor cells in the RMS [242], showing how important the polysialic acid addition is for effective migration. PSA-NCAM also contributes to the compact, regulated structure of the RMS and helps prevent neuroblasts from leaving the pathway to enter deep brain structures in an unregulated fashion [243]. The downregulation of PSA and NCAM in NPCs as they reach the olfactory bulb is related to the switch from tangential to radial migration, allowing for neuroblasts to integrate into the correct layers of the olfactory bulb [212, 241]. In brain tumors, quantifications of PSA-NCAM by ELISA in GBM biopsies showed that the PSA-NCAM content is an adverse prognosis factor for both overall survival (OS) and disease-free survival (DFS), representing a potential biomarker for the prognosis of GBM patients [36]. In medulloblastomas, the presence of PSA-NCAM levels in the CSF appears to correlate with meningeal spreading of the tumor, demonstrating its role in cancer cell migration [244, 245]. The binding of PSA to NCAM facilitates cell invasion and high levels of PSA are more frequent in diffuse astrocytomas [246]. The overexpression of polysialyltransferases increases the PSA bound to NCAM expression in rat glioblastoma C6 cells, inducing the migration of C6 cells towards the corpus callosum and facilitating glioma invasion into the brain parenchyma [247]. The activation of NCAM with the addition of polysialic acid promotes the migration of both glioma cells and NPCs, leading to their effective migration through the brain.
神经细胞粘附分子(NCAM;也称为 CD56)是一种广泛表达于中枢神经系统的细胞表面受体免疫球蛋白 [ 233 ]。NCAM 存在多种剪接变体,可以调节其介导的细胞-细胞和细胞-基质相互作用的特异性 [ 234 ]。此外,NCAM 可通过添加许多聚唾液酸(PSA)进行翻译后修饰,形成 PSA-NCAM [ 235 , 236 ]。PSA-NCAM 深度参与 NPC 沿 RMS 迁移,并作为迁移神经元前体的主要标志物 [ 237 ]。另外,NCAM 突变会导致嗅球体积减小,并在 RMS 的尾部积累神经元前体细胞,表明 NPC 的迁移缺陷 [ 238 , 239 ]。然而,当 PSA 表达被消除时,也会出现类似的表型,表明 PSA 是 PSA-NCAM 在神经元前体细胞迁移中所起作用的分子 [ 240 , 241 ]。聚唾液酸转移酶的缺失也会导致 RMS 中前体细胞的积累 [ 242 ],显示聚唾液酸添加对于有效迁移的重要性。 PSA-NCAM 也参与了室管膜细胞的紧凑、受调控结构的形成,并帮助防止神经原胚以不受调控的方式离开路径进入深脑结构 [ 243 ]。NPCs 到达嗅球时 PSA 和 NCAM 的下调与从横向迁移转变为径向迁移的开关有关,允许神经原胚整合到嗅球的正确层中 [ 212 , 241 ]。在脑肿瘤中,通过 ELISA 对 GBM 病理组织中 PSA-NCAM 的定量显示,PSA-NCAM 的含量是总体生存率(OS)和无病生存率(DFS)的不良预后因素,代表了 GBM 患者预后的潜在生物标志物 [ 36 ]。在髓母细胞瘤中,CSF 中 PSA-NCAM 水平的存在似乎与肿瘤向蛛网膜的扩散相关,表明其在癌细胞迁移中的作用 [ 244 , 245 ]。PSA 与 NCAM 的结合促进细胞侵袭,弥漫性星形细胞瘤中高水平的 PSA 更为常见 [ 246 ]。 The overexpression of polysialyltransferases 增加了 rat glioblastoma C6 细胞中 PSA 与 NCAM 表达的结合,诱导 C6 细胞向胼胝体迁移,并促进胶质瘤向脑实质的侵袭 [ 247 ]。通过多糖酸的添加激活 NCAM 促进胶质瘤细胞和 NPCs 的迁移,使其有效通过脑组织。
Matrix metalloproteinases (MMPs)
基质金属蛋白酶(MMPs)
Matrix metalloproteinases (MMPs) are enzymes capable of degrading the surrounding ECM when activated, allowing for cells to move through the parenchyma [248]. MMP activation plays a major role in neuroblast cell migration into the brain in response to injury. The activation of MMP-9 and MMP-2 has been linked to the ectopic movement of neuroblasts out of the RMS in response to stroke and traumatic brain injury [159, 249, 250]. Furthermore, increasing the expression of the MMP inducer EMMPRIN enhances the migration of cells to damaged tissue and increases the speed at which NPCs migrate [251]. MMPs are also involved in the migration of neuroblasts through the RMS, where inhibition of MMP activity leads to decreased tangential migration during early postnatal neurogenesis [252]. Some interactions of ephrinB2 with the EphB2 receptor are mediated in part by MMP-2 and MMP-9, leading to cell repulsion [253]. Additionally, MT5-MMP cleavage of N-cadherin activates B1 NSC proliferation, therefore regulating quiescence [221]. In gliomas, brain tumor cells degrade the parenchyma ECM utilizing multiple proteases, including MMPs, particularly MMP-2, MMP-9, MMP-13, and MT1-MMP [254]. The expression and activity of MMPs are often regulated by micro RNAs resulting in changes of glioma cell invasion [255–257]. While the genetic silencing of MMPs in glioma results in a decrease in cell invasion [258, 259]. The similar activity of MMPs in NPCs and BTSCs indicates the importance of moving through the ECM during their migration through the brain.
Matrix metallo 蛋白酶(MMPs)在激活时能够降解周围的 ECM,使细胞能够穿过实质[ 248 ]。MMP 激活在神经母细胞迁移至大脑以应对损伤时起着重要作用。MMP-9 和 MMP-2 的激活与中风和创伤性脑损伤后神经母细胞异常迁出室管膜区有关[ 159 , 249 , 250 ]。此外,增加 MMP 诱导剂 EMMPRIN 的表达可以促进细胞向损伤组织迁移,并增加 NPCs 的迁移速度[ 251 ]。MMPs 还参与神经母细胞通过室管膜区的迁移,在早期后 natal 神经发生过程中抑制 MMP 活性会导致切向迁移减少[ 252 ]。部分 ephrinB2 与 EphB2 受体的相互作用部分由 MMP-2 和 MMP-9 介导,导致细胞排斥[ 253 ]。此外,MT5-MMP 对 N-钙粘蛋白的切割激活了 B1 NSC 的增殖,从而调节静止状态[ 221 ]。 在胶质瘤中,脑肿瘤细胞利用多种基质金属蛋白酶(包括 MMPs,特别是 MMP-2,MMP-9,MMP-13 和 MT1-MMP)降解实质 ECM [ 254 ]。MMPs 的表达和活性通常由 micro RNAs 调节,导致胶质瘤细胞侵袭性改变 [ 255 – 257 ]。在胶质瘤中遗传沉默 MMPs 会导致细胞侵袭性下降 [ 258 , 259 ]。NPCs 和 BTSCs 中 MMPs 的相似活性表明,在它们在脑内迁移过程中通过 ECM 的重要性。
Final remarks 最后评论
Cell migration is an essential process for proper tissue organization, organogenesis, and homeostasis. It involves the orchestrated participation of both structural and functional proteins and has a crucial role in different stages of development and adult neurogenesis. Neuroblasts in the adult rodent brain are constantly migrating long distances through the RMS towards the olfactory bulb to form new interneurons. This directional migration requires a multi-cellular organization of astrocytic cells that form tubes and isolate chains of neuroblasts from the rest of the brain parenchyma. However, in the presence of brain injury, neuroblasts respond to inflammatory chemokines that induce them to exit the RMS and re-route towards the injury site without the need of astrocyte tubes. While BTSCs employ many of the NPC migration mechanisms to invade and disrupt the brain parenchyma, they present multiple contrasting features: these cancer cells are able to modify the microenvironment and regulate their cell volume to invade through intricate intercellular spaces. In addition, they take advantage of existing structures like blood vessels and myelin tracts to travel long distances in the brain. The development of safe therapeutic targets that stop GBM cell migration while sparing physiological processes requires a better understanding of the migration mechanisms used by NPCs and BTSCs. However, studying cell migration in an artificial microenvironment is suboptimal. It is necessary to develop cell migration models that replicate the brain parenchyma as closely as possible. Optimized strategies like time lapse microscopy using intravital images [260, 261] or on organotypic brain slices [262, 263] are essential for the generation of clinically relevant knowledge.
