Abstract 抽象
Background 背景
Type 2 diabetes mellitus (T2DM) is a major public health concern with growing prevalence with multiple debilitating complications. GlycaCare-II is a proprietary herbal formulation supplement for T2DM containing extracts of Cinnamomum cassia, Momordica charantia, Pterocarpus marsupium, Gymnema sylvestre, Salacia reticulata, Eugenia jambolana, and a bioavailability enhancer piperine from Piper nigrum.
2 型糖尿病 (T2DM) 是一个主要的公共卫生问题,患病率不断上升,并伴有多种使人衰弱的并发症。GlycaCare-II 是一种针对 T2DM 的专有草药配方补充剂,含有肉桂、枸杞、有袋紫檀、樟子匙羹藤、网纹香草、Eugenia jambolana 和来自黑胡椒的生物利用度增强胡椒碱的提取物。
Objective 目的
The antihyperglycemic potential of GlycaCare-II was compared against metformin in a double-blind study.
在一项双盲研究中,将 GlycaCare-II 与二甲双胍的抗高血糖潜力进行了比较。
Design 设计
It was a randomized, two-arm design on prediabetic (N = 29; 12 in metformin and 17 in GlycaCare-II arm, respectively) and newly diagnosed diabetic (N = 40; 16 in metformin and 24 in GlycaCare-II) patients for 120 days.
这是一项针对糖尿病前期(N = 29;二甲双胍组为12例,GlycaCare-II组为17例)和新诊断的糖尿病患者(N = 40;二甲双胍组为16例,GlycaCare-II组为24例)的随机双臂设计,为期120天。
Outcome measures 结果测量
Changes in diabetic panel glycosylated hemoglobin (HbA1c), fasting blood sugar (FBS), and postprandial blood sugar (PBS) were the primary endpoints. Lipid profile, liver profile, thyroid-stimulating hormone, bilirubin and creatinine were the secondary endpoints.
糖尿病组糖化血红蛋白(HbA1c)、空腹血糖(FBS)和餐后血糖(PBS)的变化是主要终点。血脂谱、肝脏谱、促甲状腺激素、胆红素和肌酐是次要终点。
Result 结果
Twice a day treatment for 120 days with GlycaCare-II led to a statistically significant change in HbA1c (p < 0.001), FBS (p < 0.001), PBS (p < 0.001) on both prediabetic and newly diagnosed diabetic patients. GlycaCare-II showed a similar potential as metformin in the treatment of T2DM. In the prediabetic group, both GlycaCare-II and metformin were comparable for all the hyperglycemic index parameters. In the case of newly diagnosed diabetic patients, GlycaCare-II showed a significantly better reduction for PBS (p = 0.026) as compared to metformin, while all other parameters in the diabetic panel were comparable. No adverse events were reported throughout the trial period.
每天两次用GlycaCare-II治疗120天,导致糖尿病前期和新诊断糖尿病患者的HbA1c(p < 0.001)、FBS(p < 0.001)、PBS(p < 0.001)发生统计学显着变化。GlycaCare-II在治疗T2DM方面显示出与二甲双胍相似的潜力。在糖尿病前期组中,GlycaCare-II和二甲双胍在所有高血糖指数参数上均具有可比性。在新诊断的糖尿病患者中,与二甲双胍相比,GlycaCare-II的PBS降低(p = 0.026)明显更好,而糖尿病组的所有其他参数都具有可比性。在整个试验期间没有报告不良事件。
Conclusion 结论
These results suggest that GlycaCare-II is effective in managing T2DM in both newly diagnosed diabetic and prediabetic patients.
这些结果表明,GlycaCare-II可有效管理新诊断的糖尿病和糖尿病前期患者的T2DM。
Similar content being viewed by others
其他人正在查看的类似内容
Introduction 介绍
Type 2 diabetes mellitus (T2DM) is a persistent hyperglycemic disorder, wherein blood glucose levels are above the normal values. Further, it is also characterized by an increase in oxidative stress [1, 2]. T2DM is a major public health concern with multiple debilitating complications. Despite considerable improvement in medical sciences, diabetes mellitus is still an incurable disease rapidly increasing in all age groups [3]. The International Diabetes Federation estimated that the global diabetes prevalence in 2019 is 463 million people, rising to 578 million by 2030 and 700 million by 2045 [4]. Hyperglycemia causes both macrovascular (coronary artery disease, peripheral arterial disease, and stroke) and microvascular complications (diabetic nephropathy, neuropathy, and retinopathy) [5]. The overall glycemic burden over time as measured by glycosylated hemoglobin (Hb1Ac) determines the risk for microvascular complications [6].
2型糖尿病(T2DM)是一种持续性高血糖疾病,其中血糖水平高于正常值。此外,它还以氧化应激的增加为特征[1,2]。T2DM是一个主要的公共卫生问题,具有多种使人衰弱的并发症。尽管医学科学有了相当大的进步,但糖尿病仍然是一种不治之症,在所有年龄组中都在迅速增加[3]。国际糖尿病联合会估计,2019年全球糖尿病患病率为4.63亿人,到2030年将上升到5.78亿人,到2045年将达到7亿人[4]。高血糖可引起大血管(冠状动脉疾病、外周动脉疾病和脑卒中)和微血管并发症(糖尿病肾病、神经病变和视网膜病变)[5]。通过糖化血红蛋白(Hb1Ac)测量的总体血糖负荷随时间变化决定了微血管并发症的风险[6]。
T2DM is treated by numerous drugs to increase glucose metabolism and insulin secretion. Biguanide, Sulphonylureas, Alpha-glucosidase inhibitors, Thiazolidinediones, and Gliptins are the commonly prescribed medication for T2DM [7]. Most of these drugs reduce circulating glucose levels and HbA1c to a similar extent but differ in their safety and pathophysiological effects [8]. Metformin is accepted as the first-line therapy for T2DM. It has an extensive safety margin, decreases hepatic glucose production, and mildly affects peripheral resistance [9]. Lactic acidosis, drowsiness, muscle pain gastrointestinal problems are a few side effects associated with metformin, while some people experience B-12 deficiency [9]. Sulfonylureas help increase insulin secretion and may also increase the responsiveness of pancreatic β-cells to glucose. They are well tolerated, although hypoglycemia and weight gain are the most common side effects. Further, their long-term durability effect is inferior to metformin [10]. The PPAR-γ agonists maintain long-term control of blood glucose levels by reducing insulin resistance and improving β-cell function. Bodyweight gain and fluid retention are the major adverse effect of this class of antidiabetic drugs [11] The glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 are peptide hormones (incretins) secreted in the small intestine, which activate insulin secretion in healthy individuals. Incretin mimetics and dipeptidyl peptidase-4 (DPP-4) inhibitors have a positive effect on sustained improvement in glycemic control and on weight gain [12]. The safety of DPP-4 or GLP-1 therapy over time is not yet clear as thyroid cancer and pancreatitis have been reported [8]. Although initial response to drugs like metformin may be good, oral hypoglycemic drugs lose their effectiveness in a significant percentage of patients [13].
T2DM通过多种药物治疗,以增加葡萄糖代谢和胰岛素分泌。双胍类、磺脲类、α-葡萄糖苷酶抑制剂、噻唑烷二酮类和格列汀类药物是治疗T2DM的常用处方药[7]。这些药物大多在相似程度上降低循环葡萄糖水平和HbA1c,但其安全性和病理生理作用不同[8]。二甲双胍被接受为T2DM的一线治疗药物。它具有广泛的安全边际,可减少肝葡萄糖的产生,并轻度影响外周阻力[9]。乳酸性酸中毒、嗜睡、肌肉疼痛、胃肠道问题是与二甲双胍相关的一些副作用,而有些人会出现 B-12 缺乏症 [ 9]。磺酰脲类药物有助于增加胰岛素分泌,还可能增加胰腺β细胞对葡萄糖的反应性。它们耐受性良好,但低血糖和体重增加是最常见的副作用。此外,它们的长期耐久性效果不如二甲双胍[10]。PPAR-γ激动剂通过降低胰岛素抵抗和改善β细胞功能来维持对血糖水平的长期控制。体重增加和体液潴留是这类抗糖尿病药物的主要不良反应 [ 11] 葡萄糖依赖性促胰岛素多肽 (GIP) 和 GLP-1 是小肠分泌的肽激素(肠促胰岛素),可激活健康个体的胰岛素分泌。肠促胰岛素模拟物和二肽基肽酶-4(DPP-4)抑制剂对持续改善血糖控制和体重增加有积极作用[12]。DPP-4或GLP-1治疗随时间推移的安全性尚不清楚,因为已有甲状腺癌和胰腺炎的报道[8]。 尽管对二甲双胍等药物的初始反应可能良好,但口服降糖药在相当一部分患者中失去疗效[ 13]。
In recent decades, there has been a collective determination for pursuing alternative medicine for the treatment of T2DM from natural or herbal sources [14]. Other factors such as patient compliance have led the way in trying out alternative, complementary medicine. Nutritional therapy is gaining importance in preventing, managing, and slowing the rate of development of diabetes complications. It is, therefore, important at all levels of diabetes prevention [15].
