Abstract 抽象的
Traumatic brain injury (TBI) leads to progressive neurodegeneration that may be caused by chronic traumatic encephalopathy (CTE). However, the precise mechanism remains unclear. Herein, the study identifies a crucial protein, axonemal dynein light intermediate polypeptide 1 (DNALI1), and elucidated its potential pathogenic role in post‐TBI neurodegeneration. The DNALI1 gene is systematically screened through analyses of Aging, Dementia, and TBI studies, confirming its elevated expression both in vitro and in vivo. Moreover, it is observed that altered DNALI1 expression under normal conditions has no discernible effect. However, upon overexpression, DNALI1 inhibits autophagosome‐lysosome fusion, reduces autophagic flux, and exacerbates cell death under pathological conditions. DNALI1 silencing significantly enhances autophagic flux and alleviates neurodegeneration in a CTE model. These findings highlight DNALI1 as a potential key target for preventing TBI‐related neurodegeneration.
创伤性脑损伤 (TBI) 会导致进行性神经变性,这可能是由慢性创伤性脑病 (CTE) 引起的。然而,确切的机制仍不清楚。在此,该研究鉴定了一种关键蛋白——轴丝动力蛋白轻中间多肽 1 (DNALI1),并阐明了其在 TBI 后神经变性中的潜在致病作用。通过对衰老、痴呆和 TBI 研究的分析,系统地筛选了DNALI1基因,证实了其在体外和体内的表达升高。此外,据观察,正常条件下 DNALI1 表达的改变没有明显的影响。然而,过度表达后,DNALI1 会抑制自噬体-溶酶体融合,减少自噬通量,并加剧病理条件下的细胞死亡。 DNALI1 沉默可显着增强 CTE 模型中的自噬通量并减轻神经退行性变。这些发现强调 DNALI1 是预防 TBI 相关神经变性的潜在关键靶点。
Keywords: autophagy, chronic traumatic encephalopathy, DNALI1, neurodegeneration, traumatic brain injury
关键词:自噬, 慢性创伤性脑病, DNALI1, 神经退行性疾病, 创伤性脑损伤
Following TBI, ciliogenesis increases under mechanical and oxidative stress, leading to the increase of DNALI1. DNALI1 prevents the clearance of phosphorylated tau by autophagy, inhibiting autophagic flux and contributing to the development of neurodegeneration.
TBI 后,纤毛发生在机械和氧化应激下增加,导致 DNALI1 增加。 DNALI1 可防止自噬清除磷酸化 tau 蛋白,从而抑制自噬通量并促进神经退行性变的发展。
1. Introduction 一、简介
Traumatic brain injury (TBI) refers to the temporary or permanent impairment of brain function caused by external mechanical forces on the head.[
1
] TBI is commonly associated with high morbidity, mortality, disability rates, and other severe adverse outcomes.[
2
] Globally, the annual incidence of TBI varies from 27 to 69 million,[
3
] and it is estimated that approximately half of the world's population will experience one or more TBIs in their lifetime. Long‐term disability affects 43% of patients hospitalized for TBI,[
4
] imposing a significant socioeconomic burden. Despite the prevalence of TBI, current treatments primarily aim to stabilize and alleviate symptoms, often neglecting potential long‐term effects.
创伤性脑损伤(TBI)是指头部受到外部机械力造成的暂时或永久性脑功能损伤。 [
1
] TBI 通常与高发病率、死亡率、致残率和其他严重不良后果相关。 [
2
]全球范围内,TBI 的年发病率从 2700 万到 6900 万不等, [
3
]据估计,世界上大约一半的人口一生中会经历一次或多次创伤性脑损伤。 43% 因 TBI 住院的患者存在长期残疾, [
4
]造成重大的社会经济负担。尽管 TBI 很普遍,但目前的治疗主要旨在稳定和缓解症状,往往忽视了潜在的长期影响。
TBI stands out as the most significant non‐genetic, non‐age‐related risk factor for dementia.[
5
] The neurodegeneration that follows head injuries or repetitive mild trauma may be caused by chronic traumatic encephalopathy (CTE) alone or in conjunction with Alzheimer's disease (AD). Various severities of TBI can result in different pathological states, and epidemiological studies underscore that 70–90% of TBIs are mild.[
6
] Though not immediately life‐threatening, repeated TBIs induce cumulative effects, ultimately elevating the risk of CTE.[
7
] CTE is a progressive tauopathy with a distinct clinical and neuropathological profile that becomes symptomatic many years after an individual experiences repeated concussive or subconcussive blows to the head.[
8
] The pathology of CTE differs from the clinical and pathological sequelae of severe single‐incident TBI, and it typically involves neurofibrillary tangles (NFTs) accumulating in the superficial gray matter.[
9
] Axon strain results in microtubule rupture and tau release, facilitating its phosphorylation at disease‐related sites, and potentially leading to neurodegeneration.[
10
] Amyloid β pathology mainly manifests in single‐incident TBI, with evident accumulation of amyloid precursor protein in damaged axons and cell bodies in both TBI animals and humans within hours.[
11
] Therefore, the clinical presentation of CTE can be different to AD, emphasizing the importance of investigating the mechanisms underpinning CTE and identifying critical factors with diagnostic, therapeutic, and prognostic value.
TBI 是痴呆症最重要的非遗传、非年龄相关的危险因素。 [
5
]头部受伤或重复性轻度创伤后的神经变性可能是由慢性创伤性脑病 (CTE) 单独引起或与阿尔茨海默病 (AD) 联合引起。不同严重程度的TBI可导致不同的病理状态,流行病学研究强调70-90%的TBI是轻度的。 [
6
]虽然不会立即危及生命,但反复的 TBI 会产生累积效应,最终增加 CTE 的风险。 [
7
] CTE 是一种进行性 tau 蛋白病,具有独特的临床和神经病理学特征,在个体多次遭受头部震荡或亚震荡打击后多年才会出现症状。 [
8
CTE的病理学不同于严重单次 TBI 的临床和病理后遗症,它通常涉及浅层灰质中积累的神经原纤维缠结 (NFT)。 [
9
]轴突应变导致微管破裂和 tau 蛋白释放,促进其在疾病相关部位的磷酸化,并可能导致神经退行性变。 [
10
] β淀粉样蛋白病理主要表现为单次TBI,在TBI动物和人类的受损轴突和细胞体中,淀粉样前体蛋白在数小时内明显积累。[
11
因此,CTE 的临床表现可能与 AD 不同,强调研究 CTE 的机制并确定具有诊断、治疗和预后价值的关键因素的重要性。
In this study, we employed a variety of bioinformatics and statistical approaches, with a particular focus on the hippocampus. By comparing data from all brain regions, we aimed to identify the key pathways or genes involved in post‐TBI neurodegeneration. Subsequently, we validated these findings using in vivo and in vitro TBI models. We conducted targeted gene manipulations using CTE animal model and evaluated the impacts on neurodegeneration and delved into potential underlying mechanisms. These results identify a promising target for preventing CTE.
在这项研究中,我们采用了各种生物信息学和统计方法,特别关注海马体。通过比较所有大脑区域的数据,我们的目的是确定参与 TBI 后神经退行性变的关键途径或基因。随后,我们使用体内和体外 TBI 模型验证了这些发现。我们使用 CTE 动物模型进行有针对性的基因操作,评估对神经退行性变的影响,并深入研究潜在的潜在机制。这些结果确定了预防 CTE 的有希望的目标。
2. Results 2. 结果
2.1.
DNALI1 is a Critical Gene to Predict Cognitive Impairment Post‐TBI
2.1.
DNALI1是预测 TBI 后认知障碍的关键基因
We obtained clinical and genetic data from 107 individuals participating in the Aging, Dementia, and Traumatic Brain Injury Study.[
12
] The study's demographics and characteristics are shown in Table S1 (Supporting Information). We categorized participants based on TBI diagnosis, dementia pathology, and dementia clinical diagnosis defined by the original study[
12
] (Figure
1a). Utilizing sequencing data from four brain regions (temporal cortex [TCx], parietal cortex [PCx], frontal white matter [FWM], and hippocampus [HIP]) we conducted a preliminary analysis employing principal component analysis, and revealing distinct gene expression patterns in the hippocampus compared to other brain regions (Figure S1a, Supporting Information). Subsequently, we performed an extensive analysis of AT8 levels (mapping tau phosphorylation at Ser202 or Thr205; Figure 1b; Figure S1b–d, Supporting Information) and the brain ptau181/tau ratio (Figure 1c; Figure S1e–g, Supporting Information) across the brain regions described above, comparing “clinical dementia” and “non‐demented” cohorts in TBI and pathology groups. Both AT8 and ptau181/tau ratio exhibited significant differences between “clinical dementia” and “non‐demented” groups exclusively within the hippocampus (Figure 1b,c), suggesting that this brain region may be the primary area affected by TBI. We have also examined changes related to Aβ pathology, detecting no significant differences among groups (Figure S2, Supporting Information), thus indicating that the pathology in the study may be consistent with CTE.
我们获得了参与衰老、痴呆和创伤性脑损伤研究的 107 名个体的临床和遗传数据。 [
12
]该研究的人口统计和特征如表所示 S1 (支持信息)。我们根据原始研究定义的 TBI 诊断、痴呆病理学和痴呆临床诊断对参与者进行分类。
12
] (数字
1a )。利用来自四个大脑区域(颞叶皮层 [TCx]、顶叶皮层 [PCx]、额叶白质 [FWM] 和海马体 [HIP])的测序数据,我们采用主成分分析进行了初步分析,并揭示了不同脑区中不同的基因表达模式。海马体与其他大脑区域的比较(图 S1a ,支持信息)。随后,我们对 AT8 水平进行了广泛的分析(映射 Ser202 或 Thr205 处的 tau 磷酸化;图 1b ;数字 S1b–d ,支持信息)和大脑ptau181/tau比值(图 1c ;数字 S1e–g ,支持信息)跨越上述大脑区域,比较 TBI 和病理组中的“临床痴呆”和“非痴呆”队列。 AT8 和 ptau181/tau 比率在“临床痴呆”组和“非痴呆”组之间仅在海马内表现出显着差异(图 1b,c ),表明该大脑区域可能是受 TBI 影响的主要区域。我们还检查了与 Aβ 病理学相关的变化,发现各组之间没有显着差异(图 S2 ,支持信息),从而表明该研究中的病理学可能与 CTE 一致。
Figure 1. 图 1.
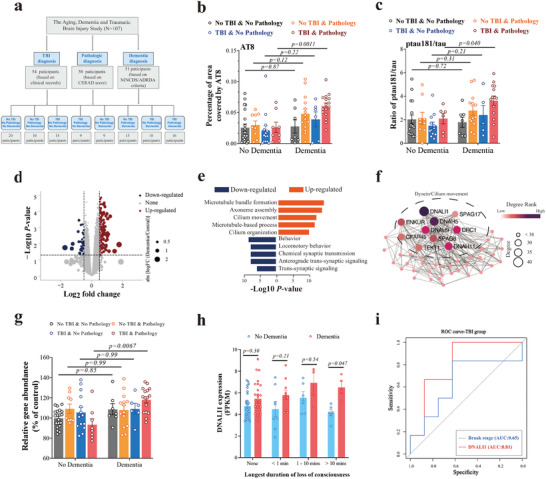
DNALI1 is the critical gene of post‐TBI neurodegeneration. a) Schematic illustration of the participants' grouping. Participants were divided according to TBI diagnosis, dementia pathological diagnosis, and dementia diagnosis. b,c) Comparison of the percentage of the area covered by AT8 (b) using histology and immunohistochemistry (IHC) and the ratio of ptau181/tau detected by Luminex assays across different groups (c). d) Sample volcano plot for participants showing –log10 (p‐value) and log2FC values for all genes, highlighting those significantly upregulated (red dots) or downregulated (blue dots) genes with dementia; non‐significant genes are marked in gray. e) Reactome enrichment for upregulated (Log (Dementia/Non‐dementia group) >0.5, and p < 0.05) and downregulated (Log (Dementia/Non‐dementia group) <0.5, and p < 0.05) genes, based on Metascape. f) Protein‐protein interaction (PPI) analysis for the MEcyan module, where node size and color represent the degree rank and degree. g) Levels of DNALI1 detected by RNA‐seq between dementia and non‐dementia groups. h) Comparison of DNALI1 expression fragments per kilobase per million (FPKM) between dementia and non‐dementia groups across different durations of loss of consciousness. i) Receiver‐operating curve (ROC) analysis to determine the discriminative power of DNALI1 expression to distinguish dementia and control in participants with TBI, with Braak stage score as a reference. The data are presented as means ± SEM. Two‐way ANOVA with Sidak's multiple comparisons test (b,c,g,h) was used. p‐values are indicated on the graphs.
DNALI1是 TBI 后神经变性的关键基因。 a) 参与者分组示意图。参与者根据TBI诊断、痴呆病理诊断和痴呆诊断进行分组。 b,c) 使用组织学和免疫组织化学 (IHC) 比较 AT8 覆盖的面积百分比 (b) 以及不同组中 Luminex 检测检测到的 ptau181/tau 比率 (c)。 d) 参与者火山图样本,显示所有基因的 –log10( p值)和 log 2 FC 值,突出显示那些患有痴呆症的显着上调(红点)或下调(蓝点)基因;非显着基因用灰色标记。 e) 上调(Log(痴呆/非痴呆组)>0.5, p < 0.05)和下调(Log(痴呆/非痴呆组)<0.5, p < 0.05)基因的反应组富集,基于元景观。 f) MEcyan 模块的蛋白质-蛋白质相互作用 (PPI) 分析,其中节点大小和颜色代表程度等级和程度。 g) 通过RNA-seq检测痴呆组和非痴呆组的DNALI1水平。 h) 不同意识丧失持续时间的痴呆组和非痴呆组之间每百万碱基DNALI1表达片段 (FPKM) 的比较。 i) 接受者操作曲线 (ROC) 分析,以确定DNALI1表达区分 TBI 参与者痴呆和控制的辨别力,以 Braak 阶段评分作为参考。数据以平均值±SEM 表示。使用带有 Sidak 多重比较检验(b、c、g、h)的双向方差分析。 p值显示在图表上。
We next examined the transcriptome changes in the cohort, employing bioinformatics analysis specifically within participants originally described as “diagnosed with TBI” and with “dementia pathology”. We then compared “clinical dementia” and “non‐demented” groups (Figure 1d–f; Figure S3, Supporting Information). Through the enrichment of differentially expressed genes (Figure 1d; Figure S3a, Supporting Information), we identified microtubule‐, axoneme‐, and cilium‐related pathways as the most upregulated (Figure 1e), consistent with the results from Gene Set Enrichment Analysis (GSEA) (Figure S3b, Supporting Information). Furthermore, all genes were categorized into modules according to their expression levels and correlation coefficients. We calculated the p‐values for gene modules associated with dementia diagnosis and Braak. The module most relevant to these phenotypes (Figure S3c, Supporting Information, Diagnose: r = 0.58, p = 0.003; Braak: r = 0.54, p = 0.006) underwent further Gene Ontology (GO) analysis (String, version 11.5) (Figure S3d, Supporting Information). The results mirrored those observed in the pathways shown in Figure 1e, underscoring the importance of these pathways.