细胞迁移是组织正常组织化、器官形成和稳态维持的一个基本过程。它涉及结构和功能蛋白的协调参与,并在发育的不同阶段和成年神经发生中起关键作用。成年啮齿动物大脑中的神经前体细胞不断通过室管膜区向嗅球迁移,形成新的中间神经元。这种定向迁移需要星形胶质细胞形成管状结构,将神经前体细胞与大脑皮质隔离开来。然而,在脑损伤存在的情况下,神经前体细胞会响应炎症趋化因子,诱导它们从室管膜区退出,并重新路由至损伤部位,而无需星形胶质细胞管状结构。虽然 BTSCs 利用许多 NPC 迁移机制侵入并破坏大脑皮质,但它们表现出多个对比特征:这些癌细胞能够修改微环境并调节细胞体积,以通过复杂的细胞间空间侵入。 此外,它们利用现有的结构如血管和髓鞘纤维,在大脑中长距离迁移。为了在停止 GBM 细胞迁移的同时保留生理过程,需要更好地了解 NPC 和 BTSC 的迁移机制,开发安全的治疗靶标。然而,在人工微环境中研究细胞迁移是次优的。必须开发尽可能接近脑实质的细胞迁移模型。优化策略如使用实时成像的体内成像[ 260 , 261 ]或在器官型脑片[ 262 , 263 ]上进行的成像对于生成临床相关知识是必不可少的。
Acknowledgements 致谢
Authors are funded by the NCI (R21CA199295, R01CA183827, R01CA195503, R01CA216855, R01CA200399, R43CA221490), NINDS (R03NS109444), Florida State Department of Health Research Grant, and the Mayo Clinic Graduate School. AQH is supported by the William J. and Charles H. Mayo Professorship and the Mayo Clinic Clinician Investigator.
作者的研究得到了 NCI(R21CA199295, R01CA183827, R01CA195503, R01CA216855, R01CA200399, R43CA221490)、NINDS(R03NS109444)、佛罗里达州卫生部研究基金以及梅奥诊所研究生院的资助。AQH 得到了威廉·J.和查尔斯·H.梅奥教授职位以及梅奥诊所临床研究员的资助。
Compliance with ethical standards
遵守伦理标准
Conflict of interest 利益冲突
The authors declare there is no conflict of interest regarding the publication of this article.
作者声明与发表本文无关的利益冲突。
Footnotes 脚注
Publisher's Note 出版商注
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
施普林格·自然对于出版地图中的管辖权争议和机构关联保持中立立场。
Natanael Zarco and Emily Norton have contributed equally to this work.
内萨内尔·扎尔科和艾米丽·诺顿对本文有同等贡献。
References 参考文献
-
1.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174IF: 44.7 Q1 . [DOI] [PubMed] [Google Scholar]
长距离神经迁移在成年哺乳动物大脑中的发生.科学. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174IF: 44.7 Q1 . [ DOI ] [ PubMed ] [ Google Scholar ] -
2.Obernier K, Alvarez-Buylla A. Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development. 2019;146(4):dev156059. doi: 10.1242/dev.156059IF: 3.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
2.Obernier K, Alvarez-Buylla A. 神经干细胞:成年哺乳动物大脑的起源、异质性和调控. 发展. 2019;146(4):dev156059. doi: 10.1242/dev.156059IF: 3.7 Q1 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
3.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
3.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. 成人哺乳动物大脑室旁增生区的细胞组成和三维组织. 神经科学杂志. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997IF: 4.4 Q1 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
4.Sawamoto K, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311(5761):629–632. doi: 10.1126/science.1119133IF: 44.7 Q1 . [DOI] [PubMed] [Google Scholar]
新神经元跟随脑脊液在成年大脑中的流动.科学. 2006;311(5761):629–632. doi: 10.1126/science.1119133IF: 44.7 Q1 . [ DOI ] [ PubMed ] [ Google Scholar ] -
5.Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119(1):55–73. doi: 10.1007/s00401-009-0624-yIF: 9.3 Q1 . [DOI] [PubMed] [Google Scholar]
5.Del Bigio MR. 脉管细胞:生物学与病理学. 神经病理学. 2010;119(1):55–73. doi: 10.1007/s00401-009-0624-yIF: 9.3 Q1 . [ DOI ] [ PubMed ] [ Google Scholar ] -
6.Johanson C, et al. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol. 2011;39(1):186–212. doi: 10.1177/0192623310394214IF: 1.4 Q3 . [DOI] [PubMed] [Google Scholar]
6.Johanson C, 等. 脉络膜向脑脊液、室管膜和大脑的分布联系:毒理学/病理学现象、室周不稳定和病灶扩散. 毒理学与病理学. 2011;39(1):186–212. doi: 10.1177/0192623310394214IF: 1.4 Q3 . [ DOI ] [ PubMed ] [ Google Scholar ] -
7.Mirzadeh Z, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004IF: 19.8 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
7.Mirzadeh Z, 等. 神经干细胞赋予成体脑神经发生区域室管膜表面独特的螺旋结构. 细胞干细胞. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004IF: 19.8 Q1 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
8.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505IF: 44.7 Q1 . [DOI] [PubMed] [Google Scholar]
8.Shen Q, 等. 内皮细胞刺激神经干细胞的自我更新和增殖神经发生. 科学. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505IF: 44.7 Q1 . [ DOI ] [ PubMed ] [ Google Scholar ] -
9.Lim DA, Alvarez-Buylla A. The Adult Ventricular-Subventricular Zone (V–SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb Perspect Biol. 2016;8(5):a018820. doi: 10.1101/cshperspect.a018820IF: 6.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
9.Lim DA, Alvarez-Buylla A. 成年室管膜-亚室管膜区(V-SVZ)和嗅球(OB)神经发生. Cold Spring Harb Perspect Biol. 2016;8(5):a018820. doi: 10.1101/cshperspect.a018820IF: 6.9 Q1 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
10.Ponti G, et al. Cell cycle and lineage progression of neural progenitors in the ventricular–subventricular zones of adult mice. Proc Natl Acad Sci USA. 2013;110(11):E1045–E1054. doi: 10.1073/pnas.1219563110IF: 9.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
10.Ponti G, 等. 成年小鼠室管膜-亚室管膜区神经前体细胞的细胞周期和谱系进展. Proc Natl Acad Sci USA. 2013;110(11):E1045–E1054. doi: 10.1073/pnas.1219563110IF: 9.4 Q1 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
11.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978–981. doi: 10.1126/science.271.5251.978IF: 44.7 Q1 . [DOI] [PubMed] [Google Scholar]
11.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. 髓神经前体细胞的链式迁移. 科学. 1996;271(5251):978–981. doi: 10.1126/science.271.5251.978IF: 44.7 Q1 . [ DOI ] [ PubMed ] [ Google Scholar ] -
12.Emsley JG, Hagg T. alpha6beta1 integrin directs migration of neuronal precursors in adult mouse forebrain. Exp Neurol. 2003;183(2):273–285. doi: 10.1016/S0014-4886(03)00209-7IF: 4.6 Q1 . [DOI] [PubMed] [Google Scholar]
12.Emsley JG, Hagg T. alpha6beta1 整合素在成年小鼠前脑神经前体细胞迁移中的作用. Exp Neurol. 2003;183(2):273–285. doi: 10.1016/S0014-4886(03)00209-7IF: 4.6 Q1 . [ DOI ] [ PubMed ] [ Google Scholar ] - 13.Martoncikova M, et al. Astrocytic and vascular scaffolding for neuroblast migration in the rostral migratory stream. Curr Neurovasc Res. 2014;11(4):321–329. doi: 10.2174/1567202611666140903121253IF: 2.0 Q3 . [DOI] [PubMed] [Google Scholar]
- 14.Musah-Eroje A, Watson S. A novel 3D in vitro model of glioblastoma reveals resistance to temozolomide which was potentiated by hypoxia. J Neurooncol. 2019;2019:1–10. doi: 10.1007/s11060-019-03107-0IF: 3.2 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeve RL, et al. Quiescent Oct4(+) neural stem cells (NSCs) repopulate ablated glial fibrillary acidic Protein(+) NSCs in the adult mouse brain. Stem Cells. 2017;35(9):2071–2082. doi: 10.1002/stem.2662IF: 4.0 Q1 . [DOI] [PubMed] [Google Scholar]
- 16.Buono KD, et al. Leukemia inhibitory factor is essential for subventricular zone neural stem cell and progenitor homeostasis as revealed by a novel flow cytometric analysis. Dev Neurosci. 2012;34(5):449–462. doi: 10.1159/000345155IF: 2.3 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim EJ, et al. Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One. 2011;6(3):e18472. doi: 10.1371/journal.pone.0018472IF: 2.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JB, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. doi: 10.1016/j.cell.2009.01.023IF: 45.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 19.Wang J, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 2008;3(11):e3769. doi: 10.1371/journal.pone.0003769IF: 2.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddick G, et al. A core regulatory circuit in glioblastoma stem cells links MAPK activation to a transcriptional program of neural stem cell identity. Sci Rep. 2017;7:43605. doi: 10.1038/srep43605IF: 3.8 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024IF: 45.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 22.Papapetrou EP. Induced pluripotent stem cells, past and future. Science. 2016;353(6303):991–992. doi: 10.1126/science.aai7626IF: 44.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35(5):865–875. doi: 10.1016/S0896-6273(02)00835-8IF: 14.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 24.Mao XG, et al. Brain tumor stem-like cells identified by neural stem cell marker CD15. Transl Oncol. 2009;2(4):247–257. doi: 10.1593/tlo.09136IF: 4.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown DV, et al. Expression of CD133 and CD44 in glioblastoma stem cells correlates with cell proliferation, phenotype stability and intra-tumor heterogeneity. PLoS One. 2017;12(2):e0172791. doi: 10.1371/journal.pone.0172791IF: 2.