近几十年来,人们集体决定寻求替代医学来治疗天然或草药来源的T2DM[14]。其他因素,如患者依从性,也引领了尝试替代补充医学的方式。营养疗法在预防、管理和减缓糖尿病并发症的发展速度方面越来越重要。因此,它在糖尿病预防的各个层面都很重要[15]。
Medicinal herbs from India and China have been widely used for more than 2000 years to treat type 2 diabetes mellitus [16]. The mechanism of action of the herbal medicines involves modifying glycemic metabolism, reducing cholesterol levels, and facilitating insulin secretion [17].
2000多年来,印度和中国的草药被广泛用于治疗2型糖尿病[16]。草药的作用机制包括改变血糖代谢、降低胆固醇水平和促进胰岛素分泌[17]。
GlycaCare-II® is the formulation containing Cinnamomum cassia, Momordica charantia, Pterocarpus marsupium, Gymnema sylvestre, Salacia reticulata, Eugenia jambolana and piperine from Piper nigrum. The cinnamaldehyde in C. cassia sensitizes the body to insulin by enhancing insulin-stimulated tyrosine phosphorylation [18], while the cinnamon polyphenols display insulin-like activity [19]. M. charantia has molecules like charantin, vicine, and polypeptide-p(insulin-like hypoglycemic protein), which possess an antihyperglycemic effect with a mechanism similar to insulin [20]. Thus, some of the proposed mechanism of M. charantia in T2DM includes insulin-like effects and reduction in glucose absorption [21].
GlycaCare-II®是含有肉桂决明子、枸杞、紫檀、匙羹藤、网纹香草、桔梗和胡椒碱的配方。决明子中的肉桂醛通过增强胰岛素刺激的酪氨酸磷酸化使人体对胰岛素敏感[18],而肉桂多酚则显示出胰岛素样活性[19]。查兰蒂亚分枝杆菌具有查兰汀、维辛和多肽-p(胰岛素样降糖蛋白)等分子,具有抗高血糖作用,其机制类似于胰岛素[20]。因此,T2DM中查兰蒂亚分枝杆菌的一些机制包括胰岛素样作用和葡萄糖吸收减少[21]。
P. marsupium extract has been documented to help in protection against oxidative stress in diabetes [22]. Pterostilbene, present in the extract, normalizes serum insulin levels and reduces oxidative stress in diabetic rats [23]. The C-glycosides present in P. marsupium was found to increase glucose uptake by skeletal muscles and could be the active constituent responsible for antihyperglycemic activity [24].
有袋假单胞菌提取物有助于防止糖尿病的氧化应激[22]。提取物中存在的紫檀芪可使糖尿病大鼠的血清胰岛素水平正常化并减少氧化应激[23]。研究发现,P. marsupium中存在的C-糖苷可增加骨骼肌对葡萄糖的摄取,可能是负责抗高血糖活性的活性成分[24]。
Gymnemic acid from Gymnema sylvestre is a mixture of at least 23 different saponins with a similar atomic arrangement to glucose. It acts as an antihyperglycemic agent by filling the receptor location, preventing sugar molecules' absorption by the intestine [25]. Salacinol and Mangiferin from Salacia reticulata inhibit the alpha-glucosidase enzyme, thus decreasing the plasma glucose level [26, 27].
来自匙羹藤的匙羹藤酸是至少 23 种不同皂苷的混合物,具有与葡萄糖相似的原子排列。它通过填充受体位置来充当抗高血糖剂,阻止糖分子被肠道吸收[25]。来自网状Salacia的Salacinol和Mangiferin抑制α-葡萄糖苷酶,从而降低血浆葡萄糖水平[26,27]。
The bark of Eugenia jambolana is rich in several bioactive compounds [28,29,30]. Its fruits contain raffinose which has hypoglycemic activities [31,32,33,34,35]. The blood-glucose-lowering effect of Eugenia jambolana may be due to increased secretion of insulin from the pancreas or by inhibition of insulin degradation [36].
Eugenia jambolana的树皮富含几种生物活性化合物[28,29,30]。它的果实含有具有降血糖活性的棉子糖[31,32,33,34,35]。Eugenia jambolana的降血糖作用可能是由于胰腺胰岛素分泌增加或抑制胰岛素降解[36]。
Piperine enhances the absorption of nutrients through epithelial cell modification and promotes permeability [37]. Piperine, through its multifaceted effect on bioavailability has an indirect impact in the treatment of T2DM [38, 39].
胡椒碱通过上皮细胞修饰增强营养物质的吸收,促进通透性[37]。胡椒碱通过其对生物利用度的多方面作用,对T2DM的治疗具有间接影响[38,39]。
Although various herbal products are in use for T2DM, only a few products have been compared with metformin, and even in the comparison, the outcome of antihyperglycemic activity was lower than that of metformin [40]. Hence, the purpose of this study was to evaluate and compare the efficacy and safety of GlycaCare-II against metformin for the management of T2DM in prediabetic and newly diagnosed patients.
尽管T2DM使用了多种草药产品,但只有少数产品与二甲双胍进行了比较,即使在比较中,抗高血糖活性的结果也低于二甲双胍[40]。因此,本研究的目的是评估和比较 GlycaCare-II 与二甲双胍在糖尿病前期和新诊断患者中治疗 T2DM 的疗效和安全性。
Materials and methods 材料与方法
Test product 测试产品
GlycaCare-II® tablets (522.5 mg) was manufactured and provided by Sami-Sabinsa Group Limited (erstwhile Sami Labs Limited), India. GlycaCare-II® contains the following ingredients:
GlycaCare-II®片剂(522.5毫克)由印度Sami-Sabinsa Group Limited(前身为Sami Labs Limited)制造和提供。GlycaCare-II®含有以下成分:
Sl.no | Ingredients 成分 | Qty/mg 数量/毫克 | Percentage (%) of Actives |
|---|---|---|---|
1 | Cinnamon Extract 肉桂提取物 | 150 | 20% polyphenols 20%多酚 |
2 | Momordica charantia Extract | 150 | 0.5% Charantin 0.5%Charantin |
3 | Pterocarpus Extract (Water-soluble) | 150 | 5% C-glycosides 5%C-糖苷 |
4 | Gymnema sylvestre Extract | 30 | 25% gymnemic acids 25%匙羹藤酸 |
5 | Salacia reticulata extract | 20 | 1% Mangiferin 1%芒果苷 |
6 | Eugenia jambolana extract | 20 | < 15% Tannins < 15% 单宁 |
7 | Piperine(Bioperine) 胡椒碱(Bioperine) | 2.5 | 95% Piperine 95% 胡椒碱 |
Cinnamon (Cinnamomum cassia) bark, Gymnema (Gymnema sylvestre) leaves, deseeded (Momordica charantia) fruits, Dried fruits of Jamun(Eugenia jambolana), and Dried Salacia bark (Salacia reticulata) were powdered and extracted with methanolic water at refluxed condition. The extract was concentrated to remove methanol, dissolved in water, and spray dried. Dried Pterocarpus wood (Pterocarpus marsupium) was extracted with water.
将肉桂(Cinnamomum cassia)树皮、匙羹藤(Gymnema sylvestre)叶、去籽(Momordica charantia)果实、Jamun(Eugenia jambolana)干果和干萨拉西亚树皮(Salacia reticulata)粉化,并在回流条件下用甲醇水提取。将提取物浓缩除去甲醇,溶于水,喷雾干燥。用水提取干燥的紫檀木(Pterocarpus marsupium)。
Metformin (GLYCIRITE) tablets (500 mg) was manufactured by Tusker Pharma India Pvt. Ltd, India.
