抽象
慢性阻塞性肺疾病 (AECOPD) 的急性加重是症状恶化的发作,对患者产生严重的不良后果。急性加重是与气道和全身炎症增加以及生理变化相关的高度异质性事件。急性加重的频率与肺功能加速下降、生活质量损害和死亡率增加有关。它们主要由呼吸道病毒和细菌触发,这些病毒和细菌感染下气道并增加气道炎症。与不频繁加重的患者相比,一部分患者似乎更容易加重,生活质量更差,疾病进展更具侵袭性。恶化也对医疗保健支出有很大影响。因此,预防和减轻急性加重是 COPD 管理的关键目标。
关键字:慢性阻塞性肺疾病、急性加重、发病机制
要点
-
•
慢性阻塞性肺疾病 (AECOPD) 的急性加重是症状恶化的发作,对患者产生严重的不良后果。
-
•
与气道和全身炎症和生理变化增加相关的高度异质性事件。
-
•
它们主要由呼吸道病毒和细菌触发,这些病毒和细菌感染下气道并增加气道炎症。
-
•
一部分患者似乎更容易恶化,生活质量较差,疾病进展更具侵袭性。
-
•
因此,预防和减轻急性加重是 COPD 管理的关键目标。
介绍
慢性阻塞性肺疾病 (AECOPD) 急性加重是指症状恶化1 的发作,对患者有严重的不良后果。2 恶化的重要原因包括气道细菌、病毒和污染;但是,还必须考虑这些触发因素的相互作用。众所周知,免疫力和宿主防御缺陷会导致更频繁的 AECOPD。恶化频率增加与肺功能加速下降、3 生活质量受损4 和死亡率增加有关。5 此外,随着慢性阻塞性肺病 (COPD) 发病率的增加,恶化给医疗保健系统带来了更大的负担,在美国每年有超过 1000 万次计划外就诊。6 在美国,COPD 治疗的直接费用每年超过 320 亿美元,7 ,8 据估计,恶化占这些医疗保健费用的 50% 至 75%。9 急性加重也是 COPD 的重要结局指标,急性治疗旨在加速恢复,而长期维持治疗旨在预防和降低其频率和严重程度。
Although half of the patients treated in the community recover to their baseline symptoms by 7 days, a study of the time course found that, despite treatment, 14% had still not fully recovered by 5 weeks. Moreover, in a small proportion of exacerbations, symptoms never returned to the baseline level.10 Consequently, a substantial number of COPD exacerbations can be prolonged, which culminates in greater morbidity associated with such an event. A key audit examining hospital admissions showed that more than one-quarter of patients experience another event during the following 8 weeks.11 In a cohort of patients with moderate to severe COPD followed up after exacerbation, 22% had a recurrent event within 50 days of the first (index) exacerbation. Such events are therefore complex, and an initial exacerbation seems to increase the susceptibility to a subsequent exacerbation.12 These recurrent events are associated with substantially increased mortality13 and this has driven financial incentives for health care services aiming to avoiding hospital readmission.14
,
15
尽管在社区接受治疗的患者中有一半在 7 天前恢复到基线症状,但一项关于时间进程的研究发现,尽管接受了治疗,但仍有 14% 的患者在 5 周内仍未完全康复。此外,在一小部分恶化中,症状从未恢复到基线水平。 10 因此,大量 COPD 恶化可能会延长,最终导致与此类事件相关的并发症发生率更高。一项检查住院情况的关键审计显示,超过四分之一的患者在接下来的 8 周内经历了另一次事件。 11 在急性加重后随访的中度至重度 COPD 患者队列中,22% 的患者在首次(指数)恶化后 50 天内发生复发事件。因此,此类事件很复杂,初始加重似乎会增加对后续加重的易感性。 12 这些反复发生的事件与死亡率的大幅增加有关 13 ,这推动了对旨在避免再次入院的医疗保健服务的经济激励。 14 15
Exacerbations Definition Exagerbations 定义
AECOPDs are transient periods of increased symptoms of dyspnea, sputum purulence, and sputum volume, but they may also encompass minor symptoms of nasal blockage/discharge, wheeze, sore throat, cough, fever, chest tightness or discomfort, fatigue/reduced energy, sleep disturbance, or limited physical activity.16 COPD exacerbations are associated with several features, including increased airway inflammation, mucus hypersecretion, and gas trapping. There is a degree of controversy over the precise definition of exacerbation events. The 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) document AECOPD definition slightly differs from this as “an acute worsening of respiratory symptoms that results in additional therapy.” This definition requires the patient to seek or use treatment and is an example of a health care use (HCU) exacerbation in which the patient or clinician decides whether treatment is warranted. The disadvantage with only considering this definition is that it risks not accounting for important events in certain key scenarios; for example, those of lesser severity that do not trigger increased treatment use, where respiratory deterioration with an alternative cause is misdiagnosed, or events in resource-poor areas with a lack of access to treatment or clinicians.
AECOPD 是呼吸困难、痰脓和痰量症状增加的短暂时期,但它们也可能包括鼻塞/分泌物、喘息、喉咙痛、咳嗽、发烧、胸闷或不适、疲劳/精力下降、睡眠障碍或身体活动受限等轻微症状。 16 COPD 恶化与多种特征有关,包括气道炎症增加、粘液分泌过多和气体潴留。关于恶化事件的精确定义存在一定程度的争议。2017 年慢性阻塞性肺病全球倡议 (GOLD) 文件 AECOPD 的定义与此略有不同,即“呼吸系统症状急性恶化,导致额外治疗”。该定义要求患者寻求或使用治疗,是医疗保健使用 (HCU) 恶化的一个例子,其中患者或临床医生决定是否需要治疗。仅考虑此定义的缺点是,它有可能不考虑某些关键情景中的重要事件;例如,那些严重程度较轻但不会引发治疗使用增加的事件,其中呼吸恶化与其他原因被误诊,或者在资源匮乏地区无法获得治疗或临床医生的事件。
The alternative to an HCU definition is to measure the increase in symptoms and to classify an exacerbation when this change crosses a threshold (regardless of whether the patient receives treatment). This approach has been widely accepted in research, using several validated patient-reported outcome (PRO) tools such as symptom/treatment diary cards and questionnaire tools such as the EXACT (Exacerbations of Chronic Obstructive Pulmonary Disease Tool) and CAT (The COPD Assessment Test). When implemented, it was discovered that a large number of events are unreported and untreated.4 Studies using symptom-based definitions typically report an incidence of exacerbations that is approximately twice as high as with HCU definitions. One reason for this is that the method captures additional milder events that the HCU definition does not.17 Although unreported exacerbations are milder than reported events, they do not seem to be inconsequential. However, the science of measuring symptoms is challenging, both in the collection of (daily) data and in their analysis. Analysis challenges include defining the threshold for exacerbation, ceiling effects, and how and when to reset the baseline symptom level in the event of incomplete exacerbation recovery.18 Two of the most extensively validated PROs in exacerbation studies are the EXACT17 and CAT,19 which seem to be valuable in the assessment of exacerbation frequency, duration, and severity and have been qualified as an exploratory end point by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).20 A particular strength of the EXACT is its ability to detect unreported events, and, in the ATTAIN (Aclidinium to Treat Airway Obstruction in COPD Patients),21 comparing a long-acting muscarinic antagonist with placebo, unreported (untreated) symptom (EXACT)-defined events had the same medium-term health consequences as reported (treated) HCU exacerbations. Moreover, the trial intervention reduced the rate of both symptom (EXACT)-defined and HCU events. However, a challenge with interpreting PROs such as the EXACT tool is the discordance between HCU exacerbations and symptom (EXACT)-defined events, with discrepancies found in both observational studies17 and clinical trials.21
HCU 定义的替代方法是测量症状的增加,并在这种变化超过阈值时对加重进行分类(无论患者是否接受治疗)。这种方法已在研究中被广泛接受,使用多种经过验证的患者报告结果 (PRO) 工具,例如症状/治疗日记卡和问卷工具,例如 EXACT(慢性阻塞性肺疾病恶化工具)和 CAT(COPD 评估测试)。实施后,发现大量事件未报告和未处理。 4 使用基于症状定义的研究通常报告加重的发生率大约是 HCU 定义的 2 倍。其中一个原因是该方法捕获了 HCU 定义没有捕获的其他较轻微的事件。 17 尽管未报告的恶化比报告的事件更轻,但它们似乎并非无关紧要。然而,测量症状的科学具有挑战性,无论是在(日常)数据的收集还是在分析方面。分析挑战包括定义恶化的阈值、天花板效应,以及如何以及何时在恶化不完全恢复的情况下重置基线症状水平。 18 在急性加重研究中,两个最广泛验证的 PRO 是 EXACT 17 和 CAT, 19 它们在评估急性加重频率、持续时间和严重程度方面似乎很有价值,并已被美国食品药品监督管理局 (FDA) 和欧洲药品管理局 (EMA) 认定为探索性终点。 20 EXACT 的一个特殊优势是它能够检测未报告的事件,并且在 ATTAIN(阿地溴铵治疗 COPD 患者的气道阻塞)中, 21 将长效毒蕈碱拮抗剂与安慰剂进行比较,未报告(未治疗)症状 (EXACT) 定义的事件与报告(治疗)的 HCU 恶化具有相同的中期健康后果。此外,试验干预降低了症状 (EXACT) 定义的 HCU 事件和 HCU 事件的发生率。然而,解释 PRO(例如 EXACT 工具)的一个挑战是 HCU 恶化与症状 (EXACT) 定义的事件之间的不一致,在观察性研究和 17 临床试验中都发现了差异。 21
A major challenge is the heterogeneous nature of the clinical presentation, and alternative causes for acute deterioration, such as heart failure, pneumothorax, pulmonary emboli, or anxiety, must be considered. Traditionally, infective exacerbations are thought to be driven by infection of the airway lumen (bronchi/bronchioles), whereas pneumonia represents alveolar infection. However, it is likely that these distinct processes overlap. A chest radiograph is not routinely performed during a COPD exacerbation,1 and consolidation may be missed if it is early in the infective process, or through the insensitivity of the test.