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128IF: 50.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 27.Uchida N, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720IF: 9.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayanlaja AA, et al. Distinct features of doublecortin as a marker of neuronal migration and its implications in cancer cell mobility. Front Mol Neurosci. 2017;10:199. doi: 10.3389/fnmol.2017.00199IF: 3.5 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doetsch F, et al. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36(6):1021–1034. doi: 10.1016/S0896-6273(02)01133-9IF: 14.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 30.Pang LY, Saunders L, Argyle DJ. Epidermal growth factor receptor activity is elevated in glioma cancer stem cells and is required to maintain chemotherapy and radiation resistance. Oncotarget. 2017;8(42):72494–72512. doi: 10.18632/oncotarget.19868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guichet P-O, et al. Asymmetric distribution of GFAP in glioma multipotent cells. PLoS One. 2016;11(3):e0151274. doi: 10.1371/journal.pone.0151274IF: 2.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, et al. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54(5):394–410. doi: 10.1002/glia.20392IF: 5.4 Q1 . [DOI] [PubMed] [Google Scholar]
- 33.Iacopino F, et al. Isolation of cancer stem cells from three human glioblastoma cell lines: characterization of two selected clones. PLoS One. 2014;9(8):e105166. doi: 10.1371/journal.pone.0105166IF: 2.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaneko Y, et al. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev Neurosci. 2000;22(1–2):139–153. doi: 10.1159/000017435IF: 2.3 Q2 . [DOI] [PubMed] [Google Scholar]
- 35.Jin X, et al. Cell surface Nestin is a biomarker for glioma stem cells. Biochem Biophys Res Commun. 2013;433(4):496–501. doi: 10.1016/j.bbrc.2013.03.021IF: 2.5 Q3 . [DOI] [PubMed] [Google Scholar]
- 36.Amoureux MC, et al. Polysialic acid neural cell adhesion molecule (PSA-NCAM) is an adverse prognosis factor in glioblastoma, and regulates olig2 expression in glioma cell lines. BMC Cancer. 2010;10:91. doi: 10.1186/1471-2407-10-91IF: 3.4 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutton SR, Pevny LH. SOX2 expression levels distinguish between neural progenitor populations of the developing dorsal telencephalon. Dev Biol. 2011;352(1):40–47. doi: 10.1016/j.ydbio.2011.01.015IF: 2.5 Q2 . [DOI] [PubMed] [Google Scholar]
- 38.Song WS, et al. Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells. J Chin Med Assoc. 2016;79(10):538–545. doi: 10.1016/j.jcma.2016.03.010IF: 1.9 Q2 . [DOI] [PubMed] [Google Scholar]
- 39.Khan Z, et al. The complexity of identifying cancer stem cell biomarkers. Cancer Invest. 2013;31(6):404–411. doi: 10.3109/07357907.2013.802800IF: 1.8 Q3 . [DOI] [PubMed] [Google Scholar]
- 40.Guerrero-Cazares H, et al. Cytoarchitecture of the lateral ganglionic eminence and rostral extension of the lateral ventricle in the human fetal brain. J Comp Neurol. 2011;519(6):1165–1180. doi: 10.1002/cne.22566IF: 2.3 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanai N, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478(7369):382–386. doi: 10.1038/nature10487IF: 50.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–1249. doi: 10.1126/science.1136281IF: 44.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 43.Sanai N, et al. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science. 2007;318(5849):393. doi: 10.1126/science.1145011IF: 44.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 44.Quinones-Hinojosa A, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–434. doi: 10.1002/cne.20798IF: 2.3 Q1 . [DOI] [PubMed] [Google Scholar]
- 45.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744. doi: 10.1038/nature02301IF: 50.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 46.Capilla-Gonzalez V, et al. Regulation of subventricular zone-derived cells migration in the adult brain. Adv Exp Med Biol. 2015;853:1–21. doi: 10.1007/978-3-319-16537-0_1. [DOI] [PubMed] [Google Scholar]
- 47.Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205(2):313–324. doi: 10.1016/j.expneurol.2007.03.016IF: 4.6 Q1 . [DOI] [PubMed] [Google Scholar]
- 48.Lee JH, et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560(7717):243–247. doi: 10.1038/s41586-018-0389-3IF: 50.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 49.Ostrom QT, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. doi: 10.1093/neuonc/noy131IF: 16.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018;44(2):139–150. doi: 10.1111/nan.12432IF: 4.0 Q1 . [DOI] [PubMed] [Google Scholar]
- 51.Thakkar JP, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. doi: 10.1158/1055-9965.EPI-14-0275IF: 3.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330IF: 96.2 Q1 . [DOI] [PubMed] [Google Scholar]
- 53.Osuka S, Van Meir EG. Overcoming therapeutic resistance in glioblastoma: the way forward. J Clin Invest. 2017;127(2):415–426. doi: 10.1172/JCI89587IF: 13.3 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lathia JD, et al. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–1217. doi: 10.1101/gad.261982.115IF: 7.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parada LF, Dirks PB, Wechsler-Reya RJ. Brain tumor stem cells remain in play. J Clin Oncol. 2017;35(21):2428–2431. doi: 10.1200/JCO.2017.73.9540IF: 42.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schonberg DL, et al. Brain tumor stem cells: molecular characteristics and their impact on therapy. Mol Aspects Med. 2014;39:82–101. doi: 10.1016/j.mam.2013.06.004IF: 8.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020IF: 48.8 Q1 . [DOI] [PubMed] [Google Scholar]
- 58.Silver DJ, Lathia JD. Revealing the glioma cancer stem cell interactome, one niche at a time. J Pathol. 2018;244(3):260–264. doi: 10.1002/path.5024IF: 5.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA. 2000;97(12):6242–6244. doi: 10.1073/pnas.97.12.6242IF: 9.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scherer HJ. The forms of growth in gliomas and their practical significance. Brain. 1940;63(1):1–35. doi: 10.1093/brain/63.1.1IF: 10.6 Q1 . [DOI] [Google Scholar]
- 61.Shiraki Y, et al. Significance of perivascular tumour cells defined by CD109 expression in progression of glioma. J Pathol. 2017;243(4):468–480. doi: 10.1002/path.4981IF: 5.6 Q1 . [DOI] [PubMed] [Google Scholar]
- 62.Wang X, et al. Reciprocal signaling between glioblastoma stem cells and differentiated tumor cells promotes malignant progression. Cell Stem Cell. 2018;22(4):514–528 e5. doi: 10.1016/j.stem.2018.03.011IF: 19.8 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236IF: 50.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 64.Smith CL, et al. Migration phenotype of brain-cancer cells predicts patient outcomes. Cell Rep. 2016;15(12):2616–2624. doi: 10.1016/j.celrep.2016.05.042IF: 7.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaneko N, et al. New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron. 2010;67(2):213–223. doi: 10.1016/j.neuron.2010.06.018IF: 14.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown JP, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874IF: 2.3 Q1 . [DOI] [PubMed] [Google Scholar]
- 67.Gleeson JG, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92(1):63–72. doi: 10.1016/S0092-8674(00)80899-5IF: 45.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 68.Moores CA, et al. Mechanism of microtubule stabilization by doublecortin. Mol Cell. 2004;14(6):833–839. doi: 10.1016/j.molcel.2004.06.009IF: 14.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 69.Couillard-Despres S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21(1):1–14. doi: 10.1111/j.1460-9568.2004.03813.xIF: 2.7 Q3 . [DOI] [PubMed] [Google Scholar]