二甲双胍(缩水甘油酸盐)片剂(500毫克)由印度Tusker Pharma India Pvt. Ltd生产。
Study design 研究设计
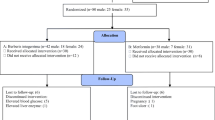
The present study was a prospective, randomized, double-blind, active-controlled clinical trial. Its primary objective was to evaluate the efficacy and safety of GlycaCare-II as monotherapy in Type 2 diabetes mellitus patients compared to metformin. Enrolled patients were initially segregated into prediabetic patients and newly diagnosed diabetic patients. The patients were randomly allocated into two treatment groups to prevent treatment bias. The patients and investigators were blinded to the treatment allocation. Out of 70 subjects screened, sixty-nine subjects enrolled in the study. All the enrolled patients were randomized to two treatment groups: Treatment 1: GlycaCare-II (522.5 mg) as active or Treatment 2: Metformin (500 mg) as the comparator. During the treatment phase, 29 prediabetic patients and 40 newly diagnosed diabetic patients with Type 2 Diabetes mellitus were randomized to receive either GlycaCare-II or metformin under each arm for a period of 120 days ± 3 days. Investigational Product (IP) was administered orally twice daily, morning and night, 20 min before food. All the participants signed informed consent before the beginning of the study after careful detailing regarding the purpose, procedure, and potential risks and benefits of the study.
本研究是一项前瞻性、随机、双盲、主动对照的临床试验。其主要目的是评估与二甲双胍相比,GlycaCare-II作为单一疗法在2型糖尿病患者中的疗效和安全性。入组患者最初分为糖尿病前期患者和新诊断的糖尿病患者。患者被随机分配到两个治疗组,以防止治疗偏倚。患者和研究人员对治疗分配不知情。在筛选的 70 名受试者中,有 69 名受试者参加了这项研究。所有入组患者被随机分配到两个治疗组:治疗 1:GlycaCare-II (522.5 mg) 作为活性或治疗 2:二甲双胍 (500 mg) 作为对照组。在治疗阶段,29 名糖尿病前期患者和 40 名新诊断的 2 型糖尿病患者被随机分配到每只手臂下接受 GlycaCare-II 或二甲双胍,为期 120 天± 3 天。研究产品 (IP) 每天口服两次,早晚,饭前 20 分钟。所有参与者在研究开始前都签署了知情同意书,仔细详细说明了研究的目的、程序以及潜在的风险和益处。
Study population 研究人群
Subjects within the age group of 30–65 years, having the ability to comply with the study protocol and willing to give written consent, were included in the study. Prediabetes was classified as per American diabetes association criteria HbA1c 5.7–6.4% and FBS between 100 mg/dL to 125 mg/dL. Newly diagnosed diabetes patients had an HbA1c value of 6.5–7.5% and FBS > 125 mg/dL [41]. Pregnant and lactating women, patients with a history of acute or chronic illness, type I diabetes, hypo-, and hyperthyroidism were excluded from the study. Also, subjects with hyperlipidemia, history of severe hepatic dysfunction or renal dysfunction, uncontrolled pulmonary dysfunction, and poorly controlled hypertension were excluded from the study. Any patient did not use concomitant medications during the course of the trial.
30-65 岁年龄组内的受试者,有能力遵守研究方案并愿意给予书面同意,被纳入研究。根据美国糖尿病协会标准,糖尿病前期被归类为 HbA1c 5.7-6.4%,FBS 在 100 mg/dL 至 125 mg/dL 之间。新诊断的糖尿病患者的HbA1c值为6.5%-7.5%,FBS>125mg/dL[41]。孕妇和哺乳期妇女、有急性或慢性疾病史、I 型糖尿病、甲状腺功能减退症和甲状腺功能亢进症的患者被排除在研究之外。此外,患有高脂血症、严重肝功能不全或肾功能不全病史、不受控制的肺功能障碍和高血压控制不佳的受试者被排除在研究之外。在试验过程中,任何患者均未使用伴随药物。
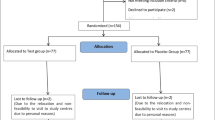
Details of the subject’s disposition are presented in the consort flow chart (Fig. 1).
受试者的性格细节在配偶流程图中呈现(图1)。
Ethics and informed consent
伦理和知情同意
The study was planned at two centers; however, executed at only one site, Levin Diabetes Specialty Hospital, Madurai. The institutional ethics committee of both the hospitals approved it. However, the trial activity was terminated at the initial phase of the study at Pristine Hospital & Research Centre (P) Ltd due to non-compliance. The study was conducted on 69 subjects instead of the proposed 140 subjects. A protocol deviation pertaining to the change in the number of subjects was filed to the ethics committee, and the changes were duly updated in the Clinical Trial Registry of India (CTRI) with registration number CTRI/2018/02/012085 on February 22, 2018, retrospectively. Written Informed Consent was taken from all the subjects before enrolling in the study.
该研究计划在两个中心进行;然而,只在一个地点执行,即马杜赖的莱文糖尿病专科医院。两家医院的机构伦理委员会都批准了它。然而,由于不合规,试验活动在Pristine Hospital & Research Centre (P) Ltd的研究初始阶段终止。该研究对 69 名受试者进行了研究,而不是拟议的 140 名受试者。与受试者数量变化有关的协议偏差已提交给伦理委员会,这些更改已于 2018 年 2 月 22 日在印度临床试验注册中心 (CTRI) 中正式更新,注册号为 CTRI/2018/02/012085,回顾性。在参加研究之前,所有受试者都获得了书面知情同意书。
Data collection, compliance, and protocol deviation
数据收集、合规性和协议偏差
This study was conducted in accordance with applicable regulations, GCP, and Standard Operating Procedures. Study monitor(s) from ClinWorld monitored the study process and data collection through periodical site visits. The monitor retrieved the CRFs (Case Report Form) upon satisfactory resolution of all the queries. Investigational Product (IP) compliance was maintained for both active tablets GlycaCare-II and comparator Glycirite tablets. IP compliances were assessed through CRF. There were no deviations observed regarding IP compliance, during the treatment.
本研究是根据适用法规、GCP 和标准操作程序进行的。来自ClinWorld的研究监督员通过定期的现场访问来监测研究过程和数据收集。监察员在所有查询得到满意解决后检索了CRF(病例报告表)。活性片剂 GlycaCare-II 和对照剂甘油酸酯片剂均保持研究产品 (IP) 合规性。通过通用报告格式对知识产权合规性进行评估。在治疗期间,没有观察到关于IP合规性的偏差。
Study outcome 研究成果
Change in diabetic panel (Glycosylated hemoglobin (HbA1c), fasting blood sugar (FBS), and postprandial blood sugar (PBS)) is the primary endpoint. In case of secondary endpoints, adverse events and change in the biochemical parameters viz lipid profile (Total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL)), liver profile (aspartate transaminase (AST), alanine transaminase (ALT)) and renal profile (Serum creatinine) were performed. All routine clinical chemistry parameters were analyzed using Erba Chem 5® Plus V2 (ERBA Diagnostics Mannheim GmbH Mallaustrasse 69–73 68,219 Mannheim, Germany). Hematology was analyzed using 6 part cell analyzer, SYSMEX, XN-150, (Mumbai, 400 078, Maharashtra, India). HbA1c was analyzed using a D-10 analyzer, BIO-RAD Laboratories (Hercules, CA, USA).
糖尿病组的变化(糖化血红蛋白 (HbA1c)、空腹血糖 (FBS) 和餐后血糖 (PBS))是主要终点。在次要终点的情况下,进行了不良事件和生化参数的变化,即脂质谱(总胆固醇 (TC)、甘油三酯 (TG)、低密度脂蛋白 (LDL)、高密度脂蛋白 (HDL)、极低密度脂蛋白 (VLDL))、肝脏谱(天冬氨酸转氨酶 (AST)、丙氨酸转氨酶 (ALT))和肾脏谱(血清肌酐)。使用Erba Chem 5® Plus V2(ERBA Diagnostics Mannheim GmbH Mallaustrasse 69–73 68,219 Mannheim, Germany)分析所有常规临床化学参数。使用 6 部分细胞分析仪 SYSMEX, XN-150, (Mumbai, 400 078, Maharashtra, India) 分析血液学。使用BIO-RAD实验室(美国加利福尼亚州赫拉克勒斯)的D-10分析仪分析HbA1c。
Efficacy and safety parameters were assessed during the patients' visits to the site. Physical examination, demographics (height, weight, body mass index (BMI)), vital signs were assessed at each visit of the subjects. Clinical efficacy parameter HbA1c was assessed at the screening visit (day − 3) and final visit (day 120 ± 3). FBS and PBS were assessed at the screening visit (day − 3), visit 3 (day 20 ± 3), visit 4 (day 40 ± 3), visit 5 (day 60 ± 3), visit 6 (day 80 ± 3), visit 7 (day 100 ± 3) and final visit (day 120 ± 3). All the biochemical parameters viz thyroid profile (thyroid-stimulating hormone-TSH), lipid profile, liver profile, and renal profile were assessed at the screening visit (day − 3) and final visit (day 120 ± 3).