Exacerbation Severity
The latest GOLD guidelines define exacerbation severity by the treatment that is required.1
-
•
Mild: treatment with short-acting bronchodilators only
-
•
Moderate: treated with short-acting bronchodilators plus antibiotics and/or oral corticosteroids
-
•
Severe: requires either hospitalization or a visit to the emergency department and may also be associated with respiratory failure.
Exacerbation Cause
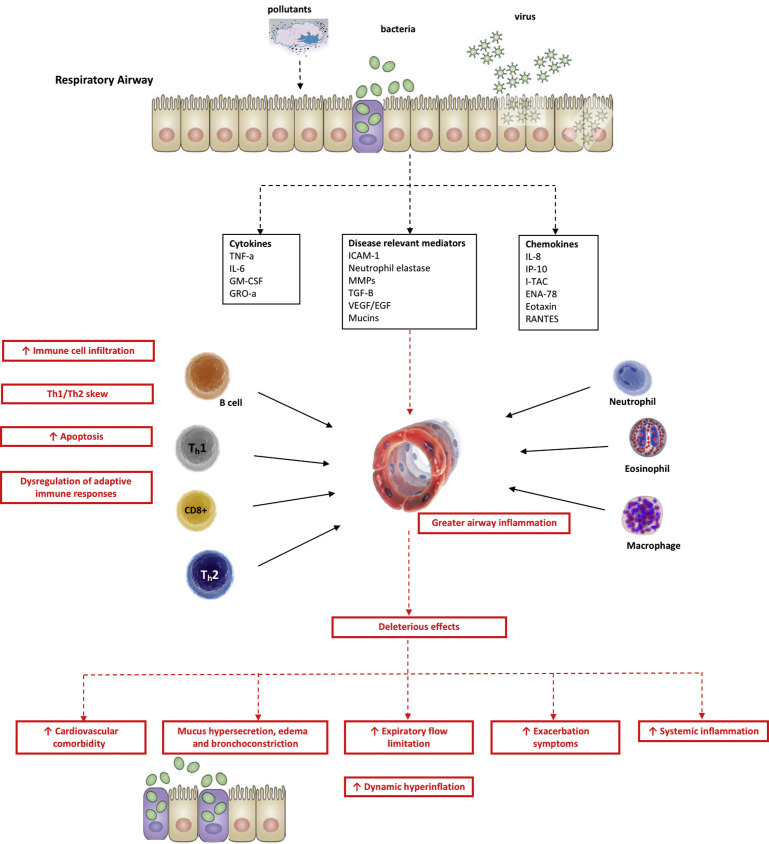
Exacerbations are airway inflammatory events that are triggered by infection in most cases. Respiratory viral infections are the predominant cause, although bacterial infections and environmental factors such as air pollution and ambient temperature trigger or worsen these events.22 , 23 Although early studies focused on bacteria as the primary cause of exacerbations, the development of highly specific molecular diagnostic techniques has highlighted the importance of viruses as key triggers for exacerbations.24, 25, 26 The primary role of different exacerbation triggers and important aspects of their interplay, including viral-bacterial coinfection, deficient host response to bacteria, and the lung microbiome in exacerbation are described here (Fig. 1 ). It has long been observed that the frequency of AECOPD doubles in winter months,27 , 28 with more than 50% of exacerbations preceded by coryzal symptoms (Table 1 ).10 , 29 , 30
Fig. 1.
Overview of AECOPD. EGF, endothelial growth factor; ENA, epithelial-derived neutrophil-activating peptide; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; IP, interferon γ–induced protein; I-TAC, interferon-inducible T-cell alpha chemoattractant; GM-CSF, granulocyte-macrophage colony–stimulating factor; GRO, growth-regulated oncogene; MMP, matrix metalloproteinase; RANTES, regulated upon activation, normal T Cell expressed and presumably secreted; TGF, transforming growth factor; Th, T helper; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Table 1.
Noteworthy studies showing the winter/summer seasonality incidence of acute exacerbations of chronic obstructive pulmonary disease
| Study Name | Study Findings |
|---|---|
| TORCH27 | 80% winter/summer excess (9% of patients exacerbating in December-February compared with 5% in June to August) in the northern hemisphere and a 71% excess (12% vs 7% of patients) in the southern hemisphere |
| POET28 | 7.63 vs 3.63 exacerbations (per 100 patient months) |
| Donaldson et al,140 2012 | 1052 exacerbations in winter vs 652 in summer. Winter exacerbations lasted longer and were more severe: 8.4% of exacerbations resulted in patients who were hospitalized, compared with 4.6% of exacerbations in the warm seasons |
| TIOSPIR141 | 6646 exacerbations in winter compared with 3198 in summer |
Viruses
Earlier studies using culture-based methods underestimated the prevalence of respiratory viruses during COPD exacerbations. However, with the advent of polymerase chain reaction (PCR) methods, the detection of viruses in COPD exacerbations increased to 22% to 64%.30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 The wide variations in virus detection are likely to be the consequence of whether patients were sampled at true onset of symptoms or sampling was delayed. Additional factors could include variation in the range of viruses tested for, sensitivity of the assays, the study period (eg, winter vs yearlong, variation in virus epidemics; eg, respiratory syncytial virus [RSV]), population (eg, community vs inpatient, uptake of the influenza vaccine), and sampling method (eg, nasopharyngeal swabs, sputum). In studies where patients reported exacerbation symptoms at onset, there is a greater prevalence of viral infection, because viral load is higher at exacerbation onset53 , 54 and may therefore be undetectable by the time patients present to hospital.29 , 30 , 34 , 41 , 50 , 55
Rhinoviruses are the most prevalent in most of these studies, accounting for up to 60% of all exacerbations.53 Influenza viruses and RSVs are also commonly detected, being identified in up to 36%52 and 28%55 of AECOPDs respectively. Parainfluenza viruses, human metapneumoviruses, coronaviruses, and adenoviruses are detected, but less frequently. Importantly, viral AECOPDs are associated with more severe symptoms, greater airflow limitation, and delayed recovery compared with exacerbations where no virus is detected.47 , 56 The greater incidence of rhinovirus in induced sputum, as opposed to nasal aspirates at exacerbation,57 further supports the theory that naturally occurring rhinovirus drive most exacerbations. Although these studies have shown an association between respiratory virus infection and exacerbations, they do not prove causation because PCR detects viral nucleic acid but it cannot prove the presence of live, replicating virus. Consequently, secondary causes cannot be excluded. However, in 2011, Mallia and colleagues54 provided novel evidence of a causal relationship between respiratory virus infection and exacerbations in patients with COPD through their experimental rhinovirus infection in patients with mild COPD. In their human model, they showed clearly that respiratory viruses produce symptoms that are typical of an exacerbation, confirming that respiratory viruses can infect the lower airway and contribute to inflammatory changes.54
Chronic viral infection is another key aspect to examine when considering the role played by viruses such as RSV. Although RSV infection has been seen at exacerbation,55 whether it alone drives the event is not entirely clear, because this virus is found incidentally within the airways of patients with COPD at stable state where it is associated with increased airway inflammation.58 Latent expression of adenoviral E1A protein in alveolar epithelial cells can potentiate the effects of lung inflammation induced by cigarette smoke.59 It is therefore plausible that chronic viral infection could contribute to disease severity in COPD, and further work is required to understand how viruses detected in the stable state relate to exacerbations.