- 70.Gleeson JG, et al. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23(2):257–271. doi: 10.1016/S0896-6273(00)80778-3IF: 14.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 71.Dobyns WB. The clinical patterns and molecular genetics of lissencephaly and subcortical band heterotopia. Epilepsia. 2010;51(Suppl 1):5–9. doi: 10.1111/j.1528-1167.2009.02433.xIF: 6.6 Q1 . [DOI] [PubMed] [Google Scholar]
- 72.Shahsavani M, et al. An in vitro model of lissencephaly: expanding the role of DCX during neurogenesis. Mol Psychiatry. 2018;23(7):1674. doi: 10.1038/mp.2017.175IF: 9.6 Q1 . [DOI] [PubMed] [Google Scholar]
- 73.Filipovic R, et al. Increasing doublecortin expression promotes migration of human embryonic stem cell-derived neurons. Stem Cells. 2012;30(9):1852–1862. doi: 10.1002/stem.1162IF: 4.0 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ocbina PJ, et al. Doublecortin is necessary for the migration of adult subventricular zone cells from neurospheres. Mol Cell Neurosci. 2006;33(2):126–135. doi: 10.1016/j.mcn.2006.06.014IF: 2.6 Q3 . [DOI] [PubMed] [Google Scholar]
- 75.Gdalyahu A, et al. DCX, a new mediator of the JNK pathway. EMBO J. 2004;23(4):823–832. doi: 10.1038/sj.emboj.7600079IF: 9.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin J, et al. JNK phosphorylates Ser332 of doublecortin and regulates its function in neurite extension and neuronal migration. Dev Neurobiol. 2010;70(14):929–942. doi: 10.1002/dneu.20833IF: 2.7 Q2 . [DOI] [PubMed] [Google Scholar]
- 77.Schaar BT, Kinoshita K, McConnell SK. Doublecortin microtubule affinity is regulated by a balance of kinase and phosphatase activity at the leading edge of migrating neurons. Neuron. 2004;41(2):203–213. doi: 10.1016/S0896-6273(03)00843-2IF: 14.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 78.Toriyama M, et al. Phosphorylation of doublecortin by protein kinase A orchestrates microtubule and actin dynamics to promote neuronal progenitor cell migration. J Biol Chem. 2012;287(16):12691–12702. doi: 10.1074/jbc.M111.316307IF: 4.0 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daou MC, et al. Doublecortin is preferentially expressed in invasive human brain tumors. Acta Neuropathol. 2005;110(5):472–480. doi: 10.1007/s00401-005-1070-0IF: 9.3 Q1 . [DOI] [PubMed] [Google Scholar]
- 80.Masui K, et al. Evaluation of sensitivity and specificity of doublecortin immunostatining for the detection of infiltrating glioma cells. Brain Tumor Pathol. 2008;25(1):1–7. doi: 10.1007/s10014-007-0225-1IF: 2.7 Q2 . [DOI] [PubMed] [Google Scholar]
- 81.Santra M, et al. Effect of doublecortin on self-renewal and differentiation in brain tumor stem cells. Cancer Sci. 2011;102(7):1350–1357. doi: 10.1111/j.1349-7006.2011.01952.xIF: 4.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9(1):86–92. doi: 10.1016/S0955-0674(97)80156-1IF: 6.0 Q1 . [DOI] [PubMed] [Google Scholar]
- 83.Khodosevich K, Monyer H. Signaling involved in neurite outgrowth of postnatally born subventricular zone neurons in vitro. BMC Neurosci. 2010;11:18. doi: 10.1186/1471-2202-11-18IF: 2.4 Q3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leong SY, et al. The Rho kinase pathway regulates mouse adult neural precursor cell migration. Stem Cells. 2011;29(2):332–343. doi: 10.1002/stem.577IF: 4.0 Q1 . [DOI] [PubMed] [Google Scholar]
- 85.Ballester-Lurbe B, et al. RhoE deficiency alters postnatal subventricular zone development and the number of calbindin-expressing neurons in the olfactory bulb of mouse. Brain Struct Funct. 2015;220(6):3113–3130. doi: 10.1007/s00429-014-0846-1IF: 2.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 86.Ota H, et al. Speed control for neuronal migration in the postnatal brain by Gmip-mediated local inactivation of RhoA. Nat Commun. 2014;5:4532. doi: 10.1038/ncomms5532IF: 14.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 87.Wong K, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107(2):209–221. doi: 10.1016/S0092-8674(01)00530-XIF: 45.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 88.Kwiatkowska A, et al. The small GTPase RhoG mediates glioblastoma cell invasion. Mol Cancer. 2012;11:65. doi: 10.1186/1476-4598-11-65IF: 27.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Danussi C, et al. RHPN2 drives mesenchymal transformation in malignant glioma by triggering RhoA activation. Cancer Res. 2013;73(16):5140–5150. doi: 10.1158/0008-5472.CAN-13-1168-TIF: 12.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirata E, et al. In vivo fluorescence resonance energy transfer imaging reveals differential activation of Rho-family GTPases in glioblastoma cell invasion. J Cell Sci. 2012;125(Pt 4):858–868. doi: 10.1242/jcs.089995IF: 3.3 Q3 . [DOI] [PubMed] [Google Scholar]
- 91.Fortin SP, et al. Cdc42 and the guanine nucleotide exchange factors Ect2 and trio mediate Fn14-induced migration and invasion of glioblastoma cells. Mol Cancer Res. 2012;10(7):958–968. doi: 10.1158/1541-7786.MCR-11-0616IF: 4.1 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Franke TF, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81(5):727–736. doi: 10.1016/0092-8674(95)90534-0IF: 45.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 93.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/S0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 94.Chen X, et al. Involvement of caspase-3/PTEN signaling pathway in isoflurane-induced decrease of self-renewal capacity of hippocampal neural precursor cells. Brain Res. 2015;1625:275–286. doi: 10.1016/j.brainres.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 95.Groszer M, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294(5549):2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 96.Ka M, et al. mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development. 2014;141(21):4076–4086. doi: 10.1242/dev.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peltier J, O’Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67(10):1348–1361. doi: 10.1002/dneu.20506IF: 2.7 Q2 . [DOI] [PubMed] [Google Scholar]
- 98.Zhang Q, et al. BDNF promotes EGF-induced proliferation and migration of human fetal neural stem/progenitor cells via the PI3K/Akt pathway. Molecules. 2011;16(12):10146–10156. doi: 10.3390/molecules161210146IF: 4.2 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choi JK, et al. Granulocyte macrophage colony-stimulating factor shows anti-apoptotic activity via the PI3K-NF-kappaB-HIF-1alpha-survivin pathway in mouse neural progenitor cells. Mol Neurobiol. 2014;49(2):724–733. doi: 10.1007/s12035-013-8550-3IF: 4.6 Q1 . [DOI] [PubMed] [Google Scholar]
- 100.Li L, et al. PTEN in neural precursor cells: regulation of migration, apoptosis, and proliferation. Mol Cell Neurosci. 2002;20(1):21–29. doi: 10.1006/mcne.2002.1115IF: 2.6 Q3 . [DOI] [PubMed] [Google Scholar]
- 101.Lachyankar MB, et al. A role for nuclear PTEN in neuronal differentiation. J Neurosci. 2000;20(4):1404–1413. doi: 10.1523/JNEUROSCI.20-04-01404.2000IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li L, et al. Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells. 2008;26(8):2193–2200. doi: 10.1634/stemcells.2007-1022IF: 4.0 Q1 . [DOI] [PubMed] [Google Scholar]
- 103.Meng X, et al. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp Neurol. 2011;227(1):210–217. doi: 10.1016/j.expneurol.2010.11.002IF: 4.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Katakowski M, et al. Phosphoinositide 3-kinase promotes adult subventricular neuroblast migration after stroke. J Neurosci Res. 2003;74(4):494–501. doi: 10.1002/jnr.10775IF: 2.9 Q2 . [DOI] [PubMed] [Google Scholar]
- 105.Kong X, et al. Tetramethylpyrazine promotes migration of neural precursor cells via activating the phosphatidylinositol 3-kinase pathway. Mol Neurobiol. 2016;53(9):6526–6539. doi: 10.1007/s12035-015-9551-1IF: 4.6 Q1 . [DOI] [PubMed] [Google Scholar]
- 106.Joy AM, et al. Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci. 2003;116(Pt 21):4409–4417. doi: 10.1242/jcs.00712IF: 3.3 Q3 . [DOI] [PubMed] [Google Scholar]