在患者访问现场期间评估了疗效和安全性参数。每次就诊时评估体格检查、人口统计学(身高、体重、体重指数 (BMI))、生命体征。在筛选访视(第 − 3 天)和最后一次访视(第 120 ± 3 天)评估临床疗效参数 HbA1c。FBS 和 PBS 在筛选访视(第 − 3 天)、第 3 天(第 20 ±第 3 天)、第 4 天(第 40 ±第 3 天)、第 5 天(第 60 ±第 3 天)、第 6 天(第 80 天± 3)、第 7 天(第 100 天± 3)和最后一次访视(第 120 ± 3 天)进行评估。在筛选访视(第 − 3 天)和最后一次访视(第 120 ± 3 天)评估所有生化参数,即甲状腺概况(促甲状腺激素-TSH)、血脂概况、肝脏概况和肾脏概况。
Statistical analysis 统计分析
All patients in the study with relevant safety and efficacy data were considered for the analysis. Efficacy and safety endpoints were analyzed for the relevant study population. A descriptive analysis of demographic characteristics was performed. Mean, and the standard deviation was derived for numeric and categorical parameters. Vital signs at each visit were also analyzed descriptively. Both primary and secondary efficacy outcomes were analyzed descriptively.
研究中所有具有相关安全性和有效性数据的患者均被考虑用于分析。分析了相关研究人群的疗效和安全性终点。对人口统计学特征进行了描述性分析。均值,并推导了数值和分类参数的标准差。还对每次就诊时的生命体征进行了描述性分析。对主要和次要疗效结局进行描述性分析。
For normally distributed data, parametric tests have been applied, and results on continuous measurements were presented as mean ± SD, and results on categorical measurements were presented in percentage (%). A statistical significance level of ≤ 5% was considered significant. Fasting and postprandial glucose levels have been evaluated using repeated-measures ANOVA. HbA1c levels at the screening visit (visit 1) and at the end of the treatment were evaluated using student's ―paired t-test.
对于正态分布的数据,应用了参数检验,连续测量结果以平均值±标准差表示,分类测量结果以百分比 (%) 表示。≤ 5% 的统计学显著性水平被认为是显著的。使用重复测量方差分析评估了空腹和餐后血糖水平。筛选访视(访视 1)和治疗结束时的 HbA1c 水平使用学生配对 t 检验进行评估。
As part of safety outcomes, adverse events, concomitant medications, and clinical laboratory data were assessed. Clinical laboratory outcomes were assessed descriptively. Mean and standard deviation were derived from the data. The p-value for each efficacy parameter and for individual laboratory parameters was calculated using the Wilcoxon test.
作为安全结局的一部分,评估了不良事件、伴随药物和临床实验室数据。临床实验室结局进行描述性评估。均值和标准差是从数据中得出的。使用 Wilcoxon 检验计算每个疗效参数和单个实验室参数的 p 值。
Results 结果
Demographics and other baseline characteristics
人口统计和其他基线特征
A total number of 69 subjects were enrolled and completed the study with an average age range of 48–52.9 years. The demographic parameters were comparable between the metformin and GlycaCare-II treatments at baseline. The other demographic parameters are as shown in Table 1.
总共招募了 69 名受试者并完成了研究,平均年龄范围为 48-52.9 岁。基线时,二甲双胍和GlycaCare-II治疗之间的人口统计学参数具有可比性。其他人口统计参数如表1所示。
表1 人口统计和基线特征
Bodyweight and BMI comparison from screening to final visit. A Body weight change in newly diagnosed diabetic group B Bodyweight change in prediabetic group C BMI change in newly diagnosed diabetic group D BMI change in prediabetic group; Values are expressed as Mean ± SE
从筛选到最终就诊的体重和 BMI 比较。A 新诊断糖尿病B组的体重变化 糖尿病前期C组的体重变化 新诊断糖尿病组的BMI变化 D 糖尿病前期组的BMI变化;值表示为平均值±标准差
Efficacy analysis of GlycaCare-II and metformin
GlycaCare-II和二甲双胍的疗效分析
Newly diagnosed diabetic patients
新诊断的糖尿病患者
Effect on HbA1c level 对 HbA1c 水平的影响
One of the primary efficacy parameters recorded for this clinical study was a change in the level of HbA1c from screening visit to final visit. In the case of newly diagnosed diabetic patients, the HbA1c level was 6.99 ± 0.32% for GlycaCare-II and 6.99 ± 0.38% for the metformin group at the screening visit. In the final visit, the HbA1c level reduced to 6.52 ± 0.19% (p < 0.001) for GlycaCare-II and 6.53 ± 0.26% (p = 0.004) for metformin. The mean changes in HbA1c were not significantly different in metformin & GlycaCare-II groups, suggesting equivalent efficacy (Fig. 3A).
该临床研究记录的主要疗效参数之一是从筛选访视到最终访视的 HbA1c 水平的变化。在新诊断的糖尿病患者中,GlycaCare-II组的HbA1c水平为6.99±0.32%,二甲双胍组的HbA1c水平为6.99±0.38%。在最后一次访视中,GlycaCare-II 的 HbA1c 水平降至 6.52 ± 0.19%(p < 0.001),二甲双胍的 HbA1c 水平降至 6.53 ± 0.26% (p = 0.004)。二甲双胍和GlycaCare-II组中HbA1c的平均变化没有显着差异,表明疗效相当(图3A)。
Efficacy parameter for GlycaCare-II and Metformin in newly diagnosed diabetic patients, FBS and PBS was measured at the screening visit (visit 1), visit 3, visit 4, visit 5, visit 6, visit 7 and visit 8and HbA1c was measured at screening and final visit (visit 8). A HbA1c level in %, B Fasting blood sugar level in mg/dL, C Postprandial blood sugar level in mg/dL. (Values are expressed as Mean ± SD, *p < 0.05 in comparison to screening visit by repeated measure ANOVA)
在筛选访视(访视 1)、访视 3、访视 4、访视 5、访视 6、访视 7 和访视 8 时测量 GlycaCare-II 和二甲双胍对新诊断糖尿病患者的疗效参数,并在筛选和最终访视(访视 8)时测量 HbA1c。A HbA1c 水平以 % 为单位,B 空腹血糖水平以 mg/dL 为单位,C 餐后血糖水平以 mg/dL 为单位。(与重复测量方差分析的筛选访视相比,值表示为平均值± SD、*p < 0.05)
Effect on fasting blood sugar
对空腹血糖的影响
FBS level recorded a reduction of 11% for GlycaCare-II, and a similar improvement in the FBS level was observed in the metformin arm with a 10% reduction at visit 8 (Fig. 3B).
GlycaCare-II 的 FBS 水平降低了 11%,二甲双胍组的 FBS 水平也有类似的改善,在第 8 次就诊时降低了 10%(图 3B)。
Effect on postprandial blood sugar
对餐后血糖的影响
A significant change in the PBS level at the final visit as compared to the screening visit was observed in both GlycaCare-II (p < 0.0001) and metformin (p = 0.0005) groups in the newly diagnosed diabetes patients. Total change in the PBS level was significant between the treatment groups (p = 0.0268). In the case of PBS level, GlycaCare-II displayed a significant therapeutic response compared to metformin with the absolute mean change of 32.75 mg/dl as against 21.06 mg/dL of metformin group. Additionally, a significant change was observed in the PBS value from visit three onwards within the group for GlycaCare-II and metformin. Further, these changes were incremental across the time points for both groups (Fig. 3C).
与筛选访视相比,在新诊断的糖尿病患者中,在 GlycaCare-II (p < 0.0001) 组和二甲双胍 (p = 0.0005) 组中观察到最后一次就诊时 PBS 水平的显着变化。治疗组间PBS水平的总变化显著(p = 0.0268)。在PBS水平方面,与二甲双胍相比,GlycaCare-II显示出显着的治疗反应,绝对平均变化为32.75 mg/dl,而二甲双胍组为21.06 mg/dL。此外,从第三次就诊开始,组内 GlycaCare-II 和二甲双胍的 PBS 值发生了显着变化。此外,这些变化在两组的时间点上都是递增的(图3C)。
Prediabetic patients 糖尿病前期患者
Effect on HbA1c level 对 HbA1c 水平的影响
The mean HbA1c changed from 6.16 ± 0.21 to 5.87 ± 0.15% (p < 0.001) in GlycaCare-II group and 6.2 ± 0.24 to 5.97 ± 0.21% (p = 0.02) in metformin group, from screening visit to final visit. The mean changes in HbA1c were not significantly different in metformin & GlycaCare-II groups, suggesting equivalent efficacy (Fig. 4A).