Impaired Antiviral Immunity in Chronic Obstructive Pulmonary Disease
It is not fully understood why patients develop an exacerbation following respiratory virus infection but never smokers do not often go on to develop significant lower respiratory symptoms. Furthermore, there is a subgroup of COPD that seems to be more susceptible to infection, irrespective of disease severity (the frequent-exacerbator phenotype).60 COPD is associated with substantial changes in innate immunity that are likely to be relevant in the pathogenesis of exacerbations. Tobacco smoking impairs mucociliary clearance,61 and the rhinovirus binding receptor intercellular adhesion molecule 1 (ICAM-1) is upregulated by bronchial epithelial cells in COPD.62 Alveolar macrophages, which are numerous and form a first line of defense in the respiratory tract, are defective in COPD, with impairments in their ability to phagocytose bacteria63 , 64 and clear dead and dying cells65 compared with alveolar macrophages from healthy smoking and nonsmoking controls.
In the human experimental rhinovirus infection model, Mallia and colleagues54 found nasal lavage viral load was significantly higher in patients with COPD following rhinovirus infection compared with age-matched healthy controls. Because all subjects were inoculated with the same virus dose, this suggests impairment in the immune response that controls viral replication in COPD. This finding supports the work by Hurst and colleagues,29 who earlier showed that exacerbation frequency was related to cold acquisition rather than the propensity to develop an exacerbation following a cold.
The most abundant cells in the airway are bronchial epithelial cells (BECs) and alveolar macrophages. Interferon (IFN) deficiency has been observed in these important cells and, therefore, proposed as a potential mechanism of increased susceptibility to rhinovirus infection. Respiratory viruses such as human rhinovirus (HRV) replicate within the respiratory epithelium triggering the production of type I (FN-α, IFN-β) and type III IFNs (IFN-λ), which limit viral replication, protein synthesis, and protein trafficking (Table 2 ).66 However, IFN deficiency remains controversial in COPD. Mallia and colleagues54 found that bronchoalveolar lavage (BAL) cells of subjects with COPD had a deficient IFN-β response to ex vivo infection with HRV-16, but did not identify any deficiency in BEC responses. In contrast, Hsu and colleagues67 recently showed impaired IFN responses to influenza virus in BECs from COPD. These findings are supported by a study that showed a decrease in expression of IFN stimulated genes in the induced sputum of COPD participants compared with healthy controls.68 However, Schneider and colleagues69 and Baines and colleagues70 showed increased IFN-λ responses to HRV-39 and HRV-1B infection of COPD BECs respectively compared with healthy controls.69 Further studies of IFN induction in response to viral infection in epithelial and BAL cells in COPD are clearly needed because this is a potential therapeutic target.
Table 2.
Inflammatory changes in viral infections in chronic obstructive pulmonary disease exacerbations
| Mediator | Naturally Occurring Infection1 | Experimental Infection in Humans |
|---|---|---|
| Chemokines | ||
| CXCL10/IP-10 | ↑ Serum + sputum38 | ↑ BAL106 |
| CXCL8/IL-8 | ↔ Serum36,142 + sputum37,57 | ↑ Sputum ↔ BAL54,106 ↑ Nasal lavage143 |
| CCL5/RANTES | ↑ Sputum38 ↔ Serum36 |
— |
| CCL2/MCP1 | ↑ Sputum38 ↔ Serum36 |
— |
| CXCL11 | ↑ Serum + sputum38 | — |
| Inflammatory Cells | ||
| Neutrophils | ↔ Sputum37 | ↑ BAL, sputum, blood54,106 |
| Lymphocytes | — | ↑ BAL54,144 |
| Eosinophils | ↑ Sputum47 | — |
| Cytokines | ||
| IL-6 | ↑ Sputum57,71 ↔ Serum36,142 |
↑ BAL ↔ Sputum54 ↑ Nasal lavage143 |
| TNF-α | ↔ Serum36 or sputum37 | ↔ BAL, sputum54 ↑ Sputum106 |
| IL-1β | ↔ Serum36 | ↑ Sputum106 |
| IL-10 | ↑ Serum36 | — |
| IL-13 | ↔ Serum36 | — |
| Type II IFN (γ) | ↑ Serum38 ↔ Serum36 |
— |
| Selected Others | ||
| Neutrophil elastase | — | ↑ Sputum ↔ BAL54,92,106 |
| MMP-9 | — | ↑ Sputum106 |
| Antimicrobial peptides (secretory leukoprotease inhibitor, elafin) | — | ↓ Sputum92 |
| Markers of oxidative stress (8-hydroxy-2′-deoxyguanosine, 3-nitrotyrosine) | — | ↑ Sputum106 |
Viral infection in COPD also leads to the production of disease-relevant proinflammatory cytokines such as interleukin (IL)-8 (CXCL8), IL-6, chemokine ligand 5 (CCL5/RANTES), tumor necrosis factor alpha (TNF-α), and IFN-γ–induced protein (IP-10/CXCL10) via the nuclear factor κB pathway leading to the recruitment of neutrophils, macrophages, natural killer cells, T cells, and dendritic cells at the site of infection enhancing viral clearance. Importantly, the magnitude of this response is greater in patients with COPD compared with healthy controls37 , 38 , 71 and may explain how increased airway inflammation contributes to lower airway symptoms in COPD exacerbations.
In general, exacerbations become both more frequent and more severe as the severity of the underlying COPD increases,72 , 73 although the reason some patients with COPD experience more frequent exacerbations than others remains unclear. The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort study identified a distinct frequent-exacerbator phenotype. This group, irrespective of disease severity, was more susceptible to exacerbations and could be identified by a previous history of 2 or more exacerbations in a preceding year.60 There is some indirect evidence that an increased susceptibility to virus infection may be a characteristic of frequent exacerbators. In studies of naturally acquired virus-induced COPD exacerbations, virus infection was detected more commonly in exacerbation-prone patients.30 , 53 Alveolar macrophages taken from such patients (defined as having had an exacerbation during a 1-year period) and exposed to bacteria or toll-like receptor ligands ex vivo showed impaired induction of CXCL8/IL-8 and TNF-α, compared with macrophages from patients who were exacerbation free for a year.73 Nevertheless, the description of frequent exacerbators remains essentially clinical and further studies are warranted to elucidate differences in the immune responses and conclusively provide an underlying mechanism to explain this phenotype.
Bacteria
Bacteria are also extremely important in the pathogenesis of COPD exacerbations. Studies using traditional sputum culturing techniques have isolated bacteria in 40% to 60% of exacerbations of COPD.25 , 74 The most frequently identified species are nontypeable Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pneumoniae, and Pseudomonas aeruginosa.44 , 47 , 75 Atypical bacteria are infrequently isolated, with Mycoplasma pneumoniae and Chlamydophila pneumoniae implicated in only 4% to 5% of episodes.74 Studies have also shown that bacterial colonization is common in COPD and is associated with greater airway inflammation and increased risk of exacerbation.12 , 56 , 75 However, it remains unclear from these studies whether exacerbations occur because of the acquisition of new bacterial strains or an outgrowth of preexisting bacteria.26
The Microbiome Changes During Chronic Obstructive Pulmonary Disease Exacerbations
In up to 50% of AECOPDs showing the hallmarks of a bacterial cause, the causative pathogens are not recovered from respiratory samples by traditional culture methods. The application of microbiome techniques, which are culture independent, is giving rise to a new understanding of the interaction between the host and the millions of microorganisms that are present on bodily surfaces. Studies identifying bacteria based on 16S ribosomal RNA gene sequences have shown that the lungs of healthy people and patients with COPD are colonized by rich, complex bacterial communities.76, 77, 78 Recently, researchers have begun to highlight the shifts in microbial communities during COPD exacerbations (Table 3 ).