- 107.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96(8):4240–4245. doi: 10.1073/pnas.96.8.4240IF: 9.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gu JJ, et al. Suppression of microRNA-130b inhibits glioma cell proliferation and invasion, and induces apoptosis by PTEN/AKT signaling. Int J Mol Med. 2018;41(1):284–292. doi: 10.3892/ijmm.2017.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jaraiz-Rodriguez M, et al. A short region of Connexin43 reduces human glioma stem cell migration, invasion, and survival through Src, PTEN, and FAK. Stem Cell Reports. 2017;9(2):451–463. doi: 10.1016/j.stemcr.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pan S, et al. Decreased expression of ARHGAP15 promotes the development of colorectal cancer through PTEN/AKT/FOXO1 axis. Cell Death Dis. 2018;9(6):673. doi: 10.1038/s41419-018-0707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuan CY, et al. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22(4):667–676. doi: 10.1016/S0896-6273(00)80727-8IF: 14.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 112.Xu D, et al. Microcephaly-associated protein WDR62 regulates neurogenesis through JNK1 in the developing neocortex. Cell Rep. 2014;6(1):104–116. doi: 10.1016/j.celrep.2013.12.016IF: 7.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 113.Hirai S, et al. The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J Neurosci. 2006;26(46):11992–12002. doi: 10.1523/JNEUROSCI.2272-06.2006IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawauchi T, et al. The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration. EMBO J. 2003;22(16):4190–4201. doi: 10.1093/emboj/cdg413IF: 9.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X, et al. Targeted deletion of the mitogen-activated protein kinase kinase 4 gene in the nervous system causes severe brain developmental defects and premature death. Mol Cell Biol. 2007;27(22):7935–7946. doi: 10.1128/MCB.00226-07IF: 3.2 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamasaki T, et al. Stress-activated protein kinase MKK7 regulates axon elongation in the developing cerebral cortex. J Neurosci. 2011;31(46):16872–16883. doi: 10.1523/JNEUROSCI.1111-11.2011IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang F, et al. A Novel c-Jun N-terminal Kinase (JNK) signaling complex involved in neuronal migration during brain development. J Biol Chem. 2016;291(22):11466–11475. doi: 10.1074/jbc.M116.716811IF: 4.0 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Westerlund N, et al. Phosphorylation of SCG10/stathmin-2 determines multipolar stage exit and neuronal migration rate. Nat Neurosci. 2011;14(3):305–313. doi: 10.1038/nn.2755IF: 21.2 Q1 . [DOI] [PubMed] [Google Scholar]
- 119.Zhou X, et al. FRK controls migration and invasion of human glioma cells by regulating JNK/c-Jun signaling. J Neurooncol. 2012;110(1):9–19. doi: 10.1007/s11060-012-0933-1IF: 3.2 Q2 . [DOI] [PubMed] [Google Scholar]
- 120.Okada M, et al. JNK contributes to temozolomide resistance of stem-like glioblastoma cells via regulation of MGMT expression. Int J Oncol. 2014;44(2):591–599. doi: 10.3892/ijo.2013.2209IF: 4.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 121.Zhao HF, et al. PI3K p110beta isoform synergizes with JNK in the regulation of glioblastoma cell proliferation and migration through Akt and FAK inhibition. J Exp Clin Cancer Res. 2016;35:78. doi: 10.1186/s13046-016-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3(9):715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 123.Vidal Perez-Trevino GS. NKCC1 cotransporters: keeping an ‘ion’ them. J Physiol. 2011;589(Pt 4):781–782. doi: 10.1113/jphysiol.2010.203919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Young SZ, et al. NKCC1 knockdown decreases neuron production through GABA(A)-regulated neural progenitor proliferation and delays dendrite development. J Neurosci. 2012;32(39):13630–13638. doi: 10.1523/JNEUROSCI.2864-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mejia-Gervacio S, Murray K, Lledo PM. NKCC1 controls GABAergic signaling and neuroblast migration in the postnatal forebrain. Neural Dev. 2011;6:4. doi: 10.1186/1749-8104-6-4IF: 4.0 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Watkins S, Sontheimer H. Hydrodynamic cellular volume changes enable glioma cell invasion. J Neurosci. 2011;31(47):17250–17259. doi: 10.1523/JNEUROSCI.3938-11.2011IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garzon-Muvdi T, et al. Regulation of brain tumor dispersal by NKCC1 through a novel role in focal adhesion regulation. PLoS Biol. 2012;10(5):e1001320. doi: 10.1371/journal.pbio.1001320IF: 7.8 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haas BR, et al. With-No-Lysine Kinase 3 (WNK3) stimulates glioma invasion by regulating cell volume. Am J Physiol Cell Physiol. 2011;301(5):C1150–C1160. doi: 10.1152/ajpcell.00203.2011IF: 5.0 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schiapparelli P, et al. NKCC1 regulates migration ability of glioblastoma cells by modulation of actin dynamics and interacting with cofilin. EBioMedicine. 2017;21:94–103. doi: 10.1016/j.ebiom.2017.06.020IF: 9.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kondapalli KC, Prasad H, Rao R. An inside job: how endosomal Na(+)/H(+) exchangers link to autism and neurological disease. Front Cell Neurosci. 2014;8:172. doi: 10.3389/fncel.2014.00172IF: 4.2 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kondapalli KC, et al. A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat Commun. 2015;6:6289. doi: 10.1038/ncomms7289IF: 14.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gomez Zubieta DM, et al. MicroRNA-135a regulates NHE9 to inhibit proliferation and migration of glioblastoma cells. Cell Commun Signal. 2017;15(1):55. doi: 10.1186/s12964-017-0209-7IF: 8.2 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith DS, Greer PL, Tsai LH. Cdk5 on the brain. Cell Growth Differ. 2001;12(6):277–283. [PubMed] [Google Scholar]
- 134.Ohshima T, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93(20):11173–11178. doi: 10.1073/pnas.93.20.11173IF: 9.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chae T, et al. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18(1):29–42. doi: 10.1016/S0896-6273(01)80044-1IF: 14.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 136.Hirasawa M, et al. Perinatal abrogation of Cdk5 expression in brain results in neuronal migration defects. Proc Natl Acad Sci USA. 2004;101(16):6249–6254. doi: 10.1073/pnas.0307322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hirota Y, et al. Cyclin-dependent kinase 5 is required for control of neuroblast migration in the postnatal subventricular zone. J Neurosci. 2007;27(47):12829–12838. doi: 10.1523/JNEUROSCI.1014-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yushan R, et al. Insights into the clinical value of cyclin-dependent kinase 5 in glioma: a retrospective study. World J Surg Oncol. 2015;13:223. doi: 10.1186/s12957-015-0629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.An JH, et al. Identification of gliotropic factors that induce human stem cell migration to malignant tumor. J Proteome Res. 2009;8(6):2873–2881. doi: 10.1021/pr900020q. [DOI] [PubMed] [Google Scholar]
- 140.Klein R. Excitatory Eph receptors and adhesive ephrin ligands. Curr Opin Cell Biol. 2001;13(2):196–203. doi: 10.1016/S0955-0674(00)00197-6IF: 6.0 Q1 . [DOI] [PubMed] [Google Scholar]
- 141.Conover JC, et al. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3(11):1091–1097. doi: 10.1038/80606IF: 21.2 Q1 . [DOI] [PubMed] [Google Scholar]
- 142.Ricard J, et al. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol Cell Neurosci. 2006;31(4):713–722. doi: 10.1016/j.mcn.2006.01.002IF: 2.6 Q3 . [DOI] [PubMed] [Google Scholar]
- 143.Todd KL, et al. EphA4 regulates neuroblast and astrocyte organization in a neurogenic Niche. J Neurosci. 2017;37(12):3331–3341. doi: 10.1523/JNEUROSCI.3738-16.2017IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Katakowski M, et al. EphB2 induces proliferation and promotes a neuronal fate in adult subventricular neural precursor cells. Neurosci Lett. 2005;385(3):204–209. doi: 10.1016/j.neulet.2005.05.060IF: 2.5 Q3 . [DOI] [PubMed] [Google Scholar]