从筛选访视到最终访视,GlycaCare-II组的平均HbA1c从6.16±0.21变为5.87±0.15%(p < 0.001),二甲双胍组的平均HbA1c从6.2±0.24变为5.97变为5.97±0.21%(p = 0.02)。二甲双胍和GlycaCare-II组的HbA1c平均变化没有显著差异,表明疗效相当(图4A)。
Efficacy parameter for GlycaCare-II and metformin in prediabetics arm, FBS and PBS was measured at the screening visit (visit 1), visit 3, visit 4, visit 5, visit 6, visit 7 and visit 8, HbA1c was measured at screening and final visit (visit 8). A HbA1c level in %, B Fasting blood sugar level in mg/dL, C Postprandial blood sugar level in mg/dL. (Values are expressed as Mean ± SD, *p < 0.05 in comparison to screening visit by repeated measure ANOVA)
在筛选访视(访视 1)、访视 3、访视 4、访视 5、访视 6、访视 7 和访视 8 时测量 GlycaCare-II 和二甲双胍在糖尿病前期组、FBS 和 PBS 中的疗效参数,在筛选和最终访视(访视 8)时测量 HbA1c。A HbA1c 水平以 % 为单位,B 空腹血糖水平以 mg/dL 为单位,C 餐后血糖水平以 mg/dL 为单位。(与重复测量方差分析的筛选访视相比,值表示为平均值± SD、*p < 0.05)
Effect on fasting blood sugar
对空腹血糖的影响
A significant change was observed in the FBS level from Day 20 onwards for both GlycaCare-II and metformin group, and the changes were incremental across the time points with resultant 15% and 11% reduction at 120 days, respectively in the pre-diabetics group (Fig. 4B).
从第20天开始,GlycaCare-II组和二甲双胍组的FBS水平都发生了显着变化,并且这些变化在糖尿病前期组中随时间点递增,在120天时分别降低了15%和11%(图4B)。
Effect on postprandial blood sugar
对餐后血糖的影响
In the prediabetes patients, the PBS level visit-wise for both GlycaCare-II and metformin showed steady augmentation over a period. The PBS level for GlycaCare-II at the screening visit was 169.59 ± 16.35 mg/dl and for the final visit 146 ± 8.66 mg/dl (p < 0.0001). For metformin it was 165.67 ± 14.89 mg/dl at screening visit (day 3) and 148 ± 8.31 mg/dl for final visit (day-120 ± 3) (p = 0.0016). Additionally, a significant change within the group was observed in the PBS level from visit five onwards for GlycaCare-II, and the changes were incremental across the time points. However, a significant change was observed for the metformin group from visit 6, with the incremental difference across the time points (Fig. 4C).
在糖尿病前期患者中,GlycaCare-II 和二甲双胍的 PBS 水平在一段时间内均呈稳定升高。筛选访视时 GlycaCare-II 的 PBS 水平为 169.59 ± 16.35 mg/dl,最后一次访视时为 146 ± 8.66 mg/dl (p < 0.0001)。对于二甲双胍,筛选访视时(第 3 天)为 165.67 ± 14.89 mg/dl,最后一次访视(第 120 ±第 3 天)为 148 ± 8.31 mg/dl (p = 0.0016)。此外,从第五次就诊开始,GlycaCare-II的PBS水平观察到组内的显着变化,并且这些变化在各个时间点都是递增的。然而,从第 6 次访问开始,二甲双胍组发生了显着变化,时间点的差异逐渐增加(图 4C)。
Safety evaluation 安全性评估
There were no adverse events or serious adverse events observed, and none of the patients consumed any concomitant medications during the study in both prediabetic and newly diagnosed diabetic arms.
在糖尿病前期和新诊断的糖尿病组中,没有观察到不良事件或严重不良事件,并且没有患者在研究期间服用任何伴随药物。
Clinical laboratory evaluation
临床实验室评估
Lipid levels 血脂水平
The reduction in TC, TG, LDL, VLDL, and the increase in HDL was highly significant in the GlycaCare-II treated subjects. Metformin did not show a significant reduction in TG and VLDL. The positive effect on lowering lipids was better with GlycaCare-II compared to metformin. This effect was not observed in prediabetic subjects (Table 2).
TC、TG、LDL、VLDL 的减少和 HDL 的增加在 GlycaCare-II 治疗的受试者中非常显着。二甲双胍没有显示出TG和VLDL的显着降低。与二甲双胍相比,GlycaCare-II对降低血脂的积极作用更好。在糖尿病前期受试者中未观察到这种效应(表2)。
表2 GlycaCare-II和二甲双胍的血脂谱
Safety parameters 安全参数
Newly diagnosed diabetic arm
新诊断的糖尿病臂
Bilirubin, and creatinine, changed significantly in metformin group in comparison to baseline. It was also noted that the total bilirubin increased significantly in the patients treated with metformin at the end of the study in comparison to the GlycaCare-II group. The vital parameters did not show any significant changes across the study period in both arms. Nevertheless, the respiratory rate of the metformin group was significantly decreased at the end of the study. These results reiterate the safety of GlycaCare-II for human consumption in newly diagnosed diabetic patients (Table 3).
与基线相比,二甲双胍组的胆红素和肌酐发生了显著变化。还注意到,与GlycaCare-II组相比,在研究结束时接受二甲双胍治疗的患者的总胆红素显着增加。在研究期间,两组的重要参数均未显示任何显着变化。然而,二甲双胍组的呼吸频率在研究结束时显着降低。这些结果重申了GlycaCare-II在新诊断的糖尿病患者中用于人类食用的安全性(表3)。
表3 GlycaCare-II和二甲双胍的安全性
Prediabetic arm 糖尿病前期手臂
Clinical laboratory evaluation was carried out on the study participants during screening and final visit. The biochemical parameters during the screening and the final visit, showed no significant change across time. The vital parameters did not show any significant changes across the study period in both the arms. These results reiterate the safety of GlycaCare II and metformin for human consumption in prediabetic patients (Table 3).
在筛选和最终访视期间对研究参与者进行了临床实验室评估。筛选和最终访视期间的生化参数随时间推移没有显着变化。在研究期间,两组的生命参数均未显示任何显着变化。这些结果重申了GlycaCare II和二甲双胍在糖尿病前期患者中用于人类的安全性(表3)。
Discussion 讨论
In the present study, we observed that a herbal formulation (GlycaCare-II), containing natural extracts of Cinnamomum cassia, Momordica charantia, Pterocarpus marsupium, Gymnema sylvestre, Salacia reticulata, Eugenia jambolana with a small quantity of piperine as bioavailability enhancer was comparable to metformin in reducing hyperglycemia and HbA1c levels on both prediabetic and newly diagnosed diabetic patients.
在本研究中,我们观察到含有肉桂、枸杞、紫檀、匙羹藤、网纹苜蓿、樟子苜蓿的天然提取物的草药制剂(GlycaCare-II)与二甲双胍在降低糖尿病前期和新诊断糖尿病患者的高血糖和HbA1c水平方面相当。
Compared with the baseline data, significant improvement in all the primary biochemical indices of hyperglycemia like HbA1c, FBS, and PBS was observed in all the subgroups after four months of treatment. The GlycaCare-II formulation was safe with no changes in blood biochemical parameters, and no adverse effects were reported during the four months of treatment. In subjects treated with metformin, a significant increase in creatinine and bilirubin levels was observed in newly diagnosed diabetic patients. Although the number of subjects was low, this trend cannot be ignored and warrants a larger cohort study. Few earlier studies on chronic therapy of metformin reported a significant change in creatinine [42, 43].
与基线数据相比,治疗4个月后,所有亚组均观察到HbA1c、FBS和PBS等所有主要高血糖生化指标均有显著改善。GlycaCare-II制剂是安全的,血液生化参数没有变化,在四个月的治疗期间没有不良反应报告。在接受二甲双胍治疗的受试者中,新诊断的糖尿病患者观察到肌酐和胆红素水平显着增加。尽管受试者数量很少,但这一趋势不容忽视,需要进行更大规模的队列研究。关于二甲双胍慢性治疗的早期研究很少报道肌酐的显著变化[42,43]。
GlycaCare-II exhibited a significant change in PBS level compared to metformin at the end of the study, especially in the newly diagnosed diabetic patients. Further, GlycaCare-II showed statistically significant improvement in lipid levels suggesting its benefit in controlling dyslipidemia. These inferences are in line with other studies, wherein individual components of this formulation were efficacious in bringing down metabolic index [18, 21, 40, 44,45,46]. The treatment of GlycaCare-II was devoid of any adverse events, and the outcome of the analysis of the laboratory parameters suggests that GlycaCare-II is safe for diabetic patients.