Table 3.
Summary of studies examining microbiome changes at chronic obstructive pulmonary disease exacerbation
| Study | Subjects and Samples | Lung Sample/Site | Key Finding |
|---|---|---|---|
| Huang et al,76 2010 | 8 intubated patients with COPD 8 tracheal aspirates |
Tracheal aspirates | Individuals have distinct airway bacterial communities Intubation duration ↓α diversity |
| Huang et al,79 2014 | 12 subjects with COPD | Sputum |
|
| Millares et al,158 2014 | 16 subjects with COPD 5 Pseudomonas colonized, 11 uncolonized |
Paired baseline and exacerbation sputum samples | No significant difference in microbiome at exacerbation between Pseudomonas colonized and uncolonized |
| Molyneux et al,82 2014 | 14 patients with COPD 17 Healthy subjects |
RV Interventional study; sputum preinfection, 5, 15, and 52 d postinfection | Rhinovirus infection led to an outgrowth of preexisting Haemophilus and Neisseria at day 15 |
| Wang et al,84 2016 BEAT-COPD |
87 patients with COPD 476 sputum samples |
Sputum at baseline, exacerbation onset, recovery | Distinct bacterial and eosinophilic exacerbation microbiome Biomarkers relate to diversity |
| Mayhew et al,81 2018 AERIS cohort |
101 patients with COPD | Sputum | ↑Proteobacteria with ↑ disease severity ↑Haemophilus with bronchiectasis ↑Dysbiosis in frequent exacerbations |
| Wang et al,80 2018 COPD-MAP |
281 patients with COPD | Sputum | Distinct microbiome for eosinophilic and bacterial exacerbations Similar taxa at baseline and exacerbation |
One of the first longitudinal studies, by Huang and colleagues,79 found that the sputum microbiome did not show any significant changes in the key characteristics of community richness, evenness, and diversity. However, substantial taxonomic composition variation was seen during exacerbations, with an increase in Proteobacteria but a decrease in Actinobacteria, Clostridia, and Bacteroidia. Furthermore, when levels of important pathogens such as H influenzae increase at AECOPD, closely related bacterial taxa were also enriched, whereas the phylogenetically distant taxa declined.79 The larger COPD-MAP and AERIS longitudinal studies found no significant change in Shannon diversity or core taxa abundancies at exacerbation, However, both studies suggested that exacerbations result from dysbiosis caused by changes in preexisting bacteria in the lung rather than complete removal or appearance of a novel species.80 , 81 Overall, these findings suggest that, although the bacteria cultured at exacerbation undoubtedly drive events, enrichment of taxa closely related to a dominant pathogen could also contribute to pathogenesis. Therefore, exacerbations can be considered polymicrobial infections.
A study of the microbiome following experimental rhinovirus infection also showed an outgrowth in Haemophilus and Neisseria that were present in lower numbers before rhinovirus infection.82 These changes were correlated with increased neutrophil concentration and neutrophil elastase levels, and were not observed in the healthy control group.82 These findings support the hypothesis that the bacteria identified at exacerbation are not newly acquired but are caused by an outgrowth of preexisting bacteria that have experienced newly favored conditions.82
Both the BEAT-COPD cohort and COPD-MAP cohorts identified distinct microbiome compositions between bacterial and eosinophilic exacerbations, suggesting that these are stable exacerbation phenotypes. The AERIS study found that individuals with concomitant bronchiectasis had a greater abundance of Haemophilus. It suggested that frequent exacerbators may have greater dysbiosis compared with infrequent exacerbators, thus providing a potential mechanism by which AECOPDs arise.
Treatment Effects on the Lung Microbiome
Events treated by antibiotics alone led to a reduction in the relative abundance of Proteobacteria, whereas treatment with corticosteroids alone led to an enrichment of multiple taxa, including members of Bacteroidetes, Firmicutes, and Proteobacteria.83 , 84 This finding was supported by an earlier study of tracheal aspirates from intubated patients in whom the investigators observed that bacterial communities became less diverse as the duration of intubation and antibiotic administration increased, suggesting that microbial communities are influenced by therapeutic interventions.76 When both steroids and antibiotics were used to treat an exacerbation, a mixed effect on the airway microbiome was seen.79
Host Response to Bacteria and Bacterial Susceptibility
A current hypothesis is that bacteria enter the lower respiratory tract by microaspiration during sleep or inhalation.85 In healthy lungs, pathogens either fill an ecological niche or are eradicated with minimal inflammation by the innate immune response. However, in patients with COPD, a combination of defective innate immunity including impaired mucociliary clearance and variation in antigenic structure among strains allow these bacteria to persist and proliferate.85
A complex host-pathogen interaction in the lower airway determines this outcome. In a mouse model, H influenzae strains associated with COPD exacerbations induced greater airway neutrophil recruitment compared with colonization-associated strains.86 Exacerbation-associated M catarrhalis strains interact differently with primary human airway epithelial cells, showing greater adherence and eliciting more IL-8.87 Sputum immunoglobulin (Ig)A levels, representing the mucosal host response to the infecting strain, were greater with colonization, whereas the systemic serum IgG host response was larger during exacerbations.88 It is thought that a robust mucosal immune response diminishes bacterial interaction with the airway epithalamium, resulting in less airway inflammation, thus favoring colonization.
Recent studies focusing on the immune response to bacterial infection have shown the development of specific antibodies to important species, including H influenzae, M catarrhalis, S pneumoniae, and P aeruginosa following exacerbations. Some of these show bactericidal and opsonophagocytic function, thereby aiding bacterial clearance.88, 89, 90 However, the multitude of strains may result in recurrent exacerbations with the same species and also creates a challenge for effective vaccine development.
Viral-Bacterial Coinfection
Coinfection with bacteria and viruses is common, occurring in 6% to 27% of exacerbations.44 , 47 , 91 The dynamics of viral and bacterial infection have been examined by Hutchinson and colleagues,32 who collected respiratory samples from patients with COPD at exacerbation onset, and also 5 to 7 days later: 36% of patients who had a virus detected at exacerbation onset went on to have a bacterial infection. George and colleagues53 reported that, when HRV was detected at exacerbation onset, 60% of patients developed a bacterial infection at 14 days. Mallia and colleagues92 found comparable results in experimental rhinovirus infection in COPD, with 60% of patients with COPD showing bacterial infection in their sputum at day 15 compared with only 10% in healthy volunteers. Those who developed a bacterial infection had prolonged respiratory symptoms and delayed recovery compared with those in whom bacteria were not detected.92
Exacerbations with coinfection with viruses and bacteria are associated with greater airflow limitation, increased airway inflammation, and delayed exacerbation recovery.47 , 56 However, mechanisms underpinning how HRV infection leads to a secondary bacterial infection have not been fully elucidated. Possible mechanisms include viral impairment of macrophage response to bacteria93, 94, 95 leading to a reduction in neutrophil recruitment and bacterial clearance96 or, alternatively, an upregulation of adhesion molecules in the bronchial epithelium.97 However, further work is needed to understand the complex pathogen-host interactions to direct further therapeutics.
Airway Inflammation and Cells of Interest
COPD is characterized by aberrant airway inflammation.1 A further increase in airway inflammation is seen in most exacerbations, but this process is not uniform and inflammation is related to exacerbation cause. Frequent exacerbators also show greater inflammation, and exacerbation nonrecovery is associated with persistent inflammation and a shorter time to the next exacerbation.12
Eosinophils
Traditionally, airway eosinophilia and T-helper cell type 2 (Th2) inflammation has been considered associated with allergic airway disorders such as asthma, and airway neutrophilia with COPD. However recent studies have reported that 20% to 40% of patients with COPD show sputum eosinophilia in the stable state.98, 99, 100 The SPIROMICS (SubPopulations and InteRmediate Outcome Measures In COPD Study) cohort has found that sputum eosinophilia at stable state is associated with more severe disease and increased exacerbation frequency.101 Interventional studies additionally suggest that high blood eosinophilia level at stable state might predict a better treatment response to inhaled corticosteroid use and could therefore be used to guide therapy.102 , 103
Acute exacerbations may be associated with further enhancement of eosinophilic airway inflammation, with up to 30% of COPD exacerbations being associated with sputum eosinophilia.38 , 99 Although there is biological plausibility for viral infection leading to sputum eosinophilia,104 studies of exacerbations to date have been conflicting.38 , 47 , 105 As a result, despite the considerable interest in the role of sputum and blood eosinophilia at stable state as biomarkers for disease outcome and steroid responsiveness, further work is needed to evaluate the significance of increased Th2 inflammation during COPD exacerbations.