- 145.Steinecke A, et al. EphA/ephrin A reverse signaling promotes the migration of cortical interneurons from the medial ganglionic eminence. Development. 2014;141(2):460–471. doi: 10.1242/dev.101691IF: 3.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 146.Nakada M, et al. The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int J Cancer. 2010;126(5):1155–1165. doi: 10.1002/ijc.24849IF: 5.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nakada M, et al. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004;64(9):3179–3185. doi: 10.1158/0008-5472.CAN-03-3667IF: 12.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 148.Nakada M, et al. Ephrin-B3 ligand promotes glioma invasion through activation of Rac1. Cancer Res. 2006;66(17):8492–8500. doi: 10.1158/0008-5472.CAN-05-4211IF: 12.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 149.Sikkema AH, et al. EphB2 activity plays a pivotal role in pediatric medulloblastoma cell adhesion and invasion. Neuro Oncol. 2012;14(9):1125–1135. doi: 10.1093/neuonc/nos130IF: 16.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Krusche B, et al. EphrinB2 drives perivascular invasion and proliferation of glioblastoma stem-like cells. Elife. 2016;5:e14845. doi: 10.7554/eLife.14845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Teng L, et al. Ligand-dependent EphB1 signaling suppresses glioma invasion and correlates with patient survival. Neuro Oncol. 2013;15(12):1710–1720. doi: 10.1093/neuonc/not128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu XS, et al. Functional response to SDF1 alpha through over-expression of CXCR152 on adult subventricular zone progenitor cells. Brain Res. 2008;1226:18–26. doi: 10.1016/j.brainres.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kokovay E, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR153 signaling. Cell Stem Cell. 2010;7(2):163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jin K, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24(1):171–189. doi: 10.1016/S1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 155.Salman H, Ghosh P, Kernie SG. Subventricular zone neural stem cells remodel the brain following traumatic injury in adult mice. J Neurotrauma. 2004;21(3):283–292. doi: 10.1089/089771504322972077. [DOI] [PubMed] [Google Scholar]
- 156.Imitola J, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102IF: 9.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Robin AM, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(1):125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 158.Itoh T, et al. The relationship between SDF-1alpha/CXCR158 and neural stem cells appearing in damaged area after traumatic brain injury in rats. Neurol Res. 2009;31(1):90–102. doi: 10.1179/174313208X332995. [DOI] [PubMed] [Google Scholar]
- 159.Mao W, et al. CXCL12/CXCR159 axis improves migration of neuroblasts along corpus callosum by stimulating MMP-2 secretion after traumatic brain injury in rats. Neurochem Res. 2016;41(6):1315–1322. doi: 10.1007/s11064-016-1831-2IF: 3.7 Q2 . [DOI] [PubMed] [Google Scholar]
- 160.Saha B, et al. Cortical lesion stimulates adult subventricular zone neural progenitor cell proliferation and migration to the site of injury. Stem Cell Res. 2013;11(3):965–977. doi: 10.1016/j.scr.2013.06.006IF: 0.8 Q4 . [DOI] [PubMed] [Google Scholar]
- 161.Zhu M, et al. Human cerebrospinal fluid regulates proliferation and migration of stem cells through insulin-like growth factor-1. Stem Cells Dev. 2015;24(2):160–171. doi: 10.1089/scd.2014.0076IF: 2.5 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.do Carmo A, et al. CXCL12/CXCR1 promotes motility and proliferation of glioma cells. Cancer Biol Ther. 2010;9(1):56–65. doi: 10.4161/cbt.9.1.10342IF: 4.4 Q2 . [DOI] [PubMed] [Google Scholar]
- 163.Gatti M, et al. Inhibition of CXCL12/CXCR163 autocrine/paracrine loop reduces viability of human glioblastoma stem-like cells affecting self-renewal activity. Toxicology. 2013;314(2–3):209–220. doi: 10.1016/j.tox.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 164.Wang S, et al. CXCL12-induced upregulation of FOXM1 expression promotes human glioblastoma cell invasion. Biochem Biophys Res Commun. 2014;447(1):1–6. doi: 10.1016/j.bbrc.2013.12.079. [DOI] [PubMed] [Google Scholar]
- 165.Goffart N, et al. Adult mouse subventricular zones stimulate glioblastoma stem cells specific invasion through CXCL12/CXCR165 signaling. Neuro Oncol. 2015;17(1):81–94. doi: 10.1093/neuonc/nou144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gravina GL, et al. The novel CXCR166 antagonist, PRX177561, reduces tumor cell proliferation and accelerates cancer stem cell differentiation in glioblastoma preclinical models. Tumour Biol. 2017;39(6):1010428317695528. doi: 10.1177/1010428317695528. [DOI] [PubMed] [Google Scholar]
- 167.Andrews WD, Barber M, Parnavelas JG. Slit-Robo interactions during cortical development. J Anat. 2007;211(2):188–198. doi: 10.1111/j.1469-7580.2007.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu W, et al. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400(6742):331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nguyen-Ba-Charvet KT, et al. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24(6):1497–1506. doi: 10.1523/JNEUROSCI.4729-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guerrero-Cazares H, et al. Brief report: Robo1 regulates the migration of human subventricular zone neural progenitor cells during development. Stem Cells. 2017;35(7):1860–1865. doi: 10.1002/stem.2628IF: 4.0 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dallol A, et al. Frequent epigenetic inactivation of the SLIT2 gene in gliomas. Oncogene. 2003;22(29):4611–4616. doi: 10.1038/sj.onc.1206687. [DOI] [PubMed] [Google Scholar]
- 172.Astuti D, et al. SLIT2 promoter methylation analysis in neuroblastoma, Wilms’ tumour and renal cell carcinoma. Br J Cancer. 2004;90(2):515–521. doi: 10.1038/sj.bjc.6601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yiin JJ, et al. Slit2 inhibits glioma cell invasion in the brain by suppression of Cdc42 activity. Neuro Oncol. 2009;11(6):779–789. doi: 10.1215/15228517-2009-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mertsch S, et al. Slit2 involvement in glioma cell migration is mediated by Robo1 receptor. J Neurooncol. 2008;87(1):1–7. doi: 10.1007/s11060-007-9484-2. [DOI] [PubMed] [Google Scholar]
- 175.Xu Y, et al. Slit2/Robo1 signaling in glioma migration and invasion. Neurosci Bull. 2010;26(6):474–478. doi: 10.1007/s12264-010-0730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Casazza A, Fazzari P, Tamagnone L. Semaphorin signals in cell adhesion and cell migration: functional role and molecular mechanisms. Adv Exp Med Biol. 2007;600:90–108. doi: 10.1007/978-0-387-70956-7_8. [DOI] [PubMed] [Google Scholar]
- 177.Melendez-Herrera E, et al. Semaphorin-3A and its receptor neuropilin-1 are predominantly expressed in endothelial cells along the rostral migratory stream of young and adult mice. Cell Tissue Res. 2008;333(2):175–184. doi: 10.1007/s00441-008-0643-3. [DOI] [PubMed] [Google Scholar]
- 178.Kong Y, et al. Structural basis for Plexin activation and regulation. Neuron. 2016;91(3):548–560. doi: 10.1016/j.neuron.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Falk J, et al. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48(1):63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 180.Tamamaki N, et al. Evidence that Sema3A and Sema3F regulate the migration of GABAergic neurons in the developing neocortex. J Comp Neurol. 2003;455(2):238–248. doi: 10.1002/cne.10476. [DOI] [PubMed] [Google Scholar]