接下来,我们检查了该队列中转录组的变化,特别是在最初被描述为“诊断患有 TBI”和“痴呆病理学”的参与者中进行了生物信息学分析。然后我们比较了“临床痴呆”和“非痴呆”组(图 1d–f ;数字 S3 ,支持信息)。通过差异表达基因的富集(图 1d ;数字 S3a ,支持信息),我们确定微管、轴丝和纤毛相关途径是上调最多的途径(图 1e ),与基因集富集分析(GSEA)的结果一致(图 S3b ,支持信息)。此外,所有基因根据其表达水平和相关系数被分类为模块。我们计算了与痴呆诊断和 Braak 相关的基因模块的p值。与这些表型最相关的模块(图 S3c ,支持信息,诊断:r = 0.58, p = 0.003; Braak:r = 0.54, p = 0.006)进行了进一步的基因本体(GO)分析(字符串,版本 11.5)(图 S3d ,支持信息)。结果反映了在图所示的路径中观察到的结果 1e ,强调了这些途径的重要性。
Through the analysis of degree rank results in the protein‐protein interaction (PPI) network, we further identified Axonemal dynein light intermediate polypeptide 1 (DNALI1) as the most critical gene in the gene module (Figure 1f, Degree = 42). Indeed, DNALI1 expression was significantly elevated in the “clinical dementia” group (TBI diagnosis and dementia pathology) (Figure 1g, p = 0.0067). When comparing DNALI1 expression at different durations of loss of consciousness, we observed a significant difference between the “clinical dementia” and “non‐demented” groups, particularly when the duration of loss of consciousness exceeded 10 min (Figure 1h, p = 0.047). Importantly, DNALI1 expression was significantly correlated with AT8 (Figure S4a, Supporting Information, r = 0.53, p = 0.011) and the ratio of ptau181/tau (Figure S4b, Supporting Information, r = 0.68, p = 0.002) in participants with TBI‐“clinical dementia”, whereas such correlations were absent in “non‐demented” participants. Furthermore, the expression of DNALI1 in TBI participants exhibited great predictive ability for dementia, which was even better than that of the Braak stage score (Figure 1i, AUC = 0.81). Collectively, these findings indicate that DNALI1 is a critical gene for the development of neurodegeneration after TBI.
通过对蛋白质-蛋白质相互作用(PPI)网络中的程度排序结果的分析,我们进一步确定了Axonemal dynein轻中间多肽1(DNALI1)是该基因模块中最关键的基因(图 1f ,度= 42)。事实上, DNALI1表达在“临床痴呆”组中显着升高(TBI 诊断和痴呆病理)(图 1g , p = 0.0067)。当比较不同意识丧失持续时间的DNALI1表达时,我们观察到“临床痴呆”组和“非痴呆”组之间存在显着差异,特别是当意识丧失持续时间超过 10 分钟时(图 1h , p = 0.047)。重要的是, DNALI1表达与 AT8 显着相关(图 S4a ,支持信息,r = 0.53, p = 0.011)和 ptau181/tau 的比率(图 S4b ,支持信息,r = 0.68, p = 0.002)在患有 TBI“临床痴呆”的参与者中,而在“非痴呆”参与者中则不存在这种相关性。此外,TBI参与者中DNALI1的表达表现出对痴呆症的强大预测能力,甚至优于Braak阶段评分(图 1i ,AUC = 0.81)。总的来说,这些发现表明DNALI1是 TBI 后神经退行性疾病发生的关键基因。
2.2. Altered DNALI1 Protein in In Vitro and In Vivo TBI Models
2.2.体外和体内 TBI 模型中 DNALI1 蛋白的改变
DNALI1 is a component of axonemal dynein, responsible for transporting cargo along the axoneme of eukaryotic cilia and flagella in conjunction with other components.[
13
] Despite limited studies connecting DNALI1 to brain diseases, we explored whether DNALI1 expression is altered under conditions mimicking TBI. Serum deprivation has been previously used to simulate neuronal stress after TBI,[
14
] and our immunofluorescence assay revealed a significant increase in DNALI1‐positive staining (Figure
2a, p
= 0.012), which was further confirmed by western blot analysis. Prolonged serum deprivation further amplified DNALI1 expression (Figure 2b), indicating its responsiveness to neuronal stress. Considering the previous correlation between AT8 and DNALI1 in human patients, we examined tau expression and phosphorylation. The expression of tau was unchanged (Figure 2c, p = 0.99), while the AT8/tau ratio increased (Figure 2c, p = 0.0094), aligning with previous findings (Figure S4a, Supporting Information).
DNALI1 是轴丝动力蛋白的一个组成部分,负责与其他组件一起沿着真核纤毛和鞭毛的轴丝运输货物。 [
13
尽管将 DNALI1 与脑部疾病联系起来的研究有限,但我们探索了 DNALI1 表达在模拟 TBI 的条件下是否会发生改变。血清剥夺以前曾被用来模拟 TBI 后的神经元应激, [
14
]并且我们的免疫荧光检测显示 DNALI1 阳性染色显着增加(图
2a , p = 0.012),通过蛋白质印迹分析进一步证实。长时间的血清剥夺进一步放大了 DNALI1 的表达(图 2b ),表明其对神经元应激的反应。考虑到之前人类患者中 AT8 和 DNALI1 之间的相关性,我们检查了 tau 表达和磷酸化。 tau的表达没有变化(图 2c , p = 0.99),而 AT8/tau 比率增加(图 2c , p = 0.0094),与之前的发现一致(图 S4a ,支持信息)。
Figure 2. 图 2.
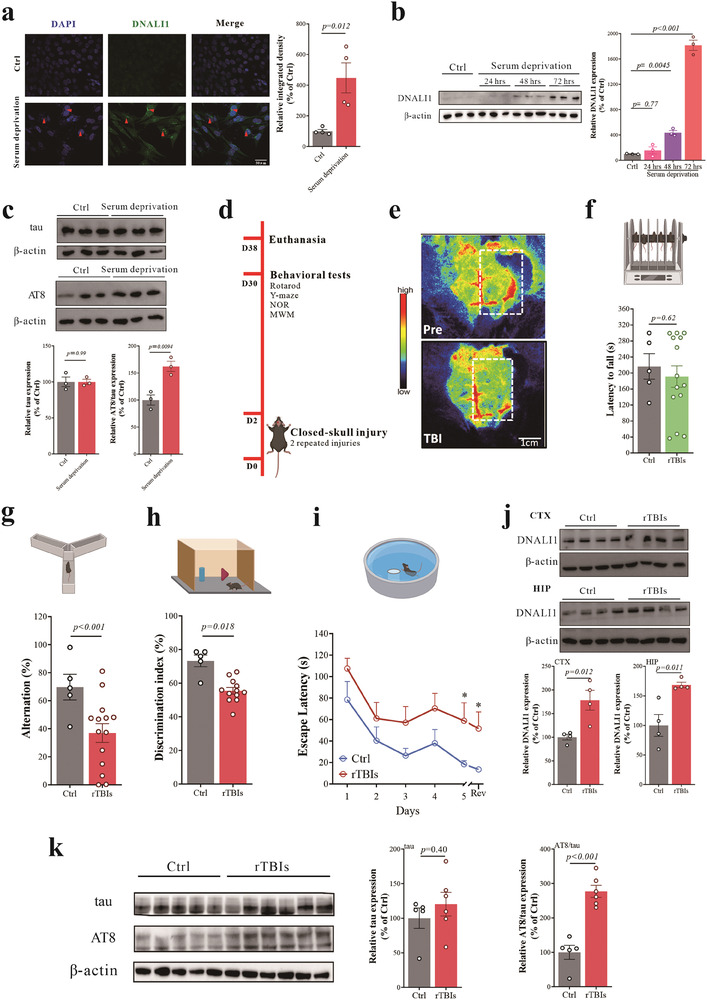
DNALI1 and tau pathology were changed significantly in vitro and in vivo. a) Immunofluorescent co‐labeling of DNALI1 (green) and nuclear (blue) with corresponding statistical results comparing control and serum deprivation models. n = 4 wells from one representative of four independent experiments. Scale bar, 50 µm, as indicated. b) Western blot analysis of DNALI1 in cells subjected to varying duration of serum deprivation (n = 3). Data are normalized to β‐actin and expressed relative to the control. c) Western blot analysis of tau and AT8 for serum deprivation model (n = 3). Data are normalized to β‐actin and expressed relative to the control. d) Diagrammatic drawing of the repeated mild closed‐head model and subsequent experiments. e) Blood flow changes in the TBI model before and after injury detected by Laser speckle imaging. Perfusion is visualized as a 2D color‐coded map of blood flow (red = high; blue = low), with a scale bar = 1 cm. f) Performance on the rotarod test analyzed one month after the brain injury. Ctrl, n = 5; TBI, n = 14. g) Performance on the Y‐Maze spontaneous alternation test analyzed one month after the brain injury. Ctrl, n = 5; TBI, n = 14. h) Performance on the Novel object recognition test analyzed one month after the brain injury. Ctrl, n = 5; TBI, n = 14. i) Performance on the Morris water maze test analyzed one month after the brain injury. Ctrl, n = 5; TBI, n = 14; Rev: Reversal learning. j) Western blot analysis of DNALI1 between control and TBI in the cortex and hippocampus (n = 4). Data are normalized to β‐actin and expressed relative to the control. k) Western blot analysis of tau and AT8 between control and TBI in the hippocampus. Control (Ctrl, n = 5; TBI, n = 6). Data are normalized to β‐actin and expressed relative to the control. Data are represented as the mean ± SEM. T‐test (a,c,f–k) and one‐way ANOVA with Tukey's multiple comparisons test (b) were used. p‐values are indicated on the graphs.
DNALI1 和 tau 病理学在体外和体内均发生显着变化。 a) DNALI1(绿色)和细胞核(蓝色)的免疫荧光联合标记以及比较对照和血清剥夺模型的相应统计结果。 n = 4 个孔,来自四个独立实验的代表。比例尺,50 µm,如图所示。 b) 对经历不同血清剥夺持续时间的细胞中的 DNALI1 进行蛋白质印迹分析 ( n = 3)。数据标准化为 β-肌动蛋白并相对于对照进行表达。 c) 血清剥夺模型中 tau 和 AT8 的蛋白质印迹分析 ( n = 3)。数据标准化为 β-肌动蛋白并相对于对照进行表达。 d)重复轻度闭头模型和后续实验的示意图。 e)激光散斑成像检测TBI模型受伤前后的血流变化。灌注可视化为血流的二维颜色编码图(红色=高;蓝色=低),比例尺= 1 cm。 f) 脑损伤一个月后分析旋转杆测试的表现。 Ctrl, n =5; TBI, n = 14。 g) 脑损伤一个月后分析 Y 迷宫自发交替测试的表现。 Ctrl, n =5; TBI, n = 14。h) 脑损伤一个月后分析的新物体识别测试的表现。 Ctrl, n =5; TBI, n = 14。 i) 脑损伤一个月后分析莫里斯水迷宫测试的表现。 Ctrl, n =5;创伤性脑损伤, n = 14; Rev:逆向学习。 j) 皮质和海马中对照和 TBI 之间 DNALI1 的蛋白质印迹分析 ( n = 4)。数据标准化为 β-肌动蛋白并相对于对照进行表达。 k) 对照和 TBI 海马中 tau 和 AT8 的蛋白质印迹分析。控制(Ctrl, n = 5;TBI, n = 6)。数据标准化为 β-肌动蛋白并相对于对照进行表达。数据表示为平均值±SEM。使用 T 检验(a、c、f-k)和带有 Tukey 多重比较检验(b)的单向方差分析。 p值显示在图表上。
Our analysis highlights the predominance of tau pathology in the human study, with Abeta pathologies remaining unaltered. To further investigate, we established an animal model of repetitive traumatic brain injuries (rTBIs) mimicking CTE. We employed closed‐skull, two mild replicate hit models with specific hit parameters (3.0 m s−1 strike velocity, 1.0 mm strike depth, and 500 ms) and an experiment time after injury of 30 days, (Figure 2d) based on a literature survey[
15
] and pre‐experimentation (Figure S5, Supporting Information). We monitored blood flow using laser speckle imaging and assessed changes in cognitive performance in the model. Blood flow in the brains of mice significantly decreased after injury (Figure 2e), confirming the success of the model. Under the parameters used in this study, the rTBIs model did not induce motor dysfunction, as evidenced by the unaltered latency to fall in the rotarod test compared to controls (Figure 2f, p = 0.62). Conversely, mice exhibited significant cognitive impairment, as evidenced by significantly reduced alternation in the Y‐maze (Figure 2g, p < 0.001), reduced discrimination index in the novel object recognition (NOR) test (Figure 2h, p
= 0.018), and prolonged escape latency in the Morris water maze (MWM) (Figure 2i). Subsequent to modeling, we found significant increases in DNALI1 expression in both the cortex and hippocampus (Figure 2j, cortex, p = 0.012; hippocampus, p = 0.011), along with an increased AT8/tau ratio (Figure 2k, p < 0.001).
我们的分析强调了 tau 病理学在人类研究中的主导地位,而 Abeta 病理学保持不变。为了进一步研究,我们建立了模仿 CTE 的重复性创伤性脑损伤 (rTBIs) 动物模型。我们采用了闭合颅骨、两个具有特定击打参数(3.0 ms -1击打速度、1.0 mm 击打深度和 500 ms)的轻度重复击打模型,以及受伤后 30 天的实验时间(图 2d )基于文献调查[
15
]和预实验(图 S5 ,支持信息)。我们使用激光散斑成像监测血流并评估模型中认知能力的变化。小鼠受伤后脑部血流量显着减少(图 2e ),证实了模型的成功。在本研究中使用的参数下,rTBIs 模型不会诱发运动功能障碍,这一点可以通过与对照组相比在旋转杆测试中跌倒的潜伏期不变来证明(图 2f , p = 0.62)。相反,小鼠表现出明显的认知障碍,Y 迷宫中的交替显着减少就证明了这一点(图 2g , p < 0.001),新物体识别(NOR)测试中的辨别指数降低(图 2h , p = 0.018),并且莫里斯水迷宫(MWM)中的逃避潜伏期延长(图 2i )。建模后,我们发现皮质和海马中 DNALI1 表达显着增加(图 2j ,皮质, p = 0.012;海马体, p = 0.011),同时 AT8/tau 比率增加(图 2k , p< 0.001)。
2.3.
DNALI1 Knockdown Prevents Neurodegeneration after Repeated Mild Closed‐Head Injury
2.3.
DNALI1敲低可预防反复轻度闭合性头部损伤后的神经退行性变
To determine whether DNALI1 contributes to neurodegeneration induced by repeated mild closed‐head injuries, we knocked‐down DNALI1 in the brain by employing stereotaxic injection of adeno‐associated virus (AAV8)‐EF‐Cas9 + AAV8‐mDNALI1‐sp.g3 (1.0 × 10^12 GC mL−1) or AAV8 empty vectors as the control (Figure
3a). This injection was administered 14 days before inducing head injury. Immunofluorescence analysis confirmed the efficacy of AAV8‐mDNALI1‐sp.g3 in attenuating the increase in DNALI1 protein expression after head injury (Figure 3b, p < 0.0001). Subsequent motor and cognitive performance assessments revealed that the knockdown of DNALI1 did not alter motor function (Figure 3c, p < 0.95) but successfully rescued the cognitive impairment resulting from repeated mild closed head injury (Figure 3d–f). Consistent with this finding, western blot analysis of total tau and its phosphorylation revealed that the AT8/tau ratio significantly decreased after AAV injection (Figure 3g, p = 0.0090), consistent with the rescue of cognitive impairment. Taken together, these results suggest that targeting DNALI1 after a head injury may offer a promising avenue to prevent further neurodegeneration.