Neutrophils
COPD exacerbations associated with bacterial pathogens show significantly more airway neutrophilic inflammation compared with nonbacterial episodes.88 Furthermore, the exacerbation severity and degree of airway bacterial concentration are related to the degree of neutrophilic inflammation.88 , 89 Important mediators of this airway neutrophilia in bacterial exacerbations include IL-8, leukotriene B4, and TNF-α.44 , 90 Studies examining bacterial exacerbations have identified an IL-1β signature comprising TNF-α, granulocyte colony–stimulating factor (Growth-regulated oncogene-α), IL-6, cluster of differentiation (CD) 40 ligand, and macrophage inflammatory protein 1 (MIP-1).92 IL-17A has been associated specifically with H influenzae exacerbations.54 Neutrophil degranulation and necrosis can cause significant damage related to the release of neutrophil elastase and matrix metalloproteinases.69 Clinical resolution of the symptoms of exacerbation is associated with a consistent decrease in mediators of neutrophilic airway inflammation, whereas nonresolving exacerbations show a sustained level of exaggerated airway inflammation.88 Studies from experimental infections also indicate that viral infection induces airway neutrophilic inflammation and innate inflammatory meditators such as IL-1β, granulocyte colony–stimulating factor (GM-CSF), CXCL8/IL-8, and TNF-α.54 , 106 , 107
Macrophages
Alveolar macrophages play a key role in the host defense against invasive pathogens by removing bacteria from the lung by phagocytosis, mediating inflammatory responses. There is increasing evidence of macrophage dysfunction in COPD.108 Alveolar macrophages and monocyte-derived macrophages show impaired phagocytosis of H influenzae, S pneumoniae, and Escherichia coli compared with healthy controls.63 , 64 , 109 Bewley and colleagues110 also found that phagocytosis of H influenzae was impaired in subjects with COPD with a history of exacerbations. Alveolar macrophages of exacerbation-prone subjects with COPD also showed impaired production of inflammatory cytokines CXCL8 and TNF-α in response to H influenzae compared with non–exacerbation-prone subjects with COPD, implicating macrophage dysfunction as a potential mechanism responsible for increased exacerbation frequency in COPD.64
Macrophages from patients with COPD stimulated ex vivo with respiratory virus produce less IFN compared with healthy subjects.54 However, in vitro studies have not necessarily supported this, with similar70 and even increased69 IFN released by cells taken from patients with COPD. In a murine model of COPD, IFN-α and IFN-β responses as a result of virus infection were reported as deficient in 1 study and viral clearance was impaired111; conversely, another study reported reduced IFN-λ (but not in IFN-β) and no difference in virus load. Therefore, it remains unclear whether production of IFN in response to virus infection is impaired in patients with COPD.
Biomarkers of Acute Exacerbations of Chronic Obstructive Pulmonary Disease
A reliable and objective biomarker of an AECOPD would be invaluable to aid in reliable diagnosis and guide appropriate treatment. The patient samples most investigated are serum or plasma, although sputum, urine, or exhaled breath may also contain useful biomarkers. Several studies have shown that the levels of a variety of immunoinflammatory cells and molecules are increased during exacerbations in respiratory samples, including exhaled breath, sputum, bronchoalveolar lavage, and bronchial biopsy (Table 4 ).
Table 4.
Common biomarkers examined in acute exacerbations of chronic obstructive pulmonary disease
| Biomarker | Study Findings |
|---|---|
| CRP |
|
| PCT |
|
| BNP | |
| Plasma fibrinogen |
|
| IL-6 |
|
| Urine metabolomics |
|
| Sputum eosinophilia | |
| Exhaled nitric oxide |
Biomarkers of Viral Exacerbations
A viral exacerbation is suggested with a history of coryzal symptoms and can subsequently be confirmed by PCR from a respiratory sample. However, a reliable biomarker would be invaluable for guiding therapy and antibiotic stewardship (see Tables 2 and 4). To date, serum CXCL10 (IP-10) seems the most promising,112 with Bafadhel and colleagues38 reporting a cutoff of 56 pg/mL to distinguish viral from nonviral exacerbations, giving a specificity of 65% and sensitivity of 75%. Quint and colleagues142 reported an area under the curve for serum IP-10 alone of 0.78 (95% confidence interval, 0.65–091) for detecting a human rhinovirus infection at exacerbation. Other biomarkers have been investigated, with levels of IL-6, monocyte chemoattractant protein-1 (MCP-1), and TNF-α all being increased in viral-associated AECOPD compared with viral-negative subjects and controls.113 Procalcitonin has also been used to try to detect viral-associated AECOPD, but the evidence so far is equivocal.114
Biomarkers of Bacterial Exacerbations
Bafadhel and colleagues38 suggested that a useful biomarker for determining bacterial-associated AECOPD was sputum IL-1β, with a cutoff of 125 pg/mL having a specificity of 80% and sensitivity of 90%. The serum biomarker best suited for distinguishing a bacterial cause in this study was C-reactive protein (CRP) at a cutoff of 10 mg/L, having a specificity of 70% and sensitivity of 60%.38 Dal Negro and colleagues115 also found that high sputum TNF-α level was associated with Pseudomonas-related exacerbations, and, in those subjects without high TNF-α level, high levels of IL-8 and IL-1β in the sputum distinguished bacterial from viral and noninfective exacerbations. An electronic nose used in the detection of cardinal volatile organic compounds has recently been used in a pilot study to distinguish bacterial from viral AECOPD,116 although development and proof of concept are needed before this technology can play a role in outpatient diagnostics.
A Danish study investigating biomarkers indicative of frequent exacerbators discovered that simultaneously increased fibrinogen, CRP, and white blood cell counts indicated an increased risk of frequent exacerbation.117 Increased plasma fibrinogen level in patients at risk of frequent exacerbation has also been replicated in further studies.118 , 119 The FDA has gone on to qualify fibrinogen as an end point of exacerbations and mortality. High levels of serum surfactant protein D have been shown to predict exacerbations when at their highest levels.120 However, the most comprehensive study to date, which included 2000 patients and examined 90 markers, in 2 separate cohorts (Spiromics and COPDGene), found no biomarker showed a significant relationship to exacerbation frequency in either cohort (after adjustment for recognized confounders: age, gender, percentage predicted forced expiratory volume in 1 second [FEV1], smoking and health status [quality of life], and self-report of gastroesophageal reflux).121
Consequences of Exacerbations
Lung function decline
Several studies have now shown that COPD exacerbations affect disease progression. Donaldson and colleagues3 showed that patients with a history of frequent exacerbations show accelerated decline, at around 25%, whereas Kanner and colleagues122 also showed that episodes of respiratory infections affect FEV1 decline. However, some of the earlier studies did not show a relationship between exacerbations and FEV1 decline.123, 124, 125 A review by Silverman126 suggested that this heterogeneity could be caused by the general/unselected or chronic bronchitis/emphysema populations studied in the early, negative studies in contrast with the COPD patient populations studied in the later, positive studies. A recent COPDGene study showed that the effect of exacerbations on decline was greatest in patients with mild (GOLD stage 1) COPD, with each event associated with an additional 23 mL/y decline.127 On occasion, lung function following an exacerbation does not fully recover, and then a group of patients who experience frequent exacerbations (because they have more events) are likely to have a faster lung function decline than patients who have zero or few exacerbations.128
Mortality
According to the latest Global Burden of Disease study estimates for 2015, COPD accounted worldwide for 3.2 million deaths.129 Exacerbations are the predominant cause of mortality, and Soler-Cataluña and colleagues5 showed that AECOPDs requiring hospitalization are independently associated with mortality (after adjusting for confounding variables such as age, FEV1, body mass index, and Charlson comorbidity index), and that the mortality risk increases with exacerbation frequency. A Canadian mortality study showed that rates after the first hospitalized COPD exacerbation were 50% at 3·6 years and 75% at 7·7 years.130 The mortality risk peaks sharply in the first 7 days after hospitalization and gradually declines over the subsequent 3 months. With every new hospitalized exacerbation, the risk of death increased, and the interval between hospitalizations decreased over time. For AECOPDs requiring hospitalization, patients with older age, higher arterial Paco 2, prolonged oral corticosteroid use, or admission to intensive care unit are more likely to die.131 In a large analysis of a UK primary care population, Rothnie and colleagues132 show a clear association between both the increasing frequency and the severity of AECOPDs and mortality.