- 181.Andrews WD, et al. Altered proliferative ability of neuronal progenitors in PlexinA1 mutant mice. J Comp Neurol. 2016;524(3):518–534. doi: 10.1002/cne.23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hirschberg A, et al. Gene deletion mutants reveal a role for semaphorin receptors of the plexin-B family in mechanisms underlying corticogenesis. Mol Cell Biol. 2010;30(3):764–780. doi: 10.1128/MCB.01458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sun T, Li W, Ling S. miR-30c and semaphorin 3A determine adult neurogenesis by regulating proliferation and differentiation of stem cells in the subventricular zones of mouse. Cell Prolif. 2016;49(3):270–280. doi: 10.1111/cpr.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Saha B, et al. Plexin-B2 regulates the proliferation and migration of neuroblasts in the postnatal and adult subventricular zone. J Neurosci. 2012;32(47):16892–16905. doi: 10.1523/JNEUROSCI.0344-12.2012IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Man J, et al. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell Rep. 2014;9(5):1812–1826. doi: 10.1016/j.celrep.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bagci T, et al. Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene. 2009;28(40):3537–3550. doi: 10.1038/onc.2009.204. [DOI] [PubMed] [Google Scholar]
- 187.Zhou X, et al. Effects of SEMA3G on migration and invasion of glioma cells. Oncol Rep. 2012;28(1):269–275. doi: 10.3892/or.2012.1796. [DOI] [PubMed] [Google Scholar]
- 188.Behar TN, et al. Neurotrophins stimulate chemotaxis of embryonic cortical neurons. Eur J Neurosci. 1997;9(12):2561–2570. doi: 10.1111/j.1460-9568.1997.tb01685.x. [DOI] [PubMed] [Google Scholar]
- 189.Ohmiya M, et al. Administration of FGF-2 to embryonic mouse brain induces hydrocephalic brain morphology and aberrant differentiation of neurons in the postnatal cerebral cortex. J Neurosci Res. 2001;65(3):228–235. doi: 10.1002/jnr.1146. [DOI] [PubMed] [Google Scholar]
- 190.Fukumitsu H, et al. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J Neurosci. 2006;26(51):13218–13230. doi: 10.1523/JNEUROSCI.4251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chiaramello S, et al. BDNF/TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur J Neurosci. 2007;26(7):1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- 192.Petridis AK, El Maarouf A. Brain-derived neurotrophic factor levels influence the balance of migration and differentiation of subventricular zone cells, but not guidance to the olfactory bulb. J Clin Neurosci. 2011;18(2):265–270. doi: 10.1016/j.jocn.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 193.Xiong J, et al. Mature brain-derived neurotrophic factor and its receptor TrkB are upregulated in human glioma tissues. Oncol Lett. 2015;10(1):223–227. doi: 10.3892/ol.2015.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Johnston AL, et al. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 2007;5(8):e212. doi: 10.1371/journal.pbio.0050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xiong J, et al. Mature BDNF promotes the growth of glioma cells in vitro. Oncol Rep. 2013;30(6):2719–2724. doi: 10.3892/or.2013.2746. [DOI] [PubMed] [Google Scholar]
- 196.Lawn S, et al. Neurotrophin signaling via TrkB and TrkC receptors promotes the growth of brain tumor-initiating cells. J Biol Chem. 2015;290(6):3814–3824. doi: 10.1074/jbc.M114.599373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li YS, et al. Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science. 1990;250(4988):1690–1694. doi: 10.1126/science.2270483. [DOI] [PubMed] [Google Scholar]
- 198.Rauvala H, Pihlaskari R. Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons. J Biol Chem. 1987;262(34):16625–16635. [PubMed] [Google Scholar]
- 199.Maeda N, Noda M. Involvement of receptor-like protein tyrosine phosphatase zeta/RPTPbeta and its ligand pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) in neuronal migration. J Cell Biol. 1998;142(1):203–216. doi: 10.1083/jcb.142.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Qin EY, et al. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell. 2017;170(5):845–859.e19. doi: 10.1016/j.cell.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang L, et al. Overexpression of heparin-binding growth-associated molecule in malignant glioma cells. Neurol Med Chir (Tokyo) 2004;44(12):637–643. doi: 10.2176/nmc.44.637. [DOI] [PubMed] [Google Scholar]
- 202.Mentlein R, Held-Feindt J. Pleiotrophin, an angiogenic and mitogenic growth factor, is expressed in human gliomas. J Neurochem. 2002;83(4):747–753. doi: 10.1046/j.1471-4159.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- 203.Ma J, et al. Co-expression of midkine and pleiotrophin predicts poor survival in human glioma. J Clin Neurosci. 2014;21(11):1885–1890. doi: 10.1016/j.jocn.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 204.Zhang L, et al. Pleiotrophin promotes vascular abnormalization in gliomas and correlates with poor survival in patients with astrocytomas. Sci Signal. 2015;8(406):ra125. doi: 10.1126/scisignal.aaa1690. [DOI] [PubMed] [Google Scholar]
- 205.Koyama-Nasu R, et al. The pleiotrophin-ALK axis is required for tumorigenicity of glioblastoma stem cells. Oncogene. 2014;33(17):2236–2244. doi: 10.1038/onc.2013.168. [DOI] [PubMed] [Google Scholar]
- 206.Powers C, et al. Pleiotrophin signaling through anaplastic lymphoma kinase is rate-limiting for glioblastoma growth. J Biol Chem. 2002;277(16):14153–14158. doi: 10.1074/jbc.M112354200. [DOI] [PubMed] [Google Scholar]
- 207.Ulbricht U, et al. Expression and function of the receptor protein tyrosine phosphatase zeta and its ligand pleiotrophin in human astrocytomas. J Neuropathol Exp Neurol. 2003;62(12):1265–1275. doi: 10.1093/jnen/62.12.1265. [DOI] [PubMed] [Google Scholar]
- 208.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 209.Belvindrah R, et al. Beta1 integrins control the formation of cell chains in the adult rostral migratory stream. J Neurosci. 2007;27(10):2704–2717. doi: 10.1523/JNEUROSCI.2991-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jacques TS, et al. Neural precursor cell chain migration and division are regulated through different beta1 integrins. Development. 1998;125(16):3167–3177. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- 211.Flanagan LA, et al. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res. 2006;83(5):845–856. doi: 10.1002/jnr.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Alfonso J, et al. Downregulation of sphingosine 1-phosphate receptor 1 promotes the switch from tangential to radial migration in the OB. J Neurosci. 2015;35(40):13659–13672. doi: 10.1523/JNEUROSCI.1353-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lathia JD, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6(5):421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ying M, et al. Kruppel-like factor-9 (KLF9) inhibits glioblastoma stemness through global transcription repression and integrin alpha6 inhibition. J Biol Chem. 2014;289(47):32742–32756. doi: 10.1074/jbc.M114.588988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Blandin AF, et al. Glioma cell dispersion is driven by alpha5 integrin-mediated cell-matrix and cell-cell interactions. Cancer Lett. 2016;376(2):328–338. doi: 10.1016/j.canlet.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 216.Haas TL, et al. Integrin alpha7 is a functional marker and potential therapeutic target in glioblastoma. Cell Stem Cell. 2017;21(1):35–50 e9. doi: 10.1016/j.stem.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 217.Tilghman J, et al. Regulation of glioblastoma tumor-propagating cells by the integrin partner tetraspanin CD151. Neoplasia. 2016;18(3):185–198. doi: 10.1016/j.neo.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhou P, et al. CD151-alpha3beta1 integrin complexes are prognostic markers of glioblastoma and cooperate with EGFR to drive tumor cell motility and invasion. Oncotarget. 2015;6(30):29675–29693. doi: 10.18632/oncotarget.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu Z, et al. EGFRvIII/integrin beta3 interaction in hypoxic and vitronectinenriching microenvironment promote GBM progression and metastasis. Oncotarget. 2016;7(4):4680–4694. doi: 10.18632/oncotarget.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11(7):502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 221.Porlan E, et al. MT5-MMP regulates adult neural stem cell functional quiescence through the cleavage of N-cadherin. Nat Cell Biol. 2014;16(7):629–638. doi: 10.1038/ncb2993. [DOI] [PubMed] [Google Scholar]