为了确定DNALI1是否会导致反复轻度闭合性头部损伤引起的神经变性,我们通过立体定位注射腺相关病毒 (AAV8)-EF-Cas9 + AAV8-mDNALI1-sp.g3 (1.0 × 10^12 GC mL −1 )或AAV8空载体作为对照(图
3a )。这种注射是在引起头部损伤前 14 天进行的。免疫荧光分析证实了 AAV8-mDNALI1-sp.g3 在减弱头部损伤后 DNALI1 蛋白表达增加方面的功效(图 3b , p< 0.0001)。随后的运动和认知表现评估显示, DNALI1的敲低并没有改变运动功能(图 3c , p < 0.95),但成功挽救了重复轻度闭合性头部损伤导致的认知障碍(图 3d–f )。与这一发现一致的是,总 tau 及其磷酸化的蛋白质印迹分析表明,注射 AAV 后 AT8/tau 比率显着下降(图 3g , p = 0.0090),与认知障碍的挽救一致。总而言之,这些结果表明,头部受伤后靶向DNALI1可能为预防进一步的神经退行性疾病提供一个有希望的途径。
Figure 3. 图 3.
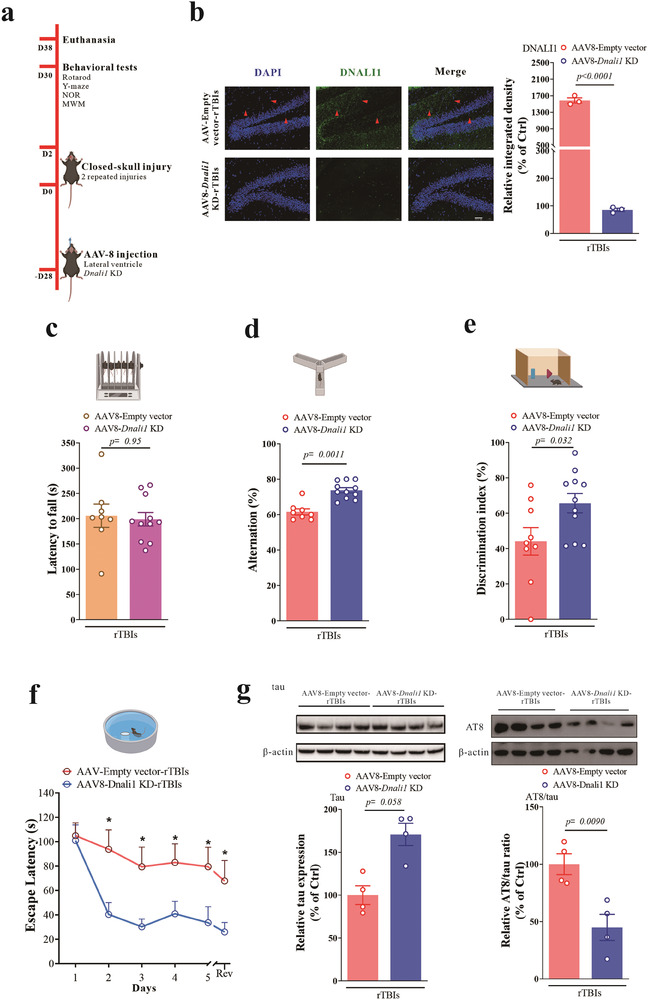
The knockdown of DNALI1 relieved cognitive impairment and the tau pathology of CTE. a) Diagrammatic representation of the brain AAV8‐DNALI1‐KD mice and subsequent experiments. b) Immunofluorescent co‐labeling of DNALI1 (green) and nuclei (blue) with corresponding statistical results after AAV injection and injury. n = 3. Scale bar, 50 µm, as indicated. c) Performance on the rotarod test analyzed after AAV injection and injury (AAV8‐empty‐TBI, n = 9; AAV8‐Dnali1 KD‐TBI, n = 11). d) Performance on the Y‐Maze spontaneous alternation test analyzed after AAV injection and injury (AAV8‐empty‐TBI, n = 9; AAV8‐Dnali1 KD‐TBI, n = 11). e) Performance on the Novel object recognition test analyzed after one after AAV injection and injury (AAV8‐empty‐TBI, n = 9; AAV8‐Dnali1 KD‐TBI, n = 11). f) Performance on the Morris water maze test analyzed after AAV injection and injury (AAV8‐empty‐TBI, n = 9; AAV8‐Dnali1 KD‐TBI, n = 11). g) Western blot analysis of tau and AT8 after AAV injection and injury in the hippocampus (n = 4). Data are normalized to β‐actin and expressed relative to the control. The data are represented as the mean ± SEM. T‐test (b–g) was used. p‐values are indicated on the graphs.
DNALI1 的敲低可缓解认知障碍和 CTE 的 tau 病理学。 a) AAV8-DNALI1-KD 小鼠大脑及后续实验的图示。 b) AAV 注射和损伤后 DNALI1(绿色)和细胞核(蓝色)的免疫荧光共标记以及相应的统计结果。 n = 3。比例尺,50 µm,如图所示。 c) AAV 注射和损伤后分析的旋转测试表现(AAV8-empty-TBI, n = 9;AAV8- Dnali1 KD-TBI, n = 11)。 d) AAV 注射和损伤后分析的 Y 迷宫自发交替测试表现(AAV8-空-TBI, n = 9;AAV8- Dnali1 KD-TBI, n = 11)。 e) 在 AAV 注射和损伤后分析新物体识别测试的表现(AAV8-empty-TBI, n = 9;AAV8- Dnali1 KD-TBI, n = 11)。 f) AAV 注射和损伤后分析莫里斯水迷宫测试的表现(AAV8-空-TBI, n = 9;AAV8- Dnali1 KD-TBI, n = 11)。 g) AAV 注射和海马损伤后 tau 和 AT8 的蛋白质印迹分析 ( n = 4)。数据标准化为 β-肌动蛋白并相对于对照进行表达。数据表示为平均值±SEM。使用 T 检验(b-g)。 p值显示在图表上。
2.4. DNALI1 Affects the Autophagosome‐Lysosome Fusion and Autophagic Flux in TBI
2.4. DNALI1 影响 TBI 中自噬体-溶酶体融合和自噬通量
Given the primary involvement of DNALI1 in axonal transport, particularly in powering the beating of cilia through axonemal dynein,[
16
] we examined differences in intraflagellar transport (IFT)‐related genes between “clinical dementia” and “non‐demented” in the Aging, Dementia and Traumatic Brain Injury Study. Notably, IFT protein 46 (IFT46), a component of the IFT complex, was significantly increased in participants with “clinical dementia” (Figure
4a, p = 0.020), accompanied by a significant positive correlation with DNALI1 (Figure 4b, r = 0.46, p = 0.0019). This correlation was not observed in non‐dementia participants, suggesting that DNALI1 may regulate IFT46 in the context of the disease.
鉴于 DNALI1 主要参与轴突运输,特别是通过轴突动力蛋白为纤毛的跳动提供动力, [
16
]我们在衰老、痴呆和创伤性脑损伤研究中检查了“临床痴呆”和“非痴呆”之间鞭毛内运输(IFT)相关基因的差异。值得注意的是,IFT 蛋白 46( IFT46 )(IFT 复合物的一个组成部分)在患有“临床痴呆”的参与者中显着增加(图
4a , p = 0.020),并与DNALI1呈显着正相关(图 4b ,r = 0.46, p = 0.0019)。在非痴呆症参与者中没有观察到这种相关性,这表明DNALI1可能在疾病背景下调节IFT46 。
Figure 4. 图 4.
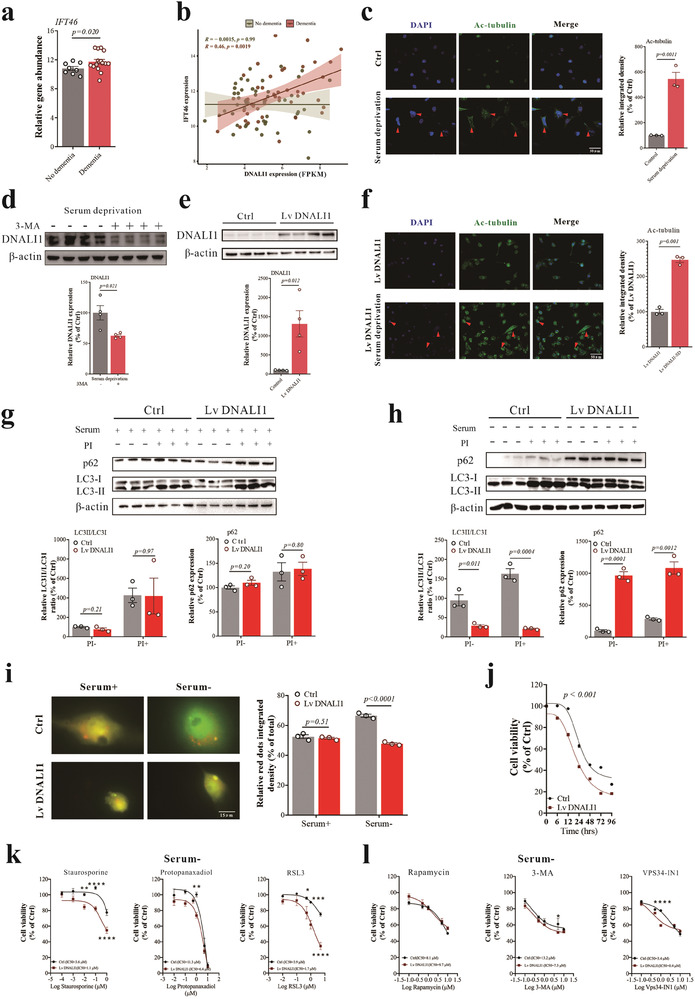
The increase of DNALI1 reduced the autophagic flux in pathological status. a) Levels of IFT46 detected by RNA‐seq between dementia and non‐dementia groups. b) Correlation between DNALI1 expression and IFT46 expression for dementia and non‐dementia groups. c) Immunofluorescent co‐labeling of Ac‐tubulin (green) and nuclei (blue) with corresponding statistical results after 72 h of serum deprivation. n = 3. Scale bar, 50 µm, as indicated. d) Western blot analysis of DNALI1 after 3‐MA pretreatment under serum deprivation status (n = 4). Data are normalized to β‐actin and expressed relative to the control. e) Western blot analysis of DNALI1 after infection by lentivirus packaged with DNALI1 overexpression plasmid (n = 4). Data are normalized to β‐actin and expressed relative to the control. f) Immunofluorescent co‐labeling of Ac‐tubulin (green) and nuclei (blue) with corresponding statistical results after DNALI1 overexpression. n = 3. Scale bar, 50 µm, as indicated. g) Western blot analysis of LC3 and p62 for normal and DNALI1 overexpression cells after protease inhibitors (20 mm NH4Cl and 100 µm Leupeptin) pretreatment under normal serum conditions (n = 3). Data are normalized to β‐actin and expressed relative to the control. h) Western blot analysis of LC3 and p62 for normal and DNALI1 overexpression cells after protease inhibitors (20 mm NH4Cl and 100 µm Leupeptin) pretreatment under serum deprivation status (n = 3). Data are normalized to β‐actin and expressed relative to the control. i) Fluorescence levels for normal and DNALI1 overexpression cells after transfection of mCherry–GFP–LC3 plasmid under normal or serum deprivation status. n = 3. Scale bar, 15 µm, as indicated. j) Cell viability of normal and DNALI1 overexpression cells at 0, 6, 12, 24, 48, 72, and 96 h after serum deprivation, n = 6 wells from one representative of three independent experiments. k) Cell viability of normal and DNALI1 overexpression cells after treatment with apoptosis activator staurosporine, necrosis activator protopanaxadiol, and ferroptosis activator RSL3 treatment under 24 h of serum deprivation pretreatment status, n = 6 wells from one representative of three independent experiments. l) Cell viability of normal and DNALI1 overexpression cells after Rapamycin, 3‐MA, or VPS34‐IN1 treatment under 24 h of serum deprivation pretreatment status, n = 6 wells from one representative of three independent experiments. Data are represented as the mean ± SEM. T‐test (a,c–f), Pearson Correlation (b), or two‐way ANOVA with Sidak's multiple comparisons test (g–l) were used. p‐values are indicated on the graphs.
DNALI1的增加降低了病理状态下的自噬通量。 a) 通过 RNA-seq 检测痴呆组和非痴呆组之间的IFT46水平。 b) 痴呆组和非痴呆组DNALI1表达和IFT46表达之间的相关性。 c) 血清剥夺 72 小时后,Ac-微管蛋白(绿色)和细胞核(蓝色)的免疫荧光联合标记以及相应的统计结果。 n = 3。比例尺,50 µm,如图所示。 d) 血清剥夺状态下 3-MA 预处理后 DNALI1 的蛋白质印迹分析 ( n = 4)。数据标准化为 β-肌动蛋白并相对于对照进行表达。 e) 用DNALI1过表达质粒包装的慢病毒感染后DNALI1的Western blot分析( n = 4)。数据标准化为 β-肌动蛋白并相对于对照进行表达。 f) DNALI1 过表达后 Ac-微管蛋白(绿色)和细胞核(蓝色)的免疫荧光共标记以及相应的统计结果。 n = 3。比例尺,50 µm,如图所示。 g) 在正常血清条件下用蛋白酶抑制剂(20 m m NH 4 Cl 和 100 µm Leupeptin)预处理后,对正常细胞和 DNALI1 过表达细胞进行 LC3 和 p62 的蛋白质印迹分析( n = 3)。数据标准化为 β-肌动蛋白并相对于对照进行表达。 h) 在血清剥夺状态下,蛋白酶抑制剂(20 m m NH 4 Cl 和 100 µm Leupeptin)预处理后,对正常细胞和 DNALI1 过表达细胞进行 LC3 和 p62 的蛋白质印迹分析( n = 3)。数据标准化为 β-肌动蛋白并相对于对照进行表达。 i) 在正常或血清剥夺状态下转染 mCherry-GFP-LC3 质粒后正常细胞和 DNALI1 过表达细胞的荧光水平。 n = 3。比例尺,15 µm,如图所示。 j) 血清剥夺后 0、6、12、24、48、72 和 96 小时正常细胞和 DNALI1 过表达细胞的细胞活力, n = 6 个孔,来自三个独立实验的一个代表。 k)在24小时去血清预处理状态下,用凋亡激活剂星孢菌素、坏死激活剂原人参二醇和铁死亡激活剂RSL3处理后,正常细胞和DNALI1过表达细胞的细胞活力, n =来自三个独立实验的一个代表的6个孔。 l) 在去血清预处理状态下 24 小时,雷帕霉素、3-MA 或 VPS34-IN1 处理后正常细胞和 DNALI1 过表达细胞的细胞活力, n = 6 个孔,来自三个独立实验的代表。数据表示为平均值±SEM。使用 T 检验 (a,c–f)、Pearson 相关性 (b) 或带有 Sidak 多重比较检验的双向方差分析 (g–l)。 p值显示在图表上。
IFT46, recognized as a core component of the intraflagellar transport machinery, plays a role in ciliogenesis. It is crucial for the IFT complex's movement along the microtubule‐based axoneme of cilia and flagella, transporting proteins and other molecules necessary for the assembly and maintenance of these structures during ciliogenesis.[
17
] Indeed, the presence of detectable primary cilia, indicative of changes in ciliogenesis, increased by 400% 72 h after serum removal (Figure 4c, p = 0.0011).