Quality of life
The relationship between COPD exacerbations and health-related quality of life was first reported by Seemungal and colleagues,4 who found that patients with frequent exacerbations (>3 per year) had a 14.8-unit higher total St George’s Respiratory Questionnaire (SGRQ) score, indicating poorer quality of life, than patients with infrequent exacerbations (≤2 per year). Patients with COPD with frequent exacerbations (>3 per year) also have a faster deterioration in SGRQ scores over time (almost 2 units per year).133 Quality of life also worsens acutely at exacerbation compared with preexacerbation levels using several difference indices. These studies include worse activity and affect SGRQ, CCQ (clinical COPD Questionnaire), EQ-5D (European Quality of Life – 5 Dimensions questionnaire), MRC (Medical Research Council) dyspnea, ADL (Activities of Daily Living), CAT (The COPD Assessment Test), and EXACT (Exacerbations of Chronic Obstructive Pulmonary Disease Tool) scores.17 , 19 , 134 Exacerbations also worsen patients’ mental health with an increase in anxiety and depression135 and feelings of fatigue.136 Hospital admission and readmission for acute exacerbations have a particularly negative impact on quality-of-life scores.4 , 137
Physical activity
Acutely at exacerbation, patients spend less time outside of their homes, and patients who experience frequent exacerbation have a faster decline in time spent outdoors compared with infrequent exacerbators.138 Peripheral muscle weakness also deteriorates during an AECOPD.139 Patients who maintain physical activity at a low level reduce the risk of hospital admission for COPD by 28% (P = .033) compared with little or no physical activity136
Summary
AECOPDs are episodes of symptom worsening that have significant adverse consequences for patients. Exacerbations are highly heterogeneous events associated with increased airway and systemic inflammation and physiologic changes. The frequency of exacerbations is associated with accelerated lung function decline, quality of life impairment, and increased mortality. They are triggered predominantly by respiratory viruses and bacteria, which infect the lower airway and increase airway inflammation. A proportion of patients seem to be more susceptible to exacerbations, with poorer quality of life and more aggressive disease progression than those who have infrequent exacerbations. Exacerbations also contribute significantly to health care expenditure. Prevention and mitigation of exacerbations are therefore key goals of COPD management.
Footnotes
Dr. Wedzicha reports grants from GSK, grants from Johnson and Johnson, other from Novartis, other from Boehringer Ingelheim, other from Astra Zeneca, other from GSK, grants from GSK, grants from Astra Zeneca, grants from Boehringer Ingelheim, grants from Novartis, outside the submitted work; Dr Ritchie reports no disclosures.
References
- 1.Vogelmeier C.F., Criner G.J., Martinez F.J., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Wedzicha J.A., Seemungal T.A. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson G.C., Seemungal T.A., Bhowmik A., et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seemungal T.A., Donaldson G.C., Paul E.A., et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 5.Soler-Cataluña J.J., Martinez-Garcia M.A., Roman Sanchez P., et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannino D.M., Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(7):502–506. doi: 10.1513/pats.200701-001FM. [DOI] [PubMed] [Google Scholar]
- 7.Guarascio A.J., Ray S.M., Finch C.K., et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toy E.L., Gallagher K.F., Stanley E.L., et al. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214–228. doi: 10.3109/15412555.2010.481697. [DOI] [PubMed] [Google Scholar]
- 9.Celli B.R., MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 10.Seemungal T.A., Donaldson G.C., Bhowmik A., et al. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 11.Hurst J.R., Donaldson G.C., Quint J.K., et al. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(5):369–374. doi: 10.1164/rccm.200807-1067OC. [DOI] [PubMed] [Google Scholar]
- 12.Perera W.R., Hurst J.R., Wilkinson T.M.A., et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29(3):527–534. doi: 10.1183/09031936.00092506. [DOI] [PubMed] [Google Scholar]
- 13.Roberts C.M., Lowe D., Bucknall C.E., et al. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57(2):137–141. doi: 10.1136/thorax.57.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stolz D., Christ-Crain M., Bingisser R., et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131(1):9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- 15.Shah T., Churpek M.M., Coca Perraillon M., et al. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anthonisen N.R., Manfreda J., Warren C.P.W., et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 17.Mackay A.J., Donaldson G.C., Patel A.R., et al. Detection and severity grading of COPD exacerbations using the exacerbations of chronic pulmonary disease tool (EXACT) Eur Respir J. 2014;43(3):735–744. doi: 10.1183/09031936.00110913. [DOI] [PubMed] [Google Scholar]
- 18.Burgel P, Contoli M, López-Campos JL, editors. Acute exacerbations of pulmonary diseases. Euro-pean Respiratory Society; 2017.
- 19.Mackay A.J., Donaldson G.C., Patel A.R., et al. Usefulness of the chronic obstructive pulmonary disease assessment test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med. 2012;185(11):1218–1224. doi: 10.1164/rccm.201110-1843OC. [DOI] [PubMed] [Google Scholar]
- 20.Mackay A.J., Kostikas K., Murray L., et al. Patient-reported outcomes for the detection, quantification, and evaluation of chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2018;198(6):730–738. doi: 10.1164/rccm.201712-2482CI. [DOI] [PubMed] [Google Scholar]
- 21.Jones P.W., Lamarca R., Chuecos F., et al. Characterisation and impact of reported and unreported exacerbations: results from ATTAIN. Eur Respir J. 2014;44(5):1156–1165. doi: 10.1183/09031936.00038814. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Sun S., Tang R., et al. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3079–3091. doi: 10.2147/COPD.S122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodhead M., Blasi F., Ewig S., et al. Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect. 2011;17(Suppl 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritchie A.I., Farne H.A., Singanayagam A., et al. Pathogenesis of viral infection in exacerbations of airway disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S115–S132. doi: 10.1513/AnnalsATS.201503-151AW. [DOI] [PubMed] [Google Scholar]
- 25.Tager I., Speizer F.E. Role of infection in chronic bronchitis. N Engl J Med. 1975;292(11):563–571. doi: 10.1056/NEJM197503132921105. [DOI] [PubMed] [Google Scholar]
- 26.Sethi S., Evans N., Grant B.J., et al. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins C.R., Celli B., Anderson J.A., et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39(1):38–45. doi: 10.1183/09031936.00194610. [DOI] [PubMed] [Google Scholar]
- 28.Rabe K.F., Fabbri L.M., Vogelmeier C., et al. Seasonal distribution of COPD exacerbations in the prevention of exacerbations with Tiotropium in COPD trial. Chest. 2013;143(3):711–719. doi: 10.1378/chest.12-1277. [DOI] [PubMed] [Google Scholar]
- 29.Hurst J.R., Donaldson G.C., Wilkinson T.M., et al. Epidemiological relationships between the common cold and exacerbation frequency in COPD. Eur Respir J. 2005;26(5):846–852. doi: 10.1183/09031936.05.00043405. [DOI] [PubMed] [Google Scholar]
- 30.Seemungal T., Harper-Owen R., Bhowmik A., et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 31.Ko F.W., Ip M., Chan P.K., et al. Viral etiology of acute exacerbations of COPD in Hong Kong. Chest. 2007;132(3):900–908. doi: 10.1378/chest.07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchinson A.F., Ghimire A.K., Thompson M.A., et al. A community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPD. Respir Med. 2007;101(12):2472–2481. doi: 10.1016/j.rmed.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Daubin C., Parienti J.J., Vabret A., et al. Procalcitonin levels in acute exacerbation of COPD admitted in ICU: a prospective cohort study. BMC Infect Dis. 2008;8:145. doi: 10.1186/1471-2334-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camargo C.A., Jr., Ginde A.A., Clark S., et al. Viral pathogens in acute exacerbations of chronic obstructive pulmonary disease. Intern Emerg Med. 2008;3(4):355–359. doi: 10.1007/s11739-008-0197-0. [DOI] [PubMed] [Google Scholar]
- 35.Bozinovski S., Hutchinson A., Thompson M., et al. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(3):269–278. doi: 10.1164/rccm.200705-678OC. [DOI] [PubMed] [Google Scholar]
- 36.Almansa R., Sanchez-Garcia M., Herrero A., et al. Host response cytokine signatures in viral and nonviral acute exacerbations of chronic obstructive pulmonary disease. J Interferon Cytokine Res. 2011;31(5):409–413. doi: 10.1089/jir.2010.0131. [DOI] [PubMed] [Google Scholar]