- 222.Yagita Y, et al. N-cadherin mediates interaction between precursor cells in the subventricular zone and regulates further differentiation. J Neurosci Res. 2009;87(15):3331–3342. doi: 10.1002/jnr.22044. [DOI] [PubMed] [Google Scholar]
- 223.Fujikake K, et al. Detachment of chain-forming neuroblasts by Fyn-mediated control of cell–cell adhesion in the postnatal brain. J Neurosci. 2018;38(19):4598–4609. doi: 10.1523/JNEUROSCI.1960-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Klingener M, et al. N-cadherin promotes recruitment and migration of neural progenitor cells from the SVZ neural stem cell niche into demyelinated lesions. J Neurosci. 2014;34(29):9590–9606. doi: 10.1523/JNEUROSCI.3699-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 225.Cao L, et al. Physiological electrical signals promote chain migration of neuroblasts by up-regulating P2Y1 purinergic receptors and enhancing cell adhesion. Stem Cell Rev. 2015;11(1):75–86. doi: 10.1007/s12015-014-9524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kim MY, et al. Bone morphogenetic protein 4 stimulates attachment of neurospheres and astrogenesis of neural stem cells in neurospheres via phosphatidylinositol 3 kinase-mediated upregulation of N-cadherin. Neuroscience. 2010;170(1):8–15. doi: 10.1016/j.neuroscience.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 227.Chen D, et al. E-cadherin maintains the activity of neural stem cells and inhibits the migration. Int J Clin Exp Pathol. 2015;8(11):14247–14251. [PMC free article] [PubMed] [Google Scholar]
- 228.Schulte JD, et al. Cadherin-11 regulates motility in normal cortical neural precursors and glioblastoma. PLoS One. 2013;8(8):e70962. doi: 10.1371/journal.pone.0070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 230.Utsuki S, et al. Relationship between the expression of E-, N-cadherins and beta-catenin and tumor grade in astrocytomas. J Neurooncol. 2002;57(3):187–192. doi: 10.1023/A:1015720220602. [DOI] [PubMed] [Google Scholar]
- 231.Noh MG, et al. Prognostic significance of E-cadherin and N-cadherin expression in Gliomas. BMC Cancer. 2017;17(1):583. doi: 10.1186/s12885-017-3591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kaur H, et al. Cadherin-11, a marker of the mesenchymal phenotype, regulates glioblastoma cell migration and survival in vivo. Mol Cancer Res. 2012;10(3):293–304. doi: 10.1158/1541-7786.MCR-11-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rutishauser U, et al. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science. 1988;240(4848):53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- 234.Cunningham BA, et al. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236(4803):799–806. doi: 10.1126/science.3576199IF: 44.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 235.Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci. 1993;13(6):2351–2358. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Finne J, et al. Occurrence of alpha 2-8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983;112(2):482–487. doi: 10.1016/0006-291X(83)91490-0. [DOI] [PubMed] [Google Scholar]
- 237.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA. 1996;93(25):14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tomasiewicz H, et al. Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron. 1993;11(6):1163–1174. doi: 10.1016/0896-6273(93)90228-J. [DOI] [PubMed] [Google Scholar]
- 239.Cremer H, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367(6462):455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 240.Ono K, et al. N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron. 1994;13(3):595–609. doi: 10.1016/0896-6273(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 241.Hu H, et al. The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron. 1996;16(4):735–743. doi: 10.1016/S0896-6273(00)80094-X. [DOI] [PubMed] [Google Scholar]
- 242.Rockle I, Hildebrandt H. Deficits of olfactory interneurons in polysialyltransferase- and NCAM-deficient mice. Dev Neurobiol. 2016;76(4):421–433. doi: 10.1002/dneu.22324. [DOI] [PubMed] [Google Scholar]
- 243.Battista D, Rutishauser U. Removal of polysialic acid triggers dispersion of subventricularly derived neuroblasts into surrounding CNS tissues. J Neurosci. 2010;30(11):3995–4003. doi: 10.1523/JNEUROSCI.4382-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Figarella-Branger DF, Durbec PL, Rougon GN. Differential spectrum of expression of neural cell adhesion molecule isoforms and L1 adhesion molecules on human neuroectodermal tumors. Cancer Res. 1990;50(19):6364–6370. [PubMed] [Google Scholar]
- 245.Figarella-Branger D, et al. Expression of adhesion molecules N. CAM, L1 and HNK1 epitope by medulloblastoma. Rev Neurol Paris. 1992;148(6–7):417–422. [PubMed] [Google Scholar]
- 246.Petridis AK, et al. Polysialic acid overexpression in malignant astrocytomas. Acta Neurochir (Wien) 2009;151(6):601–603. doi: 10.1007/s00701-009-0324-3. [DOI] [PubMed] [Google Scholar]
- 247.Suzuki M, et al. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology. 2005;15(9):887–894. doi: 10.1093/glycob/cwi071. [DOI] [PubMed] [Google Scholar]
- 248.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kang SS, et al. Inhibition of matrix metalloproteinase-9 attenuated neural progenitor cell migration after photothrombotic ischemia. Brain Res. 2008;1228:20–26. doi: 10.1016/j.brainres.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 250.Lee SR, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26(13):3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kanemitsu M, et al. EMMPRIN overexpression in SVZ neural progenitor cells increases their migration towards ischemic cortex. Exp Neurol. 2017;297:14–24. doi: 10.1016/j.expneurol.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 252.Bovetti S, et al. Subventricular zone-derived neuroblast migration to the olfactory bulb is modulated by matrix remodelling. Eur J Neurosci. 2007;25(7):2021–2033. doi: 10.1111/j.1460-9568.2007.05441.x. [DOI] [PubMed] [Google Scholar]
- 253.Lin KT, et al. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J Biol Chem. 2008;283(43):28969–28979. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Forsyth PA, et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79(11–12):1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nan Y, et al. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21. doi: 10.1016/j.brainres.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 256.Sun L, et al. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011;1389:9–18. doi: 10.1016/j.brainres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 257.Zheng X, et al. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329(2):146–154. doi: 10.1016/j.canlet.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lakka SS, et al. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23(27):4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 259.Inoue A, et al. Cancer stem-like cells of glioblastoma characteristically express MMP-13 and display highly invasive activity. Int J Oncol. 2010;37(5):1121–1131. doi: 10.3892/ijo_00000764. [DOI] [PubMed] [Google Scholar]
- 260.Lathia JD, et al. Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PLoS One. 2011;6(9):e24807. doi: 10.1371/journal.pone.0024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Vakoc BJ, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15(10):1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Guerrero-Cazares H, Chaichana KL, Quinones-Hinojosa A. Neurosphere culture and human organotypic model to evaluate brain tumor stem cells. Methods Mol Biol. 2009;568:73–83. doi: 10.1007/978-1-59745-280-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ohnishi T, et al. A novel model of glioma cell invasion using organotypic brain slice culture. Cancer Res. 1998;58(14):2935–2940. [PubMed] [Google Scholar]