在研究结束时,与二甲双胍相比,GlycaCare-II的PBS水平发生了显着变化,特别是在新诊断的糖尿病患者中。此外,GlycaCare-II的脂质水平在统计学上显示出显着的改善,表明其在控制血脂异常方面具有益处。这些推论与其他研究一致,其中该配方的单个成分可有效降低代谢指数[18,21,40,44,45,46]。GlycaCare-II的治疗没有任何不良事件,实验室参数分析的结果表明,GlycaCare-II对糖尿病患者是安全的。
Optimal treatment of type 2 diabetes mellitus requires a comprehensive and concerted approach. The management of the condition focuses on nutrition, exercise, and pharmacologic therapies to reduce the complications associated with hyperglycemia. In prediabetic and new-onset diabetes patients, nutrition therapy is of utmost importance to prevent further deterioration of the condition [47]. The American diabetic association recommends HbA1c with a cut-point ≥ 6.5% for diagnosing diabetes as an alternative to fasting plasma glucose as it provides a reliable measure of chronic glycemia and correlates well with the risk of long-term diabetes complications [48]. Further, HbA1c is also a good predictor of lipid profile, providing additional benefits of identifying cardiovascular risk among diabetes patients [49]. GlycaCare-II was comparable to metformin therapy in reducing the HbA1c levels in both prediabetic and newly diagnosed diabetic patients. In addition, GlycaCare-II was highly effective in reducing lipid levels in newly diagnosed diabetic patients, which was better than the effect of metformin. These results suggest that GlycaCare-II can be a beneficial supplement for diabetic patients with dyslipidemia, which requires further elaboration. Although the lipid levels decreased in prediabetic patients also, it was not significant, probably because of the overall lower levels in these groups.
2型糖尿病的最佳治疗需要一种全面和协调的方法。该病的治疗侧重于营养、运动和药物治疗,以减少与高血糖相关的并发症。在糖尿病前期和新发糖尿病患者中,营养治疗对于防止病情进一步恶化至关重要[47]。美国糖尿病协会(American diaticic association)推荐将HbA1c(临界值≥6.5%)作为空腹血糖的替代方法,因为它提供了慢性血糖的可靠测量方法,并且与长期糖尿病并发症的风险密切相关[48]。此外,HbA1c也是血脂谱的良好预测指标,为识别糖尿病患者的心血管风险提供了额外的好处[49]。GlycaCare-II在降低糖尿病前期和新诊断糖尿病患者的HbA1c水平方面与二甲双胍治疗相当。此外,GlycaCare-II在降低新诊断糖尿病患者的血脂水平方面非常有效,其效果优于二甲双胍。这些结果表明,GlycaCare-II可以成为血脂异常糖尿病患者的有益补充剂,这需要进一步阐述。尽管糖尿病前期患者的血脂水平也有所下降,但并不显着,可能是因为这些组的总体水平较低。
Postprandial hyperglycemia is also one of the earliest abnormalities of glucose homeostasis, and it has been suggested that lowering PPS may decrease the risk of hypoglycemia and weight gain [50]. GlycaCare-II was significantly better than metformin in reducing PPS in newly diagnosed patients, and the effect was seen at an earlier time point in prediabetic patients. These observations also suggest that GlycaCare-II may be used to successfully reduce the risk of developing diabetes in prediabetics and reduce progression in diabetic patients.
餐后高血糖也是最早的血糖稳态异常之一,有人认为降低PPS可以降低低血糖和体重增加的风险[50]。GlycaCare-II在降低新诊断患者的PPS方面明显优于二甲双胍,并且在糖尿病前期患者中观察到了较早的效果。这些观察结果还表明,GlycaCare-II可用于成功降低糖尿病前期患者患糖尿病的风险,并减少糖尿病患者的进展。
One limitation of the study was the inability to carry out the multicenter trial as per the original protocol due to non—compliance and subsequent termination of the site, resulting in smaller subgroups. We restricted the outcome parameters to only the glycemic profile as this was the first clinical study with GlycaCare-II. This can be considered a study limitation, as evaluation of insulin levels and oxidative parameters would have extended the benefits afforded by the herbal formulation.
该研究的一个局限性是,由于不依从性和随后的站点终止,无法按照原始方案进行多中心试验,导致亚组变小。我们将结局参数限制在仅血糖特征上,因为这是GlycaCare-II的首次临床研究。这可以被认为是一个研究的局限性,因为对胰岛素水平和氧化参数的评估会扩大草药配方提供的益处。
Conclusion 结论
The findings in this randomized clinical study demonstrate the potential of GlycaCare-II as an alternative safe medication in the treatment of T2DM. It was also evident that GlycaCare-II possesses a similar therapeutic response as compared to metformin. Future studies in a larger cohort may help in positioning the polyherbal formulation as an alternative to the standard treatment for type 2 diabetes. We have shown that GlycaCare-II, with its steady influence on reducing the hyperglycemic index, is comparable with metformin. GlycaCare-II also appeared to have a better effect on the changes in the lipid parameters. However, the intrinsic cumulative mechanisms of its action must be further established through a comprehensive trial involving an increase in the number of subjects.
这项随机临床研究的结果证明了 GlycaCare-II 作为治疗 T2DM 的替代安全药物的潜力。同样明显的是,与二甲双胍相比,GlycaCare-II具有相似的治疗反应。未来在更大队列中的研究可能有助于将多草药制剂定位为2型糖尿病标准治疗的替代品。我们已经证明,GlycaCare-II对降低高血糖指数具有稳定的影响,可与二甲双胍相媲美。GlycaCare-II似乎对脂质参数的变化也有更好的影响。然而,必须通过涉及受试者数量的综合试验来进一步确定其作用的内在累积机制。
Availability of data and materials
数据和材料的可用性
All the data generated are within the manuscript.
生成的所有数据都在手稿中。
Abbreviations 缩写
- AST: AST:
-
Aspartate transaminase 天冬氨酸转氨酶
- ALT: 替代:
-
Alanine transaminase 丙氨酸转氨酶
- BMI: 体重指数(BMI):
-
Body Mass Index 体重指数
- CTRI: 首席运营官:
-
Clinical Trial Registry of India
印度临床试验注册中心 - FBS: 胎牛血清:
-
Fasting blood sugar 空腹血糖
- FDA: 美国食品药品监督管理局:
-
Food and Drug Administration
美国食品药品监督管理局 - HbA1C: 血红蛋白A1C:
-
Glycosylated hemoglobin 糖基化血红蛋白
- HDL: 高密度脂蛋白:
-
High-density lipoprotein
高密度脂蛋白 - IP: IP:
-
Investigational Product 研究产品
- LDL: 低密度脂蛋白:
-
Low density lipoprotein 低密度脂蛋白
- PBS: 美国公共广播公司(PBS):
-
Postprandial blood sugar
餐后血糖 - T2DM: T2DM型:
-
Type 2 diabetes mellitus
2型糖尿病 - TC: TC型:
-
Total cholesterol 总胆固醇
- TG: TG:
-
Triglycerides 甘油 三 酯
- TSH: TSH:
-
Thyroid Stimulating Hormone
促甲状腺激素 - VLDL: VLDL:
-
Very low-density lipoprotein
极低密度脂蛋白
References 引用
American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37:S14.
美国糖尿病协会。2014年糖尿病医疗标准。糖尿病护理。2014;37:S14。Bin Abas R, Das S, Thent ZC. Herbal supplements for type 2 diabetes mellitus: a systematic review of clinical results. J Exp Appl Anim Sci. 2015;1(3):341–54.
Bin Abas R, Das S, Thent ZC.2型糖尿病的草药补充剂:临床结果的系统评价。J Exp Appl Anim Sci. 2015;1(3):341–54.Meo SA. Diabetes mellitus: health and wealth threat. Int J Diabetes Mellit. 2009;1(1):42.
喵喵喵。糖尿病:健康和财富威胁。国际糖尿病杂志2009;1(1):42.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843.