- 37.Pant S., Walters E.H., Griffiths A., et al. Airway inflammation and anti-protease defences rapidly improve during treatment of an acute exacerbation of COPD. Respirology. 2009;14(4):495–503. doi: 10.1111/j.1440-1843.2009.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bafadhel M., McKenna S., Terry S., et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 39.Singh M., Lee S.H., Porter P., et al. Human rhinovirus proteinase 2A induces TH1 and TH2 immunity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2010;125(6):1369–1378.e2. doi: 10.1016/j.jaci.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Serres G., Lampron N., La Forge J., et al. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol. 2009;46(2):129–133. doi: 10.1016/j.jcv.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McManus T.E., Marley A.M., Baxter N., et al. Respiratory viral infection in exacerbations of COPD. Respir Med. 2008;102(11):1575–1580. doi: 10.1016/j.rmed.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beckham J.D., Cadena A., Lin J., et al. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect. 2005;50(4):322–330. doi: 10.1016/j.jinf.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cameron R.J., de Wit D., Welsh T.N., et al. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med. 2006;32(7):1022–1029. doi: 10.1007/s00134-006-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perotin J.M., Dury S., Renois F., et al. Detection of multiple viral and bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a pilot prospective study. J Med Virol. 2013;85(5):866–873. doi: 10.1002/jmv.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bandi V., Jakubowycz M., Kinyon C., et al. Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzae. FEMS Immunol Med Microbiol. 2003;37(1):69–75. doi: 10.1016/S0928-8244(03)00100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu Y., Zhu J., Bandi V., et al. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(8):968–975. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- 47.Papi A., Bellettato C.M., Braccioni F., et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 48.Kherad O., Kaiser L., Bridevaux P.O., et al. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest. 2010;138(4):896–904. doi: 10.1378/chest.09-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimopoulos G., Lerikou M., Tsiodras S., et al. Viral epidemiology of acute exacerbations of chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2012;25(1):12–18. doi: 10.1016/j.pupt.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohde G., Wiethege A., Borg I., et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minosse C., Selleri M., Zaniratti M.S., et al. Frequency of detection of respiratory viruses in the lower respiratory tract of hospitalized adults. J Clin Virol. 2008;42(2):215–220. doi: 10.1016/j.jcv.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan W.C., Xiang X., Qiu D., et al. Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am J Med. 2003;115(4):272–277. doi: 10.1016/s0002-9343(03)00353-x. [DOI] [PubMed] [Google Scholar]
- 53.George S.N., Garcha D.S., Mackay A.J., et al. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J. 2014;44(1):87–96. doi: 10.1183/09031936.00223113. [DOI] [PubMed] [Google Scholar]
- 54.Mallia P., Message S.D., Gielen V., et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183(6):734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falsey A.R., Formica M.A., Hennessey P.A., et al. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(6):639–643. doi: 10.1164/rccm.200510-1681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkinson T.M., Hurst J.R., Perera W.R., et al. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129(2):317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seemungal T.A., Harper-Owen R., Bhowmik A., et al. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;16(4):677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson T.M.A., Donaldson G.C., Johnston S.L., et al. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(8):871–876. doi: 10.1164/rccm.200509-1489OC. [DOI] [PubMed] [Google Scholar]
- 59.Retamales I., Elliott W.M., Meshi B., et al. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164(3):469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 60.Hurst J.R., Vestbo J., Anzueto A., et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 61.Leopold P.L., O'Mahony M.J., Lian X.J., et al. Smoking is associated with shortened airway cilia. PLoS One. 2009;4(12):e8157. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Stefano A., Maestrelli P., Roggeri A., et al. Upregulation of adhesion molecules in the bronchial mucosa of subjects with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1994;149(3 Pt 1):803–810. doi: 10.1164/ajrccm.149.3.7509705. [DOI] [PubMed] [Google Scholar]
- 63.Taylor A.E., Finney-Hayward T.K., Quint J.K., et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35(5):1039–1047. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- 64.Berenson C.S., Kruzel R.L., Eberhardt E., et al. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis. 2013;208(12):2036–2045. doi: 10.1093/infdis/jit400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hodge S., Hodge G., Scicchitano R., et al. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol. 2003;81(4):289–296. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 66.Vareille M., Kieninger E., Edwards M.R., et al. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24(1):210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu A.C.Y., Parsons K., Moheimani F., et al. Impaired antiviral stress granule and IFN-β enhanceosome formation enhances susceptibility to influenza infection in chronic obstructive pulmonary disease epithelium. Am J Respir Cell Mol Biol. 2016;55(1):117–127. doi: 10.1165/rcmb.2015-0306OC. [DOI] [PubMed] [Google Scholar]
- 68.Hilzendeger C., da Silva J., Henket M., et al. Reduced sputum expression of interferon-stimulated genes in severe COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1485–1494. doi: 10.2147/COPD.S105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider D., Ganesan S., Comstock A.T., et al. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(3):332–340. doi: 10.1164/rccm.200911-1673OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baines K.J., Hsu A.C.Y., Tooze M., et al. Novel immune genes associated with excessive inflammatory and antiviral responses to rhinovirus in COPD. Respir Res. 2013;14(1):15. doi: 10.1186/1465-9921-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rohde G., Borg I., Wiethege A., et al. Inflammatory response in acute viral exacerbations of COPD. Infection. 2008;36(5):427–433. doi: 10.1007/s15010-008-7327-5. [DOI] [PubMed] [Google Scholar]
- 72.Burge S., Wedzicha J.A. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 73.Berenson C.S., Kruzel R.L., Eberhardt E., et al. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax. 2014;69(9):811–818. doi: 10.1136/thoraxjnl-2013-203669. [DOI] [PubMed] [Google Scholar]
- 74.Sethi S., Murphy T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 75.Hurst J.R., Wilkinson T.M., Perera W.R., et al. Relationships among bacteria, upper airway, lower airway, and systemic inflammation in COPD. Chest. 2005;127(4):1219–1226. doi: 10.1378/chest.127.4.1219. [DOI] [PubMed] [Google Scholar]
- 76.Huang Y.J., Kim E., Cox M.J., et al. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS. 2010;14(1):9–59. doi: 10.1089/omi.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Erb-Downward J.R., Thompson D.L., Han M.K., et al. Analysis of the lung microbiome in the "healthy" smoker and in COPD. PLoS One. 2011;6(2):e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cabrera-Rubio Rl, Garcia-Núñez M., Set L., et al. Microbiome diversity in the bronchial tracts of patients with chronic obstructive pulmonary disease. J Clin Microbiol. 2012;50(11):3562–3568. doi: 10.1128/JCM.00767-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Y.J., Sethi S., Murphy T., et al. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol. 2014;52(8):2813–2823. doi: 10.1128/JCM.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z., Singh R., Miller B.E., et al. Sputum microbiome temporal variability and dysbiosis in chronic obstructive pulmonary disease exacerbations: an analysis of the COPDMAP study. Thorax. 2018;73(4):331–338. doi: 10.1136/thoraxjnl-2017-210741. [DOI] [PubMed] [Google Scholar]
- 81.Mayhew D., Devos N., Lambert C., et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax. 2018;73(5):422–430. doi: 10.1136/thoraxjnl-2017-210408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molyneaux P.L., Mallia P., Cox M.J., et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(10):1224–1231. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chronic obstructive pulmonary disease | Topic | NICE.