IFT46 被认为是鞭毛内运输机制的核心组成部分,在纤毛发生中发挥作用。它对于 IFT 复合物沿着纤毛和鞭毛的微管轴丝运动至关重要,在纤毛发生过程中运输组装和维持这些结构所需的蛋白质和其他分子。 [
17
]事实上,在去除血清后 72 小时,可检测到的初级纤毛的存在增加了 400%,表明纤毛发生的变化(图 4c , p = 0.0011)。
Previous reports have indicated that ciliogenesis regulates autophagy via the Hedgehog pathway, inducing autophagy by directly acting on essential autophagy‐related proteins strategically located at the cilium's base through ciliary trafficking proteins.[
17a
] Therefore, we hypothesized that DNALI1 participated in autophagy as part of the ciliogenesis process. We identified markers of autophagy after serum deprivation, including an elevated LC3II/LC3I ratio, decreased p62 expression (Figure S6a, Supporting Information), an increased number of autophagic vesicles (Figure S6b, Supporting Information, p < 0.001), and a notably elevated fluorescence intensity of LC3 (Figure S6c, Supporting Information). Meanwhile, we found that DNALI1 expression can be reduced by pretreatment with the autophagy inhibitor 3‐MA (1 µm, 6 h) during serum deprivation (Figure 4d, p = 0.021). This suggests that DNALI1 may be functional within the autophagy process.
先前的报告表明,纤毛发生通过 Hedgehog 途径调节自噬,通过纤毛运输蛋白直接作用于战略性位于纤毛基部的重要自噬相关蛋白,从而诱导自噬。 [
17a
]因此,我们假设 DNALI1 参与自噬作为纤毛发生过程的一部分。我们鉴定了血清剥夺后自噬的标志物,包括 LC3II/LC3I 比率升高、p62 表达降低(图 S6a ,支持信息),自噬囊泡数量增加(图 S6b ,支持信息, p < 0.001),并且 LC3 的荧光强度显着升高(图 S6c ,支持信息)。同时,我们发现在血清剥夺期间用自噬抑制剂3-MA( 1μm ,6小时)预处理可以降低DNALI1的表达(图 4d , p = 0.021)。这表明 DNALI1 可能在自噬过程中发挥作用。
To further clarify the role of DNALI1 in autophagy, we employed lentiviruses to overexpress DNALI1 (Lv DNALI1) (Figure 4e, p = 0.012). This aggressive overexpression of DNALI1 led to a significant increase in ciliogenesis (Figure 4f, p = 0.001), potentially impacting autophagic flux. The characteristics of autophagic flux (LC3II/LC3I ratio and p62 expression) exhibited no significant changes between the control and DNALI1 overexpression cells when serum was added, with or without 6 h pretreatment of protease inhibitors (20 mm NH4Cl and 100 µm Leupeptin) (Figure 4g). Protease inhibitors block the degradation of autophagic cargo, promoting autophagic flux (changes in LC3‐II content after blocking lysosomal degradation).[
18
] However, we observed a significant reduction in autophagic flux upon serum removal in the DNALI1 overexpression cells (Figure 4h), indicating that the impact of elevated DNALI1 was specific to pathological conditions, such as nutrient deprivation.
为了进一步阐明DNALI1在自噬中的作用,我们采用慢病毒过表达DNALI1 (Lv DNALI1)(图 4e , p = 0.012)。 DNALI1的这种积极过度表达导致纤毛发生显着增加(图 4f , p = 0.001),可能影响自噬通量。当添加血清时,无论有或没有蛋白酶抑制剂(20 m m NH 4 Cl和100 µm )预处理6小时,自噬流特征(LC3II/LC3I比值和p62表达)在对照和DNALI1过表达细胞之间没有表现出显着变化。亮肽素)(图 4g )。蛋白酶抑制剂阻止自噬货物的降解,促进自噬通量(阻止溶酶体降解后 LC3-II 含量的变化)。 [
18
]然而,我们观察到 DNALI1 过表达细胞中血清去除后自噬通量显着减少(图 4h ),表明 DNALI1 升高的影响特定于病理条件,例如营养缺乏。
To further verify this, we transfected cells with a pH‐sensitive reporter (mCherry–GFP–LC3; a fusion of mCherry, green fluorescent protein, and LC3) that highlighted autophagosomes as yellow puncta and autophagolysosomes (post‐lysosomal fusion) as red puncta. We found that the basal levels of autophagic vacuoles were comparable in control and DNALI1 overexpression cells, consistent with previous western blotting results. However, upon serum removal, cells overexpressing DNALI1 exhibited a significant reduction in autophagolysosome content (Figure 4i, p < 0.001). The impact of DNALI1 on autophagosome‐lysosome fusion and autophagic flux also affected cell viability following serum deprivation, with DNALI1 overexpression rendering cells more susceptible (Figure 4j, p < 0.001).
为了进一步验证这一点,我们用 pH 敏感报告基因(mCherry-GFP-LC3;mCherry、绿色荧光蛋白和 LC3 的融合体)转染细胞,该报告基因将自噬体突出显示为黄色点,将自噬溶酶体(溶酶体融合后)突出显示为红色点。我们发现对照细胞和 DNALI1 过表达细胞中自噬泡的基础水平相当,这与之前的蛋白质印迹结果一致。然而,去除血清后,过表达 DNALI1 的细胞表现出自噬溶酶体含量显着减少(图 4i , p< 0.001)。 DNALI1 对自噬体-溶酶体融合和自噬通量的影响也会影响血清剥夺后的细胞活力,DNALI1 过度表达使细胞更容易受到影响(图 4j , p< 0.001)。
Autophagy is also associated with several cell death pathways, including apoptosis,[
19
] necroptosis,[
20
] and ferroptosis.[
21
] Inhibition of autophagy can lead to the accumulation of damaged organelles, prompting autophagic vacuolization and ultimately triggering cell death pathways.[
22
] Here, we found that DNALI1 overexpression also affected cell susceptibility to these cell death pathways under conditions of serum deprivation (Figure 4k), while such effects were not observed in normal serum conditions (Figure S7a, Supporting Information). Additionally, we analyzed the influence of DNALI1 overexpression on autophagy regulators. However, we only observed significant differences when VPS34‐IN1 was used (Figure 4l), likely due to its potent inhibitory effect on both VPS34 and DNALI1. Importantly, the normal serum status did not alter the serum levels (Figure S7b, Supporting Information).
自噬还与多种细胞死亡途径相关,包括细胞凋亡, [
19
]坏死性凋亡, [
20
]和铁死亡。 [
21
]自噬的抑制会导致受损细胞器的积累,促进自噬空泡化并最终触发细胞死亡途径。 [
22
]在这里,我们发现 DNALI1 过表达也会影响细胞在血清剥夺条件下对这些细胞死亡途径的敏感性(图 4k ),而在正常血清条件下没有观察到这种效应(图 S7a ,支持信息)。此外,我们还分析了 DNALI1 过表达对自噬调节因子的影响。然而,我们仅在使用 VPS34-IN1 时观察到显着差异(图 4l ),可能是由于其对 VPS34 和 DNALI1 都有有效的抑制作用。重要的是,正常的血清状态不会改变血清水平(图 S7b ,支持信息)。
To further validate the effect of DNALI1 on autophagy, we employed siRNA to inhibit DNALI1 expression (Si DNALI1) (Figure
5a, p = 0.0055). Autophagic flux, as indicated by the LC3II/LC3I ratio and p62 expression, significantly increased during serum deprivation, whereas no such effect was observed under normal serum conditions (Figure 5b). The effects of DNALI1 on autophagic flux also affected cell viability following serum deprivation, with cells exhibiting reduced DNALI1 displaying increased resistance (Figure 5c, p = 0.033). Moreover, we also found that DNALI1 reduction affected cell resistance to cell death and autophagy regulators under serum‐deprived conditions (Figure 5d,e). In contrast, only autophagy regulators were affected in normal serum (Figure S8, Supporting Information). Additionally, experiments conducted using a previously constructed AAV8‐Dnali1 KD animal model showed that LC3 and p62 immunofluorescence results indicated a significant increase in autophagic flux upon Dnali1 KD (Figure 5f,g).
为了进一步验证DNALI1对自噬的影响,我们采用siRNA抑制DNALI1的表达(Si DNALI1)(图
5a , p = 0.0055)。如 LC3II/LC3I 比率和 p62 表达所示,自噬通量在血清剥夺期间显着增加,而在正常血清条件下未观察到这种效应(图 5b )。 DNALI1 对自噬流的影响也会影响血清剥夺后的细胞活力,DNALI1 减少的细胞表现出抵抗力增加(图 5c , p = 0.033)。此外,我们还发现DNALI1的减少影响了血清剥夺条件下细胞对细胞死亡和自噬调节因子的抵抗力(图 5d,e )。相比之下,正常血清中只有自噬调节因子受到影响(图 S8 ,支持信息)。此外,使用先前构建的 AAV8- Dnali1 KD 动物模型进行的实验表明,LC3 和 p62 免疫荧光结果表明Dnali1 KD 后自噬通量显着增加(图 5f,g )。
Figure 5. 图 5.
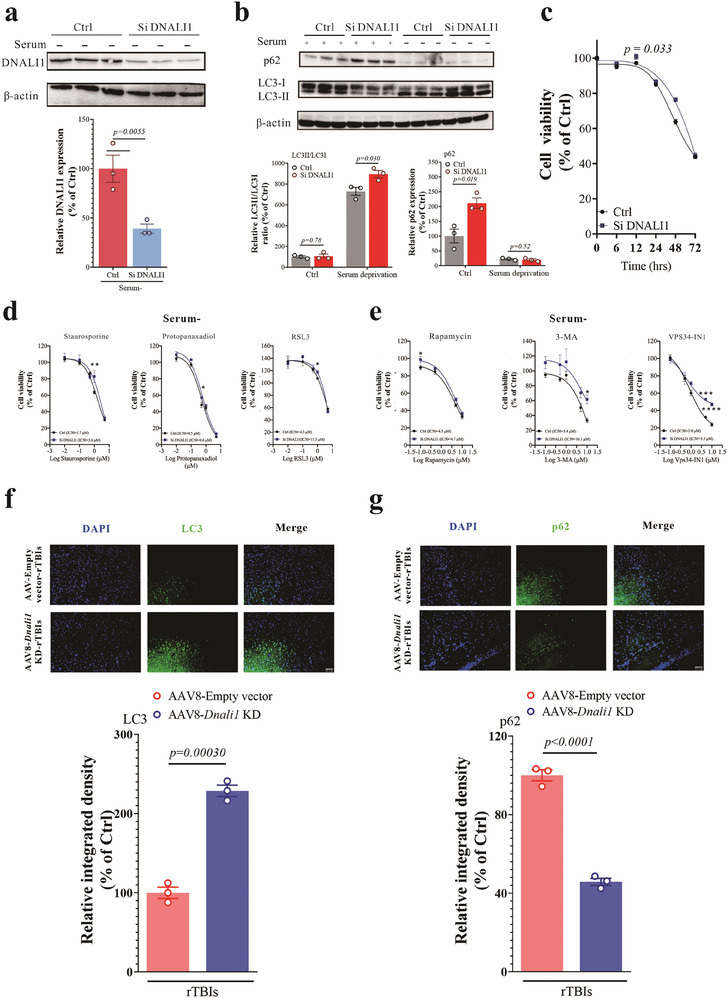
The decrease of DNALI1 increased the autophagic flux in pathological conditions. a) Western blot analysis of DNALI1 after siRNA transfection (n = 3). Data are normalized to β‐actin and expressed relative to the control. b) Western blot analysis of LC3 and p62 for normal and si‐DNALI1 cells under normal or serum deprivation status, n = 3. Data are normalized to β‐actin and expressed relative to the control. c) Cell viability of normal and si‐DNALI1 cells at 0, 6, 12, 24, 48, and 72 h after serum deprivation, n = 6 wells from one representative of three independent experiments. d) Cell viability of normal and si‐DNALI1 cells after treatment with apoptosis activator staurosporine, necrosis activator protopanaxadiol, and ferroptosis activator RSL3 under 24 h of serum deprivation pretreatment status, n = 6 wells from one representative of three independent experiments. e) Cell viability of normal and si‐DNALI1 cells after Rapamycin, 3‐MA, or VPS34‐IN1 treatment under 24 h of serum deprivation pretreatment status, n = 6 wells from one representative of three independent experiments. f) Immunofluorescent co‐labeling of LC3 (green) and nuclei (blue) with corresponding statistical results after AAV injection and injury. n = 3. Scale bar, 50 µm, as indicated. g) Immunofluorescent co‐labeling of p62 (green) and nuclei (blue) with corresponding statistical results after AAV injection and injury. n = 3. Scale bar, 50 µm, as indicated. Data are represented as the mean ± SEM. T‐test (a,f,g), or two‐way ANOVA with Sidak's multiple comparisons test (b–e) were used. p‐values are indicated on the graphs.
DNALI1 的减少增加了病理条件下的自噬通量。 a) siRNA 转染后 DNALI1 的蛋白质印迹分析 ( n = 3)。数据标准化为 β-肌动蛋白并相对于对照进行表达。 b) 在正常或血清剥夺状态下,对正常细胞和 si-DNALI1 细胞进行 LC3 和 p62 的蛋白质印迹分析, n = 3。数据针对 β-肌动蛋白进行标准化,并相对于对照进行表达。 c) 去血清后 0、6、12、24、48 和 72 小时时正常细胞和 si-DNALI1 细胞的细胞活力, n = 6 个孔,来自三个独立实验的一个代表。 d) 在去血清预处理状态 24 小时下,用凋亡激活剂星孢菌素、坏死激活剂原人参二醇和铁死亡激活剂 RSL3 处理后,正常细胞和 si-DNALI1 细胞的细胞活力, n = 6 个孔,来自三个独立实验的代表。 e) 在去血清预处理状态下,经雷帕霉素、3-MA 或 VPS34-IN1 处理 24 小时后,正常细胞和 si-DNALI1 细胞的细胞活力, n = 6 个孔,来自三个独立实验的代表。 f) AAV 注射和损伤后 LC3(绿色)和细胞核(蓝色)的免疫荧光联合标记以及相应的统计结果。 n = 3。比例尺,50 µm,如图所示。 g) AAV 注射和损伤后 p62(绿色)和细胞核(蓝色)的免疫荧光共标记以及相应的统计结果。 n = 3。比例尺,50 µm,如图所示。数据表示为平均值±SEM。使用 T 检验 (a,f,g) 或带有 Sidak 多重比较检验的双向方差分析 (b–e)。 p值显示在图表上。
3. Discussion 三、讨论
CTE, occurred after repetitive TBIs with hyperphosphorylated tau accumulation in the hippocampal region, is yet to be cured. In this study, through comprehensive screening of data derived from human samples and subsequent validation in cells and rodent models of TBI, we demonstrated that DNALI1 is a critical factor in CTE, operating through a decrease in autophagic flux. During CTE, ciliogenesis increases in response to external mechanical and oxidative stress, along with elevated DNALI1 levels, resulting in a decrease in autophagy levels. This convergence of diverse physiopathological processes creates a blockade in autophagic function, impeding the clearance of damaged organelles and deleterious material, thereby increasing phosphorylated tau protein levels and ultimately precipitating cognitive impairment. Notably, both autophagic dysfunction and cognitive decline after TBI were alleviated by DNALI1 knockdown (Figure
6
).