Saeedi P、Petersohn I、Salpea P、Malanda B、Karuranga S、Unwin N 等。2019 年全球和区域糖尿病患病率估计以及 2030 年和 2045 年的预测:来自国际糖尿病联合会糖尿病地图集的结果。糖尿病临床实践。2019;157:107843.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77–82.
福勒 MJ。糖尿病的微血管和大血管并发症。临床糖尿病。2008;26(2):77–82.Samanta S. Glycated hemoglobin and subsequent risk of microvascular and macrovascular complications. Indian J Med Sci. 2020;73:230–8.
Samanta S. 糖化血红蛋白和随后的微血管和大血管并发症风险。印度医学杂志 2020;73:230–8.Harrigan RA, Nathan MS, Beattie P. Oral agents for the treatment of type 2 diabetes mellitus: pharmacology, toxicity, and treatment. Ann Emerg Med. 2001;38(1):68–78.
Harrigan RA, Nathan MS, Beattie P. 用于治疗 2 型糖尿病的口服药物:药理学、毒性和治疗。Ann Emerg Med. 2001 年;38(1):68–78.Raz I. Guideline approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36(Supplement 2):S139–44.
Raz I. 新诊断的 2 型糖尿病患者的治疗方法指南。糖尿病护理。2013;36(增刊2):S139–44。Nasri H, Rafieian-Kopaei M. Metformin: current knowledge. J Res Med Sci. 2014;19(7):658–64.
Nasri H, Rafieian-Kopaei M. 二甲双胍:当前知识。医学科学杂志 2014;19(7):658–64.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–43.
Kahn SE、Haffner SM、Heise MA、Herman WH、Holman RR、Jones NP 等。罗格列酮、二甲双胍或格列本脲单药治疗的血糖耐久性。N Engl J Med. 2006;355(23):2427–43.Abbas A, Blandon J, Rude J, Elfar A, Mukherjee D. PPAR- γ agonist in treatment of diabetes: cardiovascular safety considerations. Cardiovasc Hematol Agents Med Chem. 2012;10(2):124–34.
Abbas A, Blandon J, Rude J, Elfar A, Mukherjee D. PPAR- γ激动剂治疗糖尿病:心血管安全考虑。心血管血红素药物 Med Chem. 2012;10(2):124–34.Macconell L, Pencek R, Li Y, Maggs D, Porter L. Exenatide once weekly: sustained improvement in glycemic control and cardiometabolic measures through 3 years. Diabetes, Metab Syndr Obes Targets Ther. 2013;6:31–41.
Macconell L, Pencek R, Li Y, Maggs D, Porter L. 艾塞那肽每周一次:血糖控制和心脏代谢指标持续改善 3 年。糖尿病,metab syndr obes 针对其他。2013;6:31–41.Tripathi KD. Essentials of medical pharmacology. New Delhi: JP Medical Ltd; 2013.
特里帕蒂KD。医学药理学要点。新德里:JP Medical Ltd;2013.Pandey A, Tripathi P, Pandey R, Srivastava R, Goswami S. Alternative therapies useful in the management of diabetes: a systematic review. J Pharm Bioallied Sci. 2011;3(4):504–12.
Pandey A, Tripathi P, Pandey R, Srivastava R, Goswami S. 可用于糖尿病管理的替代疗法:系统评价。J Pharm Bioallied Sci. 2011;3(4):504–12.Association AD. Nutrition recommendations and interventions for diabetes. A position statement of the American Diabetes Association. Diabetes Care. 2008;31(Supplement 1):S61–78.
糖尿病的营养建议和干预措施。美国糖尿病协会的立场声明。糖尿病护理。2008;31(增刊1):S61-78。Wang Z, Wang J, Chan P. Treating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbs. Evid Based Complement Alternat Med. 2013;2013:1–17.
Wang Z, Wang J, Chan P. 用传统中药和印度草药治疗 2 型糖尿病.基于 Evid 的补体 Alternat Med. 2013;2013:1–17.Liu JP, Zhang M, Wang W, Grimsgaard S. Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2002. https://doi.org/10.1002/14651858.CD003642.pub2.
Liu JP, Zhang M, Wang W, Grimsgaard S. 治疗 2 型糖尿病的中草药.Cochrane数据库系统修订版,2002年。https://doi.org/10.1002/14651858.CD003642.pub2。Basch E, Gabardi S, Ulbricht C. Bitter melon (Momordica charantia): a review of efficacy and safety. Am J Health Syst Pharm. 2003;60(4):356–9.
Basch E, Gabardi S, Ulbricht C. 苦瓜 (Momordica charantia):疗效和安全性综述。2003 年美国健康系统药学杂志;60(4):356–9.Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys. 2007;459(2):214–22.
曹 H, 波兰斯基 MM, 安德森 RA.肉桂提取物和多酚影响小鼠 3T3-L1 脂肪细胞中三胯脯素、胰岛素受体和葡萄糖转运蛋白 4 的表达。拱形生化生物物理学。2007;459(2):214–22.Uebanso T, Arai H, Taketani Y, Fukaya M, Yamamoto H, Mizuno A, et al. Extracts of Momordica charantia suppress postprandial hyperglycemia in rats. J Nutr Sci Vitaminol. 2007;53(6):482–8.
Uebanso T、Arai H、Taketani Y、Fukaya M、Yamamoto H、Mizuno A 等。Momordica charantia的提取物可抑制大鼠餐后高血糖。J Nutr Sci 维生素。2007;53(6):482–8.Chakravarthy B, Saroj G, Gambhir S, Gode K. Pancreatic beta-cell regeneration–a novel antidiabetic mechanism of Pterocarpus marsupium roxb. Indian J Pharmacol. 1980;12(2):123.
Chakravarthy B, Saroj G, Gambhir S, Gode K. 胰腺 β 细胞再生——有袋紫檀 roxb 的一种新型抗糖尿病机制。印度 J 药理学。1980;12(2):123.Singh PK, Baxi D, Banerjee S, Ramachandran A. Therapy with methanolic extract of Pterocarpus marsupium Roxb and Ocimum sanctum Linn reverses dyslipidemia and oxidative stress in alloxan-induced type I diabetic rat model. Exp Toxicol Pathol. 2012;64(5):441–8.
Singh PK, Baxi D, Banerjee S, Ramachandran A. 用紫檀有袋龙 Roxb 和 Ocimum sanctum Linn 的甲醇提取物治疗可逆转异体生诱导的 I 型糖尿病大鼠模型中的血脂异常和氧化应激。Exp 毒理病理学。2012;64(5):441–8.Tastekin B, Pelit A, Polat S, Tuli A, Sencar L, Alparslan MM, et al. Therapeutic potential of pterostilbene and resveratrol on biomechanics, biochemical, and histological parameters in streptozotocin-induced diabetic rats. Evid Based Complement Altern Med. 2018;2018:1–10.
Tastekin B、Pelit A、Polat S、Tuli A、Sencar L、Alparslan MM 等。紫檀芪和白藜芦醇对链脲佐菌素诱导的糖尿病大鼠生物力学、生化和组织学参数的治疗潜力。基于 Evid 的补体交替医学 2018 年;2018:1–10.Mishra A, Srivastava R, Srivastava SP, Gautam S, Tamrakar AK, Maurya R, et al. Antidiabetic activity of heartwood of Pterocarpus marsupium Roxb. and analysis of phytoconstituents. Indian J Exp Biol. 2013;51(5):363–74.
Mishra A、Srivastava R、Srivastava SP、Gautam S、Tamrakar AK、Maurya R 等。Pterocarpus marsupium Roxb心材的抗糖尿病活性。以及植物成分的分析。印度实验生物学杂志 2013 年;51(5):363–74.Thakur GS, Sharma R, Sanodiya BS, Pandey M, Prasad G, Bisen PS. Gymnema sylvestre: an alternative therapeutic agent for management of diabetes. J Appl Pharm Sci. 2012;2(12):1–6.
Thakur GS, Sharma R, Sanodiya BS, Pandey M, Prasad G, Bisen PS. 匙羹藤:一种用于管理糖尿病的替代治疗剂。应用药学杂志 2012;2(12):1–6.Im R, Mano H, Nakatani S, Shimizu J, Wada M. Aqueous extract of Kotahla Himbutu (Salacia reticulata) stems promotes oxygen consumption and suppresses body fat accumulation in mice. J Health Sci. 2008;54(6):645–53.