CTE 是在重复性 TBI 后发生的,且海马区 tau 蛋白过度磷酸化,目前尚未治愈。在这项研究中,通过对人类样本数据的全面筛选以及随后在 TBI 细胞和啮齿动物模型中的验证,我们证明 DNALI1 是 CTE 的关键因素,通过减少自噬通量发挥作用。在 CTE 期间,纤毛生成响应外部机械和氧化应激而增加,同时 DNALI1 水平升高,导致自噬水平下降。多种病理生理过程的融合会阻碍自噬功能,阻碍受损细胞器和有害物质的清除,从而增加磷酸化 tau 蛋白水平,最终导致认知障碍。值得注意的是, DNALI1敲低可缓解 TBI 后的自噬功能障碍和认知能力下降(图
6
)。
Figure 6. 图 6.
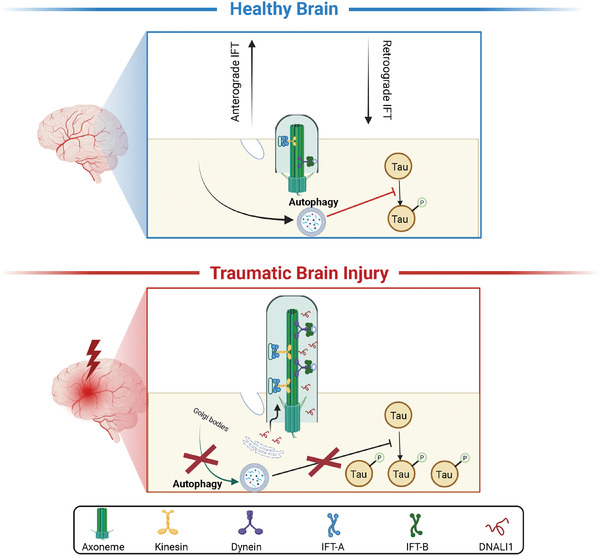
Working hypothesis of the role of DNALI1 in post‐TBI neurodegeneration. Following TBI, ciliogenesis increases under mechanical and oxidative stress, leading to the increase of DNALI1. DNALI1 prevents the clearance of phosphorylated tau by autophagy, inhibiting autophagic flux and contributing to the development of CTE. This figure was generated using BioRender.com.
DNALI1 在 TBI 后神经变性中的作用的工作假设。 TBI 后,纤毛发生在机械和氧化应激下增加,导致 DNALI1 增加。 DNALI1 可防止自噬清除磷酸化 tau 蛋白,从而抑制自噬通量并促进 CTE 的发展。该图是使用 BioRender.com 生成的。
Previous studies have highlighted a noteworthy discordance between clinical and final neuropathological diagnoses in more than one‐third of the cases.[
23
] Moreover, older individuals who remained cognitively intact proximate to death often exhibited significant AD neuropathologic changes.[
24
] Additionally, a significant proportion (∼15–30%) of patients with mild cognitive impairment (MCI) manifest evidence of neurodegeneration without amyloid deposition.[
25
] Given these complexities, we categorized participants based on their TBI diagnosis, dementia pathology, and clinical dementia diagnosis. However, while acknowledging the significance of TBI severity in this study, we were constrained by the data provided and the number of participants, preventing further subgrouping based on the severity. In addition, the differential diagnosis between CTE and AD or other types of dementia is also crucial.[
26
] In Aging, Dementia, and Traumatic Brain Injury Study, we categorized participants based on the original described TBI diagnosis, dementia pathology, and dementia clinical diagnosis. However, based on the reported history of TBI and the sole presentation of tau pathology, the cohort is more likely with CTE. In the subsequent animal experiments, a model mimicking CTE was also applied, revealing similar pathological features. Therefore, there is a clear need for improved accuracy of clinical diagnostic criterias to differentially diagnosis CTE and AD.
先前的研究强调了超过三分之一的病例的临床诊断和最终神经病理学诊断之间存在值得注意的不一致。 [
23
此外,在临死前仍保持认知完整的老年人常常表现出显着的 AD 神经病理学变化。 [
24
此外,很大一部分(~15-30%)患有轻度认知障碍(MCI)的患者表现出神经变性的证据,但没有淀粉样蛋白沉积。 [
25
]鉴于这些复杂性,我们根据参与者的 TBI 诊断、痴呆病理学和临床痴呆诊断对他们进行分类。然而,虽然承认本研究中 TBI 严重程度的重要性,但我们受到所提供的数据和参与者数量的限制,无法根据严重程度进行进一步的分组。此外,CTE与AD或其他类型痴呆症的鉴别诊断也至关重要。 [
26
]在衰老、痴呆和创伤性脑损伤研究中,我们根据最初描述的 TBI 诊断、痴呆病理学和痴呆临床诊断对参与者进行了分类。然而,根据报道的 TBI 病史和 tau 病理学的唯一表现,该队列更有可能患有 CTE。在随后的动物实验中,也应用了模拟CTE的模型,揭示了类似的病理特征。因此,显然需要提高临床诊断标准的准确性来区分 CTE 和 AD。
We identified the hippocampus as a key brain region in post‐traumatic cognitive impairment, contrasting its significance to the PCx, TCx, and FWM based on distinct gene expression patterns and differential levels of tau phosphorylation. Nevertheless, it is essential to acknowledge that other brain regions also play significant roles in cognition. For instance, the prefrontal cortex is integral to executive functions, such as decision‐making, planning, and working memory. Meanwhile, the TCx and PCx contribute to various aspects of sensory perception, language processing, and attention.[
27
] Therefore, our future research will continue to explore the intricate relationships between diverse brain regions and cognitive impairment following TBI.
我们将海马体确定为创伤后认知障碍的关键大脑区域,并根据不同的基因表达模式和不同的 tau 磷酸化水平将其与 PCx、TCx 和 FWM 的重要性进行了对比。然而,必须承认其他大脑区域在认知中也发挥着重要作用。例如,前额叶皮层是决策、计划和工作记忆等执行功能不可或缺的一部分。同时,TCx 和 PCx 有助于感官知觉、语言处理和注意力的各个方面。 [
27
]因此,我们未来的研究将继续探索不同大脑区域与 TBI 后认知障碍之间的复杂关系。
In this study, we successfully identified ciliogenesis and ciliary signaling pathways as potential contributors to cognitive impairment following TBI. Previous studies have indicated a significant increase in the length of primary cilia in the hippocampus of APP/PS1 compared to controls, and intraperitoneal administration of 5‐hydroxytryptamine receptor subtype 6 (5‐HT6) antagonist SB271046 effectively reduced cilia length and rescued cognitive impairment in APP/PS1 mice.[
28
] Among these, DNALI1, an essential component of the ciliated dynamic arm,[
29
] emerged as the key gene in our analysis, primarily responsible for cilium movement. Our findings shed light on the mechanistic underpinnings of the relationship among TBI, DNALI1, and ciliogenesis, evident through the increased presence of cells with detectable primary cilia in both the in vitro TBI model (Figure 4c) and the DNALI1 overexpression model (Figure 4f). In a human brain sequencing study,[
30
]
DNALI1 exhibited a threefold higher expression in frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD‐U) compared to controls. Furthermore, DNALI1 interacted with the major component of ubiquitinated inclusions in FTLD‐U and TDP‐43.[
31
] Given TDP‐43′s implication in synaptic and cognitive deterioration following TBI,[
32
] our work may offer insights into TDP‐43 changes, where autophagy regulates TDP‐43 through interactions with autophagosomes mediated by autophagy‐associated proteins, such as LC3 and p62, leading to its degradation.[
33
] Another transcriptome sequencing study, focusing on middle temporal gyrus tissue from 100 patients with AD and controls, also revealed that DNALI1 expression in AD cases was significantly higher than in controls. Moreover, a significant positive correlation was observed between DNALI1 and NFTs density,[
34
] a correlation consistent with our findings here. Collectively, our results position DNALI1 as a critical protein implicated in cognitive impairment.
在这项研究中,我们成功地确定了纤毛发生和纤毛信号通路是 TBI 后认知障碍的潜在因素。既往研究表明,与对照组相比,APP/PS1海马初级纤毛长度显着增加,腹腔注射5-羟色胺受体亚型6(5-HT 6 )拮抗剂SB271046可有效缩短纤毛长度并挽救认知障碍在 APP/PS1 小鼠中。 [
28
]其中,DNALI1,纤毛动力臂的重要组成部分, [
29
]在我们的分析中成为关键基因,主要负责纤毛运动。我们的研究结果揭示了 TBI、DNALI1 和纤毛发生之间关系的机制基础,这通过在体外 TBI 模型中具有可检测到的初级纤毛的细胞的增加来证明(图 4c )和DNALI1过表达模型(图 4f )。在一项人脑测序研究中, [
30
]与对照组相比, DNALI1在泛素化包涵体额颞叶变性 (FTLD-U) 中的表达量高出三倍。此外,DNALI1 与 FTLD-U 和 TDP-43 中泛素化内含物的主要成分相互作用。 [
31
]鉴于 TDP-43 对 TBI 后突触和认知功能恶化的影响, [
32
]我们的工作可能会提供对 TDP-43 变化的见解,其中自噬通过与自噬相关蛋白(例如 LC3 和 p62)介导的自噬体相互作用来调节 TDP-43,导致其降解。[
33
另一项转录组测序研究,重点关注 100 名 AD 患者和对照者的颞中回组织,也显示 AD 病例中的DNALI1表达显着高于对照者。此外, DNALI1和 NFT 密度之间观察到显着的正相关性, [
34
]相关性与我们的发现一致。总的来说,我们的结果将 DNALI1 定位为与认知障碍有关的关键蛋白质。
In our pursuit of understanding neurodegeneration after TBI, we also implemented a robust animal model (Figure 2e–i) that accurately recapitulates TBI‐induced cognitive deficits. While establishing a TBI model is relatively straightforward, the careful selection of study parameters has been identified as a key factor contributing to the observed heterogeneity in studies conducted across different laboratories.[
35
] Factors such as the number, severity, and timing of repeated concussive events also demand meticulous consideration. After testing various models of TBI, we opted for a closed skull model to circumvent primary injury occurrence. We also determined the optimal number of repeated hits in the TBI model and pinpointed the post‐injury timeframe during which animals developed the most pronounced cognitive impairment, as discerned through changes in behavioral tests. These findings carry significant implications for the design and execution of future studies exploring TBI or CTE in animal models.
为了了解 TBI 后的神经退行性变,我们还建立了一个稳健的动物模型(图 2e–i )准确地概括了 TBI 引起的认知缺陷。虽然建立 TBI 模型相对简单,但仔细选择研究参数已被认为是导致不同实验室进行的研究中观察到的异质性的关键因素。 [
35
]反复发生脑震荡事件的次数、严重程度和时间等因素也需要仔细考虑。在测试了各种 TBI 模型后,我们选择了封闭式颅骨模型来避免原发性损伤的发生。我们还确定了 TBI 模型中重复击打的最佳次数,并通过行为测试的变化确定了动物出现最明显认知障碍的受伤后时间范围。这些发现对于未来在动物模型中探索 TBI 或 CTE 的研究的设计和执行具有重要意义。
Autophagy, a self‐degradative process necessary for maintaining energy balance during critical developmental stages and in response to nutrient stress,[
36
] has gained prominence for its role in preserving neuronal homeostasis.[
37
] Most neurodegenerative diseases, characterized by abnormal protein aggregation, such as hyperphosphorylation of tau protein,[
38
] are linked to autophagy dysfunction. Here, we demonstrate that DNALI1 affects cognition by regulating autophagy under pathological conditions. Notably, DNALI1 levels showed no influence on autophagy under normal conditions, as indicated by the absence of significant differences following DNALI1 overexpression or reduction. However, under serum deprivation conditions, DNALI1 overexpression reduced autophagy levels, whereas DNALI1 inhibition enhanced them. The modulatory role of DNALI1 in autophagy further contributes to cellular sensitivity to cell death pathways. Consistent with previous findings suggesting that increased autophagy protects cells from apoptosis,[
19
] our results align with this protective mechanism. The ability of DNALI1 to regulate cell death pathways may have further implications for neurodegenerative diseases and other conditions where cell death is implicated. A recent study by Zhao et al. highlighted the impact of rTBIs on neuronal axonal microtubule assembly, leading to microtubule depolymerization and subsequent tau dissociation and spread throughout the brain.[
39
] Our study suggests that DNALI1 may represent an upstream event, with its elevation triggering tau hyperphosphorylation, potentially contributing to the observed tau spread reported by Zhao et al.
自噬是在关键发育阶段维持能量平衡和应对营养应激所必需的自我降解过程, [
36
]因其在维持神经元稳态方面的作用而受到重视。 [
37
]大多数神经退行性疾病,其特征是蛋白质聚集异常,例如 tau 蛋白过度磷酸化, [
38
]与自噬功能障碍有关。在这里,我们证明 DNALI1 通过在病理条件下调节自噬来影响认知。值得注意的是,DNALI1 水平在正常条件下对自噬没有影响,DNALI1 过表达或减少后不存在显着差异表明这一点。然而,在血清剥夺条件下,DNALI1 过度表达降低了自噬水平,而 DNALI1 抑制则增强了自噬水平。 DNALI1 在自噬中的调节作用进一步有助于细胞对细胞死亡途径的敏感性。与之前的研究结果一致,表明自噬增加可以保护细胞免于凋亡, [
19
]我们的结果与这种保护机制一致。 DNALI1 调节细胞死亡途径的能力可能对神经退行性疾病和其他涉及细胞死亡的疾病产生进一步的影响。赵等人最近的一项研究。强调了 rTBIs 对神经元轴突微管组装的影响,导致微管解聚以及随后的 tau 解离并扩散到整个大脑。[
39
]我们的研究表明,DNALI1 可能代表上游事件,其升高会触发 tau 过度磷酸化,可能导致赵等人报道的观察到的 tau 扩散。
In summary, our study strongly suggests that DNALI1 impacts the outcome of neurodegeneration after TBI by regulating autophagy. Targeting the excessive production of DNALI1 emerges as a promising approach to thwart the progression of CTE neuropathology, offering new prospects for therapeutic interventions in this challenging clinical context.
总之,我们的研究强烈表明 DNALI1 通过调节自噬影响 TBI 后神经退行性疾病的结果。针对 DNALI1 的过量产生成为阻止 CTE 神经病理学进展的一种有前景的方法,为这一充满挑战的临床环境中的治疗干预提供了新的前景。
4. Experimental Section 4. 实验部分
Reagents 试剂
Reagents were purchased from Sigma‐Aldrich unless otherwise specified.
除非另有说明,试剂均购自 Sigma-Aldrich。
Data Acquisition and Study Design
数据采集和研究设计
Publicly available datasets were obtained from the Aging, Dementia, and Traumatic Brain Injury Study (http://aging.brain‐map.org/),[
12
] which provides a systematic and extensive dataset encompassing specimen metadata, histology, immunohistochemistry, in situ hybridization, RNA sequencing, protein quantification, and isoprostane quantification for 107 individuals. Participants were categorized into eight groups based on TBI diagnosis, dementia pathology (CERAD>1), and clinical dementia diagnosis (using the NINCDS/ADRDA criteria).
公开可用的数据集来自衰老、痴呆和创伤性脑损伤研究( http://aging.brain‐map.org/ ), [
12
]它提供了系统且广泛的数据集,涵盖 107 名个体的标本元数据、组织学、免疫组织化学、原位杂交、RNA 测序、蛋白质定量和异前列烷定量。根据 TBI 诊断、痴呆病理学 (CERAD>1) 和临床痴呆诊断(使用 NINCDS/ADRDA 标准)将参与者分为八组。
Bioinformatics Analysis 生物信息学分析
Differential expression was assessed using a linear model and the Bioconductor limma package[
40
] in R, version 3.6.3 (R Foundation) based on a |fold change| ≥ 0.5 and p‐value < 0.05. The results were input into the Metascape database[
41
] (https://metascape.org/gp/index.html) to explore Gene Ontology (GO) pathways. Heatmaps were generated using the Pheatmap package[
42
] in R, version 3.6.3 (R Foundation), with z‐scores calculated for each gene row using the mean expression of biological replicates. Volcano plots, illustrating differentially expressed genes, were generated using the Enhanced Volcano package[
43
] in R, version 3.6.3 (R Foundation). The network diagram for PPI was calculated and visualized using Cytoscape (https://www.cytoscape.org/), and hub genes were ranked using cytoHubba.[
44
] Gene Set Enrichment Analysis (GSEA) (version 4.0.3, https://www.broadinstitute.org/gsea/)[
45
] was employed to identify overrepresented GO pathways (MSigDB, version 7.1) for upregulated or downregulated genes. Co‐expression networks for RNA‐seq data and their correlation values with phenotypic data were constructed and calculated using the weighted gene co‐expression network analysis (WGCNA) package[
46
] in R, version 3.6.3 (R Foundation).
使用线性模型和 Bioconductor limma 包评估差异表达[
40
]在 R 版本 3.6.3 (R Foundation) 中基于 |折叠更改| ≥ 0.5 且p值 < 0.05。结果被输入Metascape数据库[
41
] ( https://metascape.org/gp/index.html )探索基因本体(GO)途径。热图是使用 Pheatmap 包生成的[
42
]在 R 版本 3.6.3(R Foundation)中,使用生物重复的平均表达为每个基因行计算 z 分数。使用增强型火山包生成火山图,说明差异表达的基因。
43
]在 R 版本 3.6.3(R 基金会)中。使用 Cytoscape 计算并可视化 PPI 的网络图( https://www.cytoscape.org/ ),并使用 cytoHubba 对 hub 基因进行排序。 [
44
]基因集富集分析 (GSEA)(版本 4.0.3, https://www.broadinstitute.org/gsea/ ) [
45
]用于识别上调或下调基因的过度表达的 GO 途径(MSigDB,版本 7.1)。使用加权基因共表达网络分析(WGCNA)包构建并计算了RNA-seq数据的共表达网络及其与表型数据的相关值[
46
]在 R 版本 3.6.3(R 基金会)中。
Cell Culture 细胞培养
The neuronal cell line STHdhQ7/Q7 (a gift from Dr. Boxun Lu, Fudan University) was cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal calf serum and 100 Units/ml penicillin/streptomycin (all purchased from Thermo Fisher Scientific, USA).
神经元细胞系STHdhQ7/Q7(复旦大学陆伯勋博士赠品)在添加有10%胎牛血清和100单位/ml青霉素/链霉素的Dulbecco's Modified Eagle's Medium(DMEM)中培养(均购自Thermo Fisher)科学,美国)。
In Vitro TBI Model 体外 TBI 模型
The serum deprivation model was employed to replicate neuronal stress after TBI, as previously described.[
14
] Briefly, cells were seeded in 6/96‐well plates (5×104 cells/mL). After plating, the cell culture medium was removed, washed three times with DPBS (Thermo Fisher Scientific, USA), and then replaced with a serum‐free medium. The duration of serum deprivation was adjusted according to experimental requirements, as indicated in the figure legends.
如前所述,血清剥夺模型用于复制 TBI 后的神经元应激。 [
14
]简而言之,将细胞接种到6/96孔板中(5×10 4 个细胞/mL)。铺板后,除去细胞培养基,用DPBS(Thermo Fisher Scientific,美国)洗涤3次,然后更换为无血清培养基。根据实验要求调整血清剥夺的持续时间,如图图例所示。
Cell Viability Assay 细胞活力测定
Cells were seeded into 96‐well plates (5×104 cells/mL) and treated with the selected compounds [Rapamycin (Cat No. S1039, Selleck Chemicals), 3‐Methyladenine (Cat No. S2767, Selleck Chemicals), VPS34‐IN1 (Cat No. S7980, Selleck Chemicals), RSL3 (Cat No. S8155, Selleck Chemicals), Staurosporine (Cat No. S1421, Selleck Chemicals), (20S)‐Protopanaxadiol (Cat No. S4746, Selleck Chemicals)] after plating. Cell viability was assessed at different time points after treatment (24 h unless otherwise specified) using the Cell Counting Kit‐8 (CCK‐8) cytotoxicity assay (Bimake, B34304), as previously described.[
47
]
将细胞接种到 96 孔板(5×10 4 个细胞/mL)中,并用选定的化合物[雷帕霉素(货号 S1039,Selleck Chemicals)、3-甲基腺嘌呤(货号 S2767,Selleck Chemicals)、VPS34- 进行处理。 IN1(货号 S7980,Selleck Chemicals),RSL3(货号 S8155, Selleck Chemicals)、星形孢菌素(货号 S1421,Selleck Chemicals)、(20S)-原人参二醇(货号 S4746,Selleck Chemicals)] 电镀后。使用细胞计数试剂盒-8(CCK-8)细胞毒性测定(Bimake, B34304 ),如前所述。 [
47
]
Transmission Electron Microscopy
透射电子显微镜
After treatment, cells were collected into a 1.5 mL EP tube, and a low‐speed centrifugation step was employed to settle the cells at the bottom of the EP tube. Subsequently, the supernatants were removed. The collected cell pellets were fixed with 2.5% glutaraldehyde for 4 h at 4 °C and post‐fixed in 1% osmium tetroxide for 2 h at 20 °C. Then, they were dehydrated using a gradient of ethanol (50–100%) and acetone, embedded in epoxy resin, and polymerized for 48 h at 60 °C. Ultrathin sections (80 nm) were cut and stained with uranyl acetate and lead citrate prior to transmission electron microscopy (HT7700, HITACHI). Images were captured using a SlowScan CCD camera and the iTEM software (Ver 01.07, Olympus Soft Imaging Solutions). The quantification of autophagic bodies followed established procedures as previously described.[
48
]
处理后,将细胞收集至1.5 mL EP管中,并采用低速离心步骤使细胞沉降在EP管底部。随后,除去上清液。收集的细胞沉淀用2.5%戊二醛在4℃下固定4小时,并在1%四氧化锇中在20℃下固定2小时。然后,使用乙醇(50-100%)和丙酮的梯度对它们进行脱水,包埋在环氧树脂中,并在60°C下聚合48小时。在透射电子显微镜(HT7700,HITACHI)之前切割超薄切片(80 nm)并用乙酸双氧铀和柠檬酸铅染色。使用 SlowScan CCD 相机和 iTEM 软件(版本 01.07,Olympus Soft Imaging Solutions)捕获图像。自噬体的定量遵循先前描述的既定程序。 [
48
]
siRNA Transfection siRNA转染
Cells were seeded onto 6/96‐well plates (5×104 cells/mL) and transfected with siRNA. The target sequences for the siRNA used in this study are shown in Table S2 (Supporting Information). The final siRNA concentration used was 10 nm, and the transfection was carried out using RNAiMax (Invitrogen, Cat No. 13778150) following the manufacturer's specifications. Follow‐up experiments were continued 24 h after transfection, as described previously.[
49
]
将细胞接种到6/96孔板(5×10 4 个细胞/mL)并用siRNA转染。本研究中使用的 siRNA 的靶序列如表所示 S2 (支持信息)。使用的最终siRNA浓度为10 nm ,并使用RNAiMax(Invitrogen,目录号13778150)按照制造商的说明书进行转染。如前所述,转染后 24 小时继续进行后续实验。 [
49
]
Animals 动物
Eight‐week‐old male C57BL/6 mice were obtained from Beijing HFK Bioscience Co., Ltd. and housed in a specific pathogen‐free facility at the State Key Laboratory of Biotherapy (Sichuan University, China). The mice underwent an adaptive feeding period of one week prior to the commencement of the experiments. All mouse‐related procedures were conducted in accordance with the Institutional Guidelines of the Animal Care and Use Committee (K2018071, Sichuan University, China).
8周龄雄性C57BL/6小鼠获自北京华富康生物科技有限公司,饲养在生物治疗国家重点实验室(中国四川大学)的无特定病原体设施中。实验开始前,小鼠经历了一周的适应性喂养期。所有小鼠相关程序均按照动物护理和使用委员会的机构指南(K2018071,四川大学,中国)进行。
Repetitive Traumatic Brain Injuries
重复性脑外伤
A mouse model involving closed skulls and repetitive mild head injuries was used, as described previously.[
15
,
50
] Briefly, mice were placed in a stereotaxic frame following anesthesia with isoflurane, 4% for induction and 1–2% for maintenance. The injury was induced using a 3 mm blunt metal impactor tip positioned at 1.8 mm caudal to bregma and 2.0 mm left of midline. The injury was triggered by an electromagnetically controlled cortical impact device (Custom Design & Fabrication, Inc, USA) with 3.0 m s−1 for strike velocity, 1.0 mm for strike depth, and 500 ms for dwell time to the exposed skull. Post‐impact, the skin was sutured, disinfected with iodophor, and the mice were allowed to recover from anesthesia on a warming pad before being returned to their home cages. A second identical injury procedure was performed 24 h later. Sham injuries followed the same procedures and anesthesia, excluding the delivery of an impact.
如前所述,使用了包含闭合头骨和重复性轻度头部损伤的小鼠模型。 [
15
,
50
]简而言之,小鼠在异氟烷麻醉后被放置在立体定位架中,4% 用于诱导,1-2% 用于维持。使用位于前囟尾部 1.8 毫米、中线左侧 2.0 毫米的 3 毫米钝金属冲击器尖端诱发损伤。伤害是由电磁控制的皮质冲击装置(美国定制设计与制造公司)触发的,冲击速度为 3.0 m s -1 ,冲击深度为 1.0 mm,暴露头骨的停留时间为 500 ms。撞击后,缝合皮肤,用碘伏消毒,让小鼠在温暖的垫子上从麻醉中恢复,然后再返回它们的笼子。 24 小时后进行第二次相同的损伤手术。假手术伤害遵循相同的程序和麻醉,但不包括撞击。
Y‐Maze Spontaneous Alternation Test
Y 迷宫自发交替测试
The Y‐maze spontaneous alternation test was used to assess spatial working memory, as described previously.[
51
] Briefly, one month after model establishment, each mouse was placed naive to the Y maze (39.5×8.5×13 cm, Sansbio co. ltd, China), at the same end of one arm and allowed to move freely through the maze during an 8‐minute session. The entire session was video‐recorded (Basler acA640‐120gm, Basler Vision Technology), and the number of arm entries for each mouse was subsequently calculated.
如前所述,Y 迷宫自发交替测试用于评估空间工作记忆。 [
51
]简而言之,模型建立一个月后,将每只小鼠置于 Y 迷宫(39.5×8.5×13 cm,Sansbio co. ltd,中国),在一只手臂的同一端,并允许其在迷宫中自由移动。 8 分钟的会议。整个过程被视频记录(Basler acA640-120gm,Basler Vision Technology),随后计算每只小鼠的手臂进入次数。
Morris Water Maze Test 莫里斯水迷宫测试
The Morris water maze (MWM) test was conducted to assess spatial learning, as previously described.[
52
] Briefly, a circular water tank (diameter 80 cm) was filled with milk powder‐stained water, housing a concealed round platform (diameter 7 cm) positioned 1 cm below the water surface at the center of a specific quadrant. The test consisted of a place navigation test (five days) and a spatial probe test (one day). During the place navigation test stage, mice started from one of the four quadrants facing the pool wall and ended upon reaching the platform. If mice failed to locate the platform within 120 s, they were guided to it. In the subsequent spatial probe test, the platform was removed, and task performance was recorded for 120 s. Mouse movements in the water pool were recorded by a video camera (Basler acA640‐120gm, Basler Vision Technology), and task‐related metrics, including swimming paths, speed, and time spent in each quadrant, were recorded using WMT‐100 software (Chengdu Techman Software Co. Ltd, China).
如前所述,进行莫里斯水迷宫(MWM)测试是为了评估空间学习。 [
52
]简而言之,一个圆形水箱(直径80厘米)充满了奶粉染色的水,里面有一个隐藏的圆形平台(直径7厘米),位于特定象限中心水面以下1厘米处。该测试包括地点导航测试(五天)和空间探测测试(一天)。在位置导航测试阶段,小鼠从面向池壁的四个象限之一开始,并在到达平台时结束。如果小鼠未能在 120 秒内找到平台,它们就会被引导到平台。在随后的空间探测测试中,平台被移除,并记录任务表现120秒。通过摄像机(Basler acA640-120gm,Basler Vision Technology)记录小鼠在水池中的运动,并使用 WMT-100 软件记录与任务相关的指标,包括游泳路径、速度和在每个象限中花费的时间(中国成都达明软件有限公司)。
Novel Object Recognition Test
新物体识别测试
The novel object recognition (NOR) test was performed to evaluate memory retention, as described previously.[
52
] Briefly, 24 h before the test, mice underwent a 5‐minute habituation period in the arenas (50 cm × 50 cm plastic container, Sansbio co. ltd, China) without objects. On the subsequent day, the mice re‐entered the arena from the same starting point (facing the bottom‐left corner) for the training and testing stages. In the first stage of the test, animals were confronted with two identical objects for 10 min. In the second stage, 1 h after the familiarization period, the animals were exposed to two dissimilar objects in the same open field for 5 min: one familiar object used in the first phase and another novel object. The time spent exploring each object in stage two was detected using Supermaze (ver 3.3, Shanghai XinRuan Information Technology co. ltd, China). The discrimination index (DI) was calculated using the following equation: DI = TN/ (TN + TF), where TN represents the exploration time devoted to the novel object and TF is the exploration time for the familiar object.
如前所述,进行新物体识别(NOR)测试来评估记忆保留情况。 [
52
]简而言之,测试前24小时,小鼠在没有物体的竞技场(50 cm×50 cm塑料容器,Sansbio co. ltd,中国)中经历5分钟的习惯期。第二天,小鼠从同一起点(面向左下角)重新进入竞技场进行训练和测试阶段。在测试的第一阶段,动物面对两个相同的物体 10 分钟。在第二阶段,熟悉期后1小时,将动物在同一开放场地中暴露于两个不同的物体5分钟:一个用于第一阶段的熟悉物体和另一个新物体。使用 Supermaze(3.3 版,上海欣软信息技术有限公司,中国)检测第二阶段探索每个对象所花费的时间。使用以下等式计算辨别指数(DI):DI = TN/(TN + TF),其中TN表示用于新物体的探索时间,TF是用于熟悉物体的探索时间。
Rotarod Treadmill Test 旋转跑步机测试
The rotarod treadmill test was conducted to assess motor coordination, as described previously.[
53
] One month after establishing the model, animals were placed on a rotarod treadmill (RWD, Shenzhen, China) in the accelerating rotor mode (10 speeds ranging from 4 to 40 rpm for 5 min). The interval from the moment the animal mounted the rod to when it fell off was recorded as the retention time, and mice that remained on the accelerating rotating rod for 300 s were recorded as survivors. Animals underwent training for two days, with three trials per day, before model establishment, and the mean duration on the rod was recorded to obtain stable baseline values. Performance on the rotarod test was measured three times a month after repeated mild closed‐head injuries.