Im R, Mano H, Nakatani S, Shimizu J, Wada M. Kotahla Himbutu (Salacia reticulata) 茎的水提取物促进耗氧量并抑制小鼠体内脂肪堆积。健康科学杂志 2008;54(6):645–53.Stohs SJ, Ray S. Anti-diabetic and anti-hyperlipidemic effects and safety of Salacia reticulata and related species. Phytother Res. 2015;29(7):986–95.
Stohs SJ, Ray S. 网状Salacia reticulata及相关物种的抗糖尿病和抗高脂血症作用及安全性。Phytother Res. 2015 年;29(7):986–95.Bhatia I, Bajaj K. Chemical constituents of the seeds and bark of Syzygium cumini. Planta Med. 1975;28(08):346–52.
Bhatia I, Bajaj K. Syzygium cumini 种子和树皮的化学成分。Planta Med. 1975 年;28(08):346–52.Chaudhuri AN, Pal S, Gomes A, Bhattacharya S. Anti-inflammatory and related actions of Syzygium cumin seed extract. Phytother Res. 1990;4(1):5–10.
Chaudhuri AN, Pal S, Gomes A, Bhattacharya S. Syzygium 孜然籽提取物的抗炎和相关作用。Phytother Res. 1990;4(1):5–10.Srivastava H. Paper chromatography of fruit juices. J Sci Ind Res. 1953;12B:363–5.
Srivastava H.果汁的纸层析法。J Sci Ind Res. 1953年;12乙:363-5。Bhargava K, Dayal R, Seshadri T. Chemical components of Eugenia jambolana stem bark. Current Science 1974.
Bhargava K, Dayal R, Seshadri T. Eugenia jambolana 茎皮的化学成分。当代科学 1974.Grover J, Vats V, Rathi S. Anti-hyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. J Ethnopharmacol. 2000;73(3):461–70.
Grover J, Vats V, Rathi S. Eugenia jambolana 和 Tinospora cordifolia 在实验性糖尿病中的抗高血糖作用及其对参与碳水化合物代谢的关键代谢酶的影响。J 民族药理学。2000;73(3):461–70.Lewis Y, Dwarakanath C, Johar D. Acids and sugars in Eugenia jambolana. J Sci Ind Res. 1956;15:280–1.
Lewis Y, Dwarakanath C, Johar D. Eugenia jambolana 中的酸和糖。J Sci Ind Res. 1956年;15:280–1.Morton JF. Fruits of warm climates. Winterville, NC: JF Morton; 1987.
莫顿 JF。温暖气候的果实。北卡罗来纳州温特维尔:JF Morton;1987.Sacks BN, Brown SK, Stephens D, Pedersen NC, Wu JT, Berry O. Y chromosome analysis of dingoes and southeast Asian village dogs suggests a neolithic continental expansion from Southeast Asia followed by multiple Austronesian dispersals. Mol Biol Evol. 2013;30(5):1103–18.
Sacks BN、Brown SK、Stephens D、Pedersen NC、Wu JT、Berry O. Y 对野狗和东南亚乡村犬的染色体分析表明,新石器时代的大陆从东南亚扩张,随后是多个南岛语族的扩散。Mol Biol Evol.2013;30(5):1103–18.Aybar MJ, Riera ANS, Grau A, Sánchez SS. Hypoglycemic effect of the water extract of Smallantus sonchifolius (yacon) leaves in normal and diabetic rats. J Ethnopharmacol. 2001;74(2):125–32.
Aybar MJ, Riera ANS, Grau A, Sánchez SS. Smallantus sonchifolius(雪莲果)叶水提取物对正常和糖尿病大鼠的降血糖作用。J 民族药理学。2001;74(2):125–32.Annamalai A, Manavalan R. Effect of “Trikatu” and its individual components and piperine on gastrointestinal tracts. Indian Drugs. 1990;27(12):595–604.
Annamalai A, Manavalan R. “Trikatu”及其单个成分和胡椒碱对胃肠道的影响。印度毒品。1990;27(12):595–604.Johri RK, Thus N, Khajuria A, Zutshi U. Piperine-mediated changes in the permeability of rat intestinal epithelial cells: the status of γ-glutamyl transpeptidase activity, uptake of amino acids and lipid peroxidation. Biochem Pharmacol. 1992;43(7):1401–7.
Johri RK, Thus N, Khajuria A, Zutshi U. 胡椒碱介导的大鼠肠上皮细胞通透性变化:γ-谷氨酰转肽酶活性、氨基酸摄取和脂质过氧化的状态。生化药理学。1992;43(7):1401–7.Majeed M, Badmaev V, Rajendran R. Inventors use of piperine as a bioavailability enhancer patent US Patent 5744161. April 28, 1998.
Majeed M, Badmaev V, Rajendran R. 发明人使用胡椒碱作为生物利用度增强剂专利 美国专利 5744161.1998年4月28日。Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth D, et al. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36(5):340–4.
Mang B、Wolters M、Schmitt B、Kelb K、Lichtinghagen R、Stichtenoth D 等。肉桂提取物对 2 型糖尿病患者血浆葡萄糖、HbA1c 和血脂的影响。欧元 J 临床投资。2006;36(5):340–4.American Diabetic Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90.
美国糖尿病协会。糖尿病的诊断和分类。糖尿病护理。2014;37(增刊 1):S81–90。Fang L, Lu J-X, Tang J-L, Li L, Lu H-J, Hou X-H, et al. Relationship of plasma creatinine and lactic acid in type 2 diabetic patients without renal dysfunction. Chin Med J. 2009;122(21):2547–53.
方玲, 卢建军, 唐建玲, 李玲, 陆汉军, 侯旭华, 等.无肾功能不全的2型糖尿病患者血浆肌酐与乳酸的关系。中国医学杂志 2009;122(21):2547–53.Vecchio S, Giampreti A, Petrolina V, Lonati D, Protti A, Papa P, et al. Metformin accumulation: lactic acidosis and high plasmatic metformin levels in a retrospective case series of 66 patients on chronic therapy. Clin Toxicol. 2014;52(2):129–35.
Ahmad F, Khalid P, Khan MM, Rastogi AK, Kidwai JR. Insulin like activity in (−) epicatechin. Acta diabetologia latina. 1989;26(4):291–300.
Hannan J, Ojo O, Ali L, Rokeya B, Khaleque J, Akhter M, et al. Actions underlying antidiabetic effects of Ocimum sanctum leaf extracts in animal models of type 1 and type 2 diabetes. Eur J Med Plants. 2015:1–12.
Jahromi MF, Ray AB, Chansouria J. Antihyperlipidemic effect of flavonoids from Pterocarpus marsupium. J Nat Prod. 1993;56(7):989–94.
George CM, Brujin LL, Will K, Howard-Thompson A. Management of blood glucose with noninsulin therapies in type 2 diabetes. Am Fam Physician. 2015;92(1):27–34.
International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–34.
Khan HA, Sobki SH, Khan SA. Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin Exp Med. 2007;7(1):24–9.
Association AD. Postprandial blood glucose. Diabetes Care. 2001;24(4):775–8.
Acknowledgements
We would like to thank the Clinical Trial Investigator of the study, Dr. Ravikumar Sethuraman and Levin Speciality Hospital.
Funding
This work was sponsored by Sami-Sabinsa Group Limited/Sabinsa Corporation. There was no external funding involved.
Author information
Authors and Affiliations
Contributions
Conceptualization, MM, AM, and KN; Methodology, SP; Validation, SP, and LM; Investigation, SP; Resources, MM and AM; Data Curation, SP; Writing—Original Draft Preparation, SP; Writing—Review & Editing, MM, AM, KN, and LM; Supervision, MM; Project Administration, AM; Funding Acquisition, MM.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial was approved by Institutional Ethics Committee, Levin Speciality Hospital Madurai before the conduct of the study. Written informed consent form was taken from all the subjects before enrolling in the study.
Consent for publication
Not applicable.
Competing interests
Dr. Muhammed Majeed is the Founder and Managing Director of Sami-Sabinsa Group Limited and Sabinsa Corporation. The authors declare that this study received funding from Sami-Sabinsa Group Limited/ Sabinsa Corporation. The funder was involved in conceptualizing the project and providing resources. The funder was not involved in study design, data collection, and analysis of results, but was part of reviewing the manuscript and decision to publish. All the authors are affiliated with Sami-Sabinsa Group Limited or Sabinsa Corporation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Majeed, M., Majeed, A., Nagabhusahnam, K. et al. A randomized, double-blind clinical trial of a herbal formulation (GlycaCare-II) for the management of type 2 diabetes in comparison with metformin. Diabetol Metab Syndr 13, 132 (2021). https://doi.org/10.1186/s13098-021-00746-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-021-00746-0