如前所述,进行旋转跑步机测试是为了评估运动协调性。 [
53
]模型建立一个月后,将动物置于旋转跑步机(RWD,深圳,中国)上,以加速转子模式(从 4 到 40 rpm 的 10 种速度,持续 5 分钟)。从动物登上杆到脱落的时间记录为停留时间,在加速旋转杆上保持300秒的小鼠记录为幸存者。模型建立前,动物接受为期两天的训练,每天进行3次试验,并记录在杆上的平均持续时间以获得稳定的基线值。在重复轻度闭合性头部损伤后,每月测量三次旋转杆测试的表现。
Immunofluorescence 免疫荧光
For immunofluorescence of brain tissue sections, mice were anesthetized with pentobarbitone and perfused with ice‐cold PBS (0.01 m, pH 7.4). Brains were isolated, fixed in 4% paraformaldehyde in PBS overnight, and then sectioned into 4 µm paraffin sections. After staining with primary antibodies (Anti‐DNALI1, Abcam, ab155490, 1:500; Anti‐Acetylated Tubulin, Proteintech, 66200‐1‐Ig, 1:250) and fluorescent‐tagged secondary antibodies (Alexa Fluor 488 Goat Anti‐Mouse IgG, Jackson ImmunoResearch, 115‐005, 1:500; Cy3 Goat Anti‐Mouse IgG, Jackson ImmunoResearch, 115–165, 1:500), the nuclei were counterstained with 4,6‐diamidino‐2‐phenylindole (DAPI), and coverslips were applied. The labeled sections were captured using a confocal laser‐scanning microscope (Nikon ECLIPSE Ti‐S). Brightness and contrast adjustments were uniformly applied to the captured images using CaseViewer software, version 2.4 (3DHIESTECH Ltd).
对于脑组织切片的免疫荧光,用戊巴比妥麻醉小鼠并用冰冷的 PBS(0.01 m ,pH 7.4)灌注。分离大脑,在 PBS 中的 4% 多聚甲醛中固定过夜,然后切成 4 µm 石蜡切片。用一抗(抗 DNALI1,Abcam,ab155490,1:500;抗乙酰化微管蛋白,Proteintech,66200-1-Ig,1:250)和荧光标记二抗(Alexa Fluor 488 山羊抗小鼠 IgG)染色后,杰克逊免疫研究,115‐005,1:500; IgG,杰克逊免疫研究,115-165,1:500),细胞核用 4,6-二脒基-2-苯基吲哚 (DAPI) 复染,并盖上盖玻片。使用共焦激光扫描显微镜(Nikon ECLIPSE Ti-S)捕获标记的切片。使用 CaseViewer 软件 2.4 版(3DHIESTECH Ltd)对捕获的图像统一进行亮度和对比度调整。
For immunofluorescence, cells were seeded on cell‐climbing slides. After treatment, cells were fixed with 4% paraformaldehyde at room temperature for 30 min. Subsequently, cells were blocked for 1 h at room temperature with 10% normal goat serum (Solarbio, SL038) dissolved in 0.2% Triton X‐100 PBS and incubated with the primary antibody (DNALI1, Abcam, ab155490, 1:500) overnight at 4 °C in a humidified chamber. The following day, the secondary antibody (Alexa Fluor 488 AffiniPure Alpaca Anti‐Rabbit IgG (H+L), Jackson ImmunoResearch, 611‐545‐215, 1:500) and DAPI were used for imaging. Images were captured under a fluorescence microscope (OLYMPUS VS200, JPN) and quantified using ImageJ (, version 1.5.1 (NIH, USA).
对于免疫荧光,将细胞接种在细胞攀爬载玻片上。处理后,用4%多聚甲醛在室温下固定细胞30分钟。随后,用溶解在 0.2% Triton X-100 PBS 中的 10% 正常山羊血清 (Solarbio, SL038) 在室温下封闭细胞 1 小时,并与一抗 (DNALI1, Abcam, ab155490, 1:500) 一起在 1:500 下孵育过夜。 4°C 加湿室中。第二天,使用二抗(Alexa Fluor 488 AffiniPure Alpaca Anti-Rabbit IgG (H+L), Jackson Nutrition, 611-545-215, 1:500)和 DAPI 进行成像。在荧光显微镜(OLYMPUS VS200,日本)下捕获图像并使用 ImageJ(1.5.1 版(NIH,美国)进行定量)。
Western Blot 蛋白质印迹
Samples were homogenized in cell lysis buffer (P0013, Beyotime) supplemented with the protease inhibitor phenylmethylsulfonyl fluoride (1:100, ST507, Beyotime) and centrifuged at 12000 × g for 30 min. The supernatant was collected, and the total protein concentration was determined using a BCA protein assay kit (P0011, Beyotime). Equal amounts of protein were separated on 4–12% bis‐Tris gels with MOPs running buffer at 140 V for 1.5 h and then transferred to nitrocellulose membranes using a Trans‐Blot system at 100 V for 1 h. Then, the membranes were washed with 1 × TBST for 5 min at room temperature (20 °C), shaken, and blocked with 5% skim milk in 1 × TBST for 1 h at room temperature. The following antibodies were diluted in 1 × TBST as indicated: 1:3000 for anti‐LC3 (14600‐1‐AP, Proteintech), 1:3000 for anti‐P62 (18420‐1‐AP, Proteintech), 1:10 000 for anti‐mouse IgG (A9044, Sigma‐Aldrich), 1:10 000 for anti‐rabbit IgG (A0545, Sigma‐Aldrich), 1:2000 for anti‐DNALI1 (ab155490, Abcam & 17601‐1‐AP, Proteintech); 1:1000 for anti‐Tau5 (ab80579, Abcam); 1:1000 for Anti‐Phospho‐Tau (Ser202, Thr205) (MN1020, Thermo Fisher Scientific). All uncropped images of western blots are shown in Figure S9 (Supporting Information).
将样品在补充有蛋白酶抑制剂苯甲基磺酰氟(1:100,ST507,Beyotime)的细胞裂解缓冲液(P0013,Beyotime)中匀浆,并在 12000 × g 下离心 30 分钟。收集上清液,使用BCA蛋白测定试剂盒(P0011,Beyotime)测定总蛋白浓度。将等量的蛋白质在 4-12% bis-Tris 凝胶上用 MOP 运行缓冲液在 140 V 下分离 1.5 小时,然后使用 Trans-Blot 系统在 100 V 下转移到硝酸纤维素膜上 1 小时。然后用 1×TBST 室温(20℃)洗膜 5 min,振摇,用 5%脱脂牛奶溶于 1×TBST 室温封闭 1 h。以下抗体按所示在 1 × TBST 中稀释:抗 LC3(14600-1-AP,Proteintech)为 1:3000,抗 P62(18420-1-AP,Proteintech)为 1:3000,1:10 000用于抗小鼠 IgG (A9044, Sigma-Aldrich),1:10 000 用于抗兔 IgG (A0545, Sigma-Aldrich),1:2000 用于抗 DNALI1 (ab155490, Abcam & 17601-1-AP, Proteintech); 1:1000 抗 Tau5(ab80579,Abcam); 1:1000 抗 Phospho-Tau(Ser202、Thr205)(MN1020,Thermo Fisher Scientific)。所有未裁剪的蛋白质印迹图像如图所示 S9 (支持信息)。
Adeno‐Associated Viral Vector Construction and Preparation
腺相关病毒载体的构建和制备
To knockdown murine DNALI1 with a recombinant adeno‐associated virus (rAAV) in vivo, sgRNAs were designed using online tools (https://crispr.mit.edu/ and https://crispr.cos.uni‐heidelberg.de/). Off‐target effects were assessed using resources available at https://asia.ensembl.org/. sgRNA sequences with fewer off‐target sites were selected for further analysis. The target sequences of the sgRNA used in this study are shown in Table S3 (Supporting Information). The sgRNAs for murine DNALI1 knockdown were inserted into the plasmid, which was used to produce mDNALI1‐sp. g3‐rAAV8 (Obio Technology, Shanghai, China) was used to knockdown DNALI1, and the AAV8‐empty vector was used as a control. All rAAV8 vectors were generated using the triple‐plasmid co‐transfection method in human embryonic kidney 293 cells. After 72 h post‐transfection, the rAAV8 vectors were collected, purified through two rounds of CsCl gradient ultracentrifugation, followed by silver staining and genome copy titration. The viral vectors were aliquoted and stored at −80 °C before use.
为了在体内用重组腺相关病毒(rAAV)敲低小鼠 DNALI1,使用在线工具设计了 sgRNA( https://crispr.mit.edu/ 和 https://crispr.cos.uni‐heidelberg.de/ )。使用可用资源评估脱靶效应 https://asia.ensembl.org/ 。选择脱靶位点较少的 sgRNA 序列进行进一步分析。本研究中使用的sgRNA的靶序列见表 S3 (支持信息)。用于敲低小鼠 DNALI1 的 sgRNA 被插入质粒中,用于产生 mDNALI1-sp。 g3-rAAV8(Obio Technology,上海,中国)用于敲低DNALI1,AAV8-空载体用作对照。所有 rAAV8 载体均采用三质粒共转染方法在人胚肾 293 细胞中生成。转染72小时后,收集rAAV8载体,通过两轮CsCl梯度超速离心纯化,然后进行银染和基因组拷贝滴定。使用前将病毒载体等分并储存在-80°C。
AAV Injection 腺病毒注射液
For stereotaxic injection, mice were anesthetized by means of intraperitoneal injection of pentobarbital (100 mg k−1g, P11011, Bioreagent) and fixed on a stereotaxic plate (RWD, Shenzhen, China). A hole was drilled into the bone using a hand drill (RWD, Shenzhen, China). Eight microliters of the virus, with a total titer of 1.0 × 1012 genome copies, were injected into the lateral ventricle (AP = −0.6 mm, ML = −1.0 mm, DV = 2 mm) relative to the bregma. The needle was then held in place for an additional 10 min. After injection, the needle was withdrawn, and the wound was sutured.
对于立体定位注射,通过腹腔注射戊巴比妥(100 mg k -1 g,P11011,生物试剂)麻醉小鼠并固定在立体定位板上(RWD,深圳,中国)。使用手钻(RWD,中国深圳)在骨头上钻一个孔。将总效价为 1.0 × 10 12基因组拷贝的 8 微升病毒注射到相对于前囟的侧脑室(AP = -0.6 mm,ML = -1.0 mm,DV = 2 mm)。然后将针继续固定 10 分钟。注射后,拔出针,缝合伤口。
Statistical Analysis 统计分析
Data are presented as individual values. Statistical analysis was conducted using GraphPad Prism 8.0 software (GraphPad Software, Inc., USA), and Student's t‐test was used to analyze statistical differences between two groups. One‐way ANOVA or repeated‐measures two‐way ANOVA with Tukey's post hoc test was used when appropriate. Values are presented as mean ± SEM, and individual data points represent individual samples or animals. Further details of the statistical analysis can be found in the figure legends. p‐value < 0.05 was considered statistically significant.
数据以单独值的形式呈现。使用GraphPad Prism 8.0软件(GraphPad Software,Inc.,美国)进行统计分析,并使用Student's t检验分析两组之间的统计差异。在适当的时候使用单因素方差分析或重复测量双向方差分析和 Tukey 事后检验。值以平均值±SEM表示,各个数据点代表各个样品或动物。统计分析的更多详细信息可以在图形图例中找到。 p值<0.05被认为具有统计学意义。
Conflict of Interest 利益冲突
The authors declare no conflict of interest.
作者声明不存在利益冲突。
Author Contributions 作者贡献
P.L. conceived and supervised the study. P.L. and X.D. designed and raised funds for this study. X.D. performed data analysis; X.D. and Q.W. performed cell biology experiments; X.D., B.D., S.C., and Y.C. performed animal experiments; W.L. coordinated the experiments; and X.D., K.L., S.Q., Q.Z.T., W.L., and P.L. integrated the data and drafted the manuscript. All authors have edited and approved the manuscript.
PL 构思并监督了这项研究。 PL 和 XD 为本研究设计并筹集资金。 XD进行数据分析; XD和QW进行细胞生物学实验; XD、BD、SC、YC进行动物实验; WL 协调实验; XD、KL、SQ、QZT、WL、PL整合数据并起草稿件。所有作者均已编辑并批准了手稿。
Supporting information 支持信息
Supporting Information 支持信息
Acknowledgements 致谢
The authors thank Dr. Boxun Lu (Fudan University, China) for providing the STHdhQ7/Q7 cells. This work was supported by the National Key Research and Development Project of China (2021YFC2500100); National Natural Science Foundation of China (82071191, 82301362); National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2023LC005); the West China Hospital 1.3.5 project for disciplines of excellence (ZYYC23016); the Key Research Projects of Sichuan Province (24SYSX0093); Major Science & Technology Program of Sichuan Province (2022ZDZX0021); Natural Science Foundation of Jiangsu Province (BK20230277); Natural Science Foundation of Jiangsu Higher Education Institutions of China (23KJB180023); Suzhou Science and Technology Bureau project (SZM2023037) and the Gusu Talent Program.
作者感谢陆博勋博士(中国复旦大学)提供 STHdhQ7/Q7 细胞。该工作得到国家重点研发计划项目(2021YFC2500100)的支持;国家自然科学基金(82071191、82301362);四川大学华西医院国家老年医学临床研究中心(Z2023LC005);华西医院1.3.5卓越学科建设项目(ZYYC23016);四川省重点科研项目(24SYSX0093);四川省重大科技计划项目(2022ZDZX0021);江苏省自然科学基金(BK20230277);江苏省高等学校自然科学基金项目(23KJB180023);苏州市科技局项目(SZM2023037)和姑苏人才计划。
Ding X., Cao S., Wang Q., Du B., Lu K., Qi S., Cheng Y., Tuo Q., Liang W., Lei P., DNALI1 Promotes Neurodegeneration after Traumatic Brain Injury via Inhibition of Autophagosome‐Lysosome Fusion. Adv. Sci.
2024, 11, 2306399. 10.1002/advs.202306399IF: 14.3 Q1
丁X.,曹S.,王Q.,杜B.,卢K.,齐S.,程Y.,拓Q.,梁W.,雷P.,DNALI1通过抑制促进创伤性脑损伤后神经退行性变自噬体-溶酶体融合。副词。科学。 2024 年 11 月 2306399。10.1002/advs.202306399IF:14.3 第一季度
Contributor Information 贡献者信息
Weibo Liang, Email: liangweibo@scu.edu.cn.
梁伟波,Email:liangweibo@scu.edu.cn。
Peng Lei, Email: peng.lei@scu.edu.cn.
彭蕾,Email:peng.lei@scu.edu.cn。
Data Availability Statement
数据可用性声明
The data that support the findings of this study are available from the corresponding author upon reasonable request.
支持本研究结果的数据可根据合理要求从通讯作者处获得。
References 参考
-
1.
Blennow K., Brody D. L., Kochanek P. M., Levin H., McKee A., Ribbers G. M., Yaffe K., Zetterberg H., Nat. Rev. Dis. Primers
2016, 2, 16084.
[DOI] [PubMed] [Google Scholar]
1. Blennow K.、Brody DL、Kochanek PM、Levin H.、McKee A.、Ribbers GM、Yaffe K.、Zetterberg H.、Nat。牧师迪斯。引物 2016, 2, 16084. [ DOI ] [ PubMed ] [ Google Scholar ] -
2.a) Iaccarino C., Carretta A., Nicolosi F., Morselli C., J Neurosurg Sci
2018, 62, 535;
[DOI] [PubMed] [Google Scholar]; b) N. C. f. Health
,
Centers for Disease Control and Prevention
, 2021.
2.a ) Iaccarino C.、Carretta A.、Nicolosi F.、Morselli C.、神经外科杂志 2018, 62, 535; [ DOI ] [ PubMed ] [ Google Scholar ]; b) NC健康,疾病控制与预防中心,2021 年。 -
3.
Dewan M. C., Rattani A., Gupta S., Baticulon R. E., Hung Y. C., Punchak M., Agrawal A., Adeleye A. O., Shrime M. G., Rubiano A. M., Rosenfeld J. V., Park K. B., J Neurosurg
2018, 130, 1080.
[DOI] [PubMed] [Google Scholar]
3. Dewan MC、Rattani A.、Gupta S.、Baticulon RE、Hung YC、Punchak M.、Agrawal A.、Adeleye AO、Shrime MG、Rubiano AM、Rosenfeld JV、Park KB、J Neurosurg 2018, 130, 1080。 [ DOI ] [ PubMed ] [ Google Scholar ] -
4.
Ma V. Y., Chan L., Carruthers K. J., Arch. Phys. Med. Rehabil.
2014, 95, 986.
[DOI] [PMC free article] [PubMed] [Google Scholar]
4. Ma VY、Chan L.、Carruthers KJ、Arch。物理。医学。康复。 2014, 95, 986. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
5.
Shin M. K., Vázquez‐Rosa E., Koh Y., Dhar M., Chaubey K., Cintrón‐Pérez C. J., Barker S., Miller E., Franke K., Noterman M. F., Seth D., Allen R. S., Motz C. T., Rao S. R., Skelton L. A., Pardue M. T., Fliesler S. J., Wang C., Tracy T. E., Gan L., Liebl D. J., Savarraj J. P. J., Torres G. L., Ahnstedt H., McCullough L. D., Kitagawa R. S., Choi H. A., Zhang P., Hou Y., Chiang C. W., et al., Cell
2021, 184, 2715.
[DOI] [PMC free article] [PubMed] [Google Scholar]
5. Shin MK、Vázquez-Rosa E.、Koh Y.、Dhar M.、Chaubey K.、Cintrón-Pérez CJ、Barker S.、Miller E.、Franke K.、Noterman MF、Seth D.、Allen RS、 Motz CT、Rao SR、Skelton LA、Pardue MT、Fliesler SJ、Wang C.、Tracy TE、Gan L.、Liebl DJ、Savarraj JPJ、Torres GL、 Ahnstedt H.、McCullough LD、Kitakawa RS、Choi HA、Zhang P.、Hou Y.、Chiang CW 等人,Cell 2021, 184, 2715。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
6.
Cassidy J. D., Carroll L. J., Peloso P. M., Borg J., von Holst H., Holm L., Kraus J., Coronado V. G., J Rehabil Med
2004, 36, 28.
[DOI] [PubMed] [Google Scholar]
6. Cassidy JD、Carroll LJ、Peloso PM、Borg J.、von Holst H.、Holm L.、Kraus J.、Coronado VG、J Rehabil Med 2004, 36, 28。 DOI ] [ PubMed ] [ Google Scholar ] - 7. Belanger H. G., Spiegel E., Vanderploeg R. D., J Int Neuropsychol Soc 2010, 16, 262. [DOI] [PubMed] [Google Scholar]
- 8. DeKosky S. T., Blennow K., Ikonomovic M. D., Gandy S., Nat. Rev. Neurol. 2013, 9, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Ghajari M., Hellyer P. J., Sharp D. J., Brain 2017, 140, 333; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jiang L., Ding X., Wang W., Yang X., Li T., Lei P., Biomolecules 2022, 12, 1194; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ding X. L., Tuo Q. Z., Lei P., J Alzheimers Dis 2021, 80, 1353. [DOI] [PubMed] [Google Scholar]
- 10.a) Ahmadzadeh H., Smith D. H., Shenoy V. B., Biophys. J. 2014, 106, 1123; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mangiafico S. P., Tuo Q.‐Z., Li X.‐L., Liu Y., Haralambous C., Ding X.‐L., Ayton S., Wang Q., Laybutt D. R., Chan J. Y., Zhang X., Kos C., Thomas H. E., Loudovaris T., Yang C.‐H., Joannides C. N., Lamont B. J., Dai L., He H.‐H., Dong B., Andrikopoulos S., Bush A. I., Lei P., Mol. Psychiatry 2023, 28, 3982; [DOI] [PubMed] [Google Scholar]; c) Lei P., Ayton S., Sci. Bull. 2023, 68, 2507; [DOI] [PubMed] [Google Scholar]; d) Chen K., Tang F., Du B., Yue Z. Z., Jiao L. L., Ding X. L., Tuo Q. Z., Meng J., He S. Y., Dai L., Lei P., Wei X. W., MedComm 2023, 4, e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ciallella J. R., Ikonomovic M. D., Paljug W. R., Wilbur Y. I., Dixon C. E., Kochanek P. M., Marion D. W., DeKosky S. T., J Neurotrauma 2002, 19, 1555. [DOI] [PubMed] [Google Scholar]
- 12. Miller J. A., Guillozet‐Bongaarts A., Gibbons L. E., Postupna N., Renz A., Beller A. E., Sunkin S. M., Ng L., Rose S. E., Smith K. A., Szafer A., Barber C., Bertagnolli D., Bickley K., Brouner K., Caldejon S., Chapin M., Chua M. L., Coleman N. M., Cudaback E., Cuhaciyan C., Dalley R. A., Dee N., Desta T., Dolbeare T. A., Dotson N. I., Fisher M., Gaudreault N., Gee G., Gilbert T. L., et al., Elife 2017, 6, e31126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts A. J., Biochem. Soc. Trans. 2018, 46, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kondo A., Shahpasand K., Mannix R., Qiu J., Moncaster J., Chen C. H., Yao Y., Lin Y. M., Driver J. A., Sun Y., Wei S., Luo M. L., Albayram O., Huang P., Rotenberg A., Ryo A., Goldstein L. E., Pascual‐Leone A., McKee A. C., Meehan W., Zhou X. Z., Lu K. P., Nature 2015, 523, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Xiong Y., Mahmood A., Chopp M., Nat. Rev. Neurosci. 2013, 14, 128; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hoogenboom W. S., Branch C. A., Lipton M. L., Pharmacol. Ther. 2019, 198, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a) Hou Y., Witman G. B., Exp. Cell Res. 2015, 334, 26; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lechtreck K. F., Trends Biochem. Sci. 2015, 40, 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Pampliega O., Orhon I., Patel B., Sridhar S., Díaz‐Carretero A., Beau I., Codogno P., Satir B. H., Satir P., Cuervo A. M., Nature 2013, 502, 194; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lee M. S., Hwang K. S., Oh H. W., Ji‐Ae K., Kim H. T., Cho H. S., Lee J. J., Yeong Ko J., Choi J. H., Jeong Y. M., You K. H., Kim J., Park D. S., Nam K. H., Aizawa S., Kiyonari H., Shioi G., Park J. H., Zhou W., Kim N. S., Kim C. H., Dev Biol 2015, 400, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loos B., du Toit A., Hofmeyr J. H., Autophagy 2014, 10, 2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thorburn A., Apoptosis 2008, 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su Z., Yang Z., Xu Y., Chen Y., Yu Q., Mol Cancer 2015, 14, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao W., Wang X., Zhou Y., Wang X., Yu Y., Signal Transduct. Target. Ther. 2022, 7, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boya P., González‐Polo R. A., Casares N., Perfettini J. L., Dessen P., Larochette N., Métivier D., Meley D., Souquere S., Yoshimori T., Pierron G., Codogno P., Kroemer G., Mol. Cell. Biol. 2005, 25, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selvackadunco S., Langford K., Shah Z., Hurley S., Bodi I., King A., Aarsland D., Troakes C., Al‐Sarraj S., J Neural Transm 2019, 126, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a) Hyman B. T., Phelps C. H., Beach T. G., Bigio E. H., Cairns N. J., Carrillo M. C., Dickson D. W., Duyckaerts C., Frosch M. P., Masliah E., Mirra S. S., Nelson P. T., Schneider J. A., Thal D. R., Thies B., Trojanowski J. Q., Vinters H. V., Montine T. J., Alzheimers Dement. 2012, 8, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Potter P. E., Rauschkolb P. K., Pandya Y., Sue L. I., Sabbagh M. N., Walker D. G., Beach T. G., Acta Neuropathol. 2011, 122, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wisse L. E. M., Butala N., Das S. R., Davatzikos C., Dickerson B. C., Vaishnavi S. N., Yushkevich P. A., Wolk D. A., Neurobiol Aging 2015, 36, 3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gavett B. E., Stern R. A., Cantu R. C., Nowinski C. J., McKee A. C., Alzheimers Res. Ther. 2010, 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedman N. P., Robbins T. W., Neuropsychopharmacology 2022, 47, 72.34408280 [Google Scholar]
- 28. Hu L., Wang B., Zhang Y., Alzheimers Res. Ther. 2017, 9, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rashid S., Breckle R., Hupe M., Geisler S., Doerwald N., Neesen J., Mol. Reprod. Dev. 2006, 73, 784. [DOI] [PubMed] [Google Scholar]
- 30. Mishra M., Paunesku T., Woloschak G. E., Siddique T., Zhu L. J., Lin S., Greco K., Bigio E. H., Acta Neuropathol. 2007, 114, 81. [DOI] [PubMed] [Google Scholar]
- 31. Neumann M., Sampathu D. M., Kwong L. K., Truax A. C., Micsenyi M. C., Chou T. T., Bruce J., Schuck T., Grossman M., Clark C. M., McCluskey L. F., Miller B. L., Masliah E., Mackenzie I. R., Feldman H., Feiden W., Kretzschmar H. A., Trojanowski J. Q., Lee V. M., Science 2006, 314, 130. [DOI] [PubMed] [Google Scholar]
- 32. Gao F., Hu M., Zhang J., Hashem J., Chen C., Acta Neuropathol. 2022, 144, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar S., Phaneuf D., P. Cordeau, Jr. , Boutej H., Kriz J., Julien J. P., Mol Neurodegener 2021, 16, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piras I. S., Krate J., Delvaux E., Nolz J., Mastroeni D. F., Persico A. M., Jepsen W. M., Beach T. G., Huentelman M. J., Coleman P. D., J Alzheimers Dis 2019, 70, 691. [DOI] [PubMed] [Google Scholar]
- 35. Namjoshi D. R., Good C., Cheng W. H., Panenka W., Richards D., Cripton P. A., Wellington C. L., Dis Model Mech 2013, 6, 1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mizushima N., Komatsu M., Cell 2011, 147, 728. [DOI] [PubMed] [Google Scholar]
- 37. Choi A. M., Ryter S. W., Levine B., N. Engl. J. Med. 2013, 368, 651. [DOI] [PubMed] [Google Scholar]
- 38. Nah J., Yuan J., Jung Y. K., Mol. Cells 2015, 38, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao X., Zeng W., Xu H., Sun Z., Hu Y., Peng B., McBride J. D., Duan J., Deng J., Zhang B., Kim S. J., Zoll B., Saito T., Sasaguri H., Saido T. C., Ballatore C., Yao H., Wang Z., Trojanowski J. Q., Brunden K. R., Lee V. M., He Z., Sci. Transl. Med. 2023, 15, eabo6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W., Smyth G. K., limma powers differential expression analyses for RNA‐sequencing and microarray studies, 2013. [DOI] [PMC free article] [PubMed]
- 41. Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A. H., Tanaseichuk O., Benner C., Chanda S. K., Nat. Commun. 2019, 10, 1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kolde R., pheatmap: Pretty Heatmaps 2015.
- 43. Blighe K., Rana S., Lewis M., https://github.com/kevinblighe/EnhancedVolcano (accessed: July 2018).
- 44. Chin C. H., Chen S. H., Wu H. H., Ho C. W., Ko M. T., Lin C. Y., BMC Syst. Biol. 2014, 8, S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Proc. Natl. Acad. Sci. USA 2005, 102, 15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langfelder P., Horvath S., BMC Bioinformatics 2008, 9, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.a) Tuo Q. Z., Liu Y., Xiang Z., Yan H. F., Zou T., Shu Y., Ding X. L., Zou J. J., Xu S., Tang F., Gong Y. Q., Li X. L., Guo Y. J., Zheng Z. Y., Deng A. P., Yang Z. Z., Li W. J., Zhang S. T., Ayton S., Bush A. I., Xu H., Dai L., Dong B., Lei P., Signal Transduct Target Ther 2022, 7, 59; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jiao L., Li X., Luo Y., Wei J., Ding X., Xiong H., Liu X., Lei P., Front Cell Neurosci 2022, 16, 995084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.a) Backues S. K., Chen D., Ruan J., Xie Z., Klionsky D. J., Autophagy 2014, 10, 155; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Guo J., Tuo Q. Z., Lei P., J. Neurochem. 2023, 165, 487. [DOI] [PubMed] [Google Scholar]
- 49. Tang F., Zhou L. Y., Li P., Jiao L. L., Chen K., Guo Y. J., Ding X. L., He S. Y., Dong B., Xu R. X., Xiong H., Lei P., Neurotherapeutics 2023, 20, 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siebold L., Obenaus A., Goyal R., Exp Neurol 2018, 310, 48. [DOI] [PubMed] [Google Scholar]
- 51.a) Lei P., Ayton S., Finkelstein D. I., Spoerri L., Ciccotosto G. D., Wright D. K., Wong B. X., Adlard P. A., Cherny R. A., Lam L. Q., Roberts B. R., Volitakis I., Egan G. F., McLean C. A., Cappai R., Duce J. A., Bush A. I., Nat. Med. 2012, 18, 291; [DOI] [PubMed] [Google Scholar]; b) Wang Q., Zhang X., Guo Y. J., Pang Y. Y., Li J. J., Zhao Y. L., Wei J. F., Zhu B. T., Tang J. X., Jiang Y. Y., Meng J., Yue J. R., Lei P., Zool Res 2023, 44, 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tuo Q. Z., Lei P., Jackman K. A., Li X. L., Xiong H., Li X. L., Liuyang Z. Y., Roisman L., Zhang S. T., Ayton S., Wang Q., Crouch P. J., Ganio K., Wang X. C., Pei L., Adlard P. A., Lu Y. M., Cappai R., Wang J. Z., Liu R., Bush A. I., Mol. Psychiatry 2017, 22, 1520. [DOI] [PubMed] [Google Scholar]
- 53. Luoqian J., Yang W., Ding X., Tuo Q.‐z., Xiang Z., Zheng Z., Guo Y.‐J., Li L., Guan P., Ayton S., Cell Mol. Immunol. 2022, 19, 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