- 84.Wang Z., Bafadhel M., Haldar K., et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47(4):1082–1092. doi: 10.1183/13993003.01406-2015. [DOI] [PubMed] [Google Scholar]
- 85.Dickson R.P., Erb-Downward J.R., Huffnagle G.B. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013;7(3):245–257. doi: 10.1586/ers.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chin C.L., Manzel L.J., Lehman E.E., et al. Haemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizers. Am J Respir Crit Care Med. 2005;172(1):85–91. doi: 10.1164/rccm.200412-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parameswaran G.I., Wrona C.T., Murphy T.F., et al. Moraxella catarrhalis acquisition, airway inflammation and protease-antiprotease balance in chronic obstructive pulmonary disease. BMC Infect Dis. 2009;9:178. doi: 10.1186/1471-2334-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy T.F., Brauer A.L., Grant B.J.B., et al. Moraxella catarrhalis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):195–199. doi: 10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bogaert D., van der Valk P., Ramdin R., et al. Host-pathogen interaction during pneumococcal infection in patients with chronic obstructive pulmonary disease. Infect Immun. 2004;72(2):818–823. doi: 10.1128/IAI.72.2.818-823.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sethi S., Wrona C., Grant B.J.B., et al. Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(4):448–453. doi: 10.1164/rccm.200308-1181OC. [DOI] [PubMed] [Google Scholar]
- 91.Hurst J.R., Perera W.R., Wilkinson T.M.A., et al. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(1):71–78. doi: 10.1164/rccm.200505-704OC. [DOI] [PubMed] [Google Scholar]
- 92.Mallia P., Footitt J., Sotero R., et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(11):1117–1124. doi: 10.1164/rccm.201205-0806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oliver B.G., Lim S., Wark P., et al. Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax. 2008;63(6):519–525. doi: 10.1136/thx.2007.081752. [DOI] [PubMed] [Google Scholar]
- 94.Cooper G.E., Pounce Z.C., Wallington J.C., et al. Viral inhibition of bacterial phagocytosis by human macrophages: redundant role of CD36. PLoS One. 2016;11(10):e0163889. doi: 10.1371/journal.pone.0163889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Finney L.J., Belchamber K.B.R., Fenwick P.S., et al. Human rhinovirus impairs the innate immune response to bacteria in alveolar macrophages in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2019;199(12):1496–1507. doi: 10.1164/rccm.201806-1095OC. [DOI] [PubMed] [Google Scholar]
- 96.Unger B.L., Faris A.N., Ganesan S., et al. Rhinovirus attenuates non-typeable Hemophilus influenzae-stimulated IL-8 responses via TLR2-dependent degradation of IRAK-1. PLoS Pathog. 2012;8(10):e1002969. doi: 10.1371/journal.ppat.1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J.H., Kwon H.J., Jang Y.J. Rhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneously. Laryngoscope. 2009;119(7):1406–1411. doi: 10.1002/lary.20498. [DOI] [PubMed] [Google Scholar]
- 98.Leigh R., Pizzichini M.M.M., Morris M.M., et al. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27(5):964–971. doi: 10.1183/09031936.06.00072105. [DOI] [PubMed] [Google Scholar]
- 99.Eltboli O., Bafadhel M., Hollins F., et al. COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulm Med. 2014;14(1):112. doi: 10.1186/1471-2466-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brightling C.E., Monteiro W., Ward R., et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356(9240):1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 101.Hastie A.T., Martinez F.J., Curtis J.L., et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi: 10.1016/S2213-2600(17)30432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pascoe S., Locantore N., Dransfield M.T., et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 103.Pavord I.D., Lettis S., Locantore N., et al. Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD. Thorax. 2016;71(2):118–125. doi: 10.1136/thoraxjnl-2015-207021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edwards M.R., Strong K., Cameron A., et al. Viral infections in allergy and immunology: how allergic inflammation influences viral infections and illness. J Allergy Clin Immunol. 2017;140(4):909–920. doi: 10.1016/j.jaci.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saetta M., Di Stefano A., Maestrelli P., et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150(6):1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 106.Footitt J., Mallia P., Durham A.L., et al. Oxidative and nitrosative stress and histone deacetylase-2 activity in exacerbations of chronic obstructive pulmonary disease. Chest. 2016;149(1):62–73. doi: 10.1378/chest.14-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mallia P., Message S.D., Contoli M., et al. Neutrophil adhesion molecules in experimental rhinovirus infection in COPD. Respir Res. 2013;14(1):72. doi: 10.1186/1465-9921-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hiemstra P.S. Altered macrophage function in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(Suppl):S180–S185. doi: 10.1513/AnnalsATS.201305-123AW. [DOI] [PubMed] [Google Scholar]
- 109.Berenson C.S., Wrona C.T., Grove L.J., et al. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;174(1):31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bewley M.A., Belchamber K.B.R., Chana K.K., et al. Differential effects of p38, MAPK, PI3K or rho kinase inhibitors on bacterial phagocytosis and efferocytosis by macrophages in COPD. PLoS One. 2016;11(9):e0163139. doi: 10.1371/journal.pone.0163139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sajjan U., Ganesan S., Comstock A.T., et al. Elastase- and LPS-exposed mice display altered responses to rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2009;297(5):L931–L944. doi: 10.1152/ajplung.00150.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yin T., Zhu Z., Mei Z., et al. Analysis of viral infection and biomarkers in patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2018;12(3):1228–1239. doi: 10.1111/crj.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng J., Shi Y., Xiong L., et al. The expression of IL-6, TNF-α, and MCP-1 in respiratory viral infection in acute exacerbations of chronic obstructive pulmonary disease. J Immunol Res. 2017;2017:8539294. doi: 10.1155/2017/8539294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pantzaris N.-D., Spilioti D.-X., Psaromyalou A., et al. The use of serum procalcitonin as a diagnostic and prognostic biomarker in chronic obstructive pulmonary disease exacerbations: a literature review update. J Clin Med Res. 2018;10(7):545–551. doi: 10.14740/jocmr3458w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dal Negro R.W., Micheletto C., Tognella S., et al. A two-stage logistic model based on the measurement of pro-inflammatory cytokines in bronchial secretions for assessing bacterial, viral, and non-infectious origin of COPD exacerbations. COPD. 2005;2(1):7–16. doi: 10.1081/copd-200050680. [DOI] [PubMed] [Google Scholar]
- 116.van Geffen W.H., Bruins M., Kerstjens H.A.M. Diagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronic nose: a pilot study. J Breath Res. 2016;10(3):036001. doi: 10.1088/1752-7155/10/3/036001. [DOI] [PubMed] [Google Scholar]
- 117.Pais R. Biomarkers for predicting COPD exacerbations. Thorax. 2014;69(8):767. [Google Scholar]
- 118.Faner R., Agusti A. Fibrinogen and COPD: now what? Chronic Obstr Pulm Dis. 2015;2(1):1–3. doi: 10.15326/jcopdf.2.1.2014.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duvoix A., Dickens J., Haq I., et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68(7):670–676. doi: 10.1136/thoraxjnl-2012-201871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leung J.M., Sin D.D. Biomarkers in airway diseases. Can Respir J. 2013;20(3):180–182. doi: 10.1155/2013/204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keene J.D., Jacobson S., Kechris K., et al. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med. 2017;195(4):473–481. doi: 10.1164/rccm.201607-1330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kanner R.E., Anthonisen N.R., Connett J.E. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(3):358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 123.Casanova C., de Torres J.P., Aguirre-Jaime A., et al. The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med. 2011;184(9):1015–1021. doi: 10.1164/rccm.201105-0831OC. [DOI] [PubMed] [Google Scholar]
- 124.Celli B.R., Thomas N.E., Anderson J.A., et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178(4):332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 125.Howard P. A long-term follow-up of respiratory symptoms and ventilatory function in a group of working men. Br J Ind Med. 1970;27(4):326–333. doi: 10.1136/oem.27.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Silverman E.K. Exacerbations in chronic obstructive pulmonary disease: do they contribute to disease progression? Proc Am Thorac Soc. 2007;4(8):586–590. doi: 10.1513/pats.200706-068TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dransfield M.T., Kunisaki K.M., Strand M.J., et al. Acute exacerbations and lung function loss in smokers with and without COPD. Am J Respir Crit Care Med. 2016;195(3) doi: 10.1164/rccm.201605-1014OC. rccm.201605-2016201014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Donaldson G.C., Law M., Kowlessar B., et al. Impact of prolonged exacerbation recovery in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(8):943–950. doi: 10.1164/rccm.201412-2269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gbd Chronic Respiratory Disease Collaborators JB, Abajobir A.A., Abate K.H., et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suissa S., Dell'Aniello S., Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Groenewegen K.H., Schols A.M.W.J., Wouters E.F.M. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459–467. doi: 10.1378/chest.124.2.459. [DOI] [PubMed] [Google Scholar]
- 132.Rothnie K.J., Müllerová H., Smeeth L., et al. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice–based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464–471. doi: 10.1164/rccm.201710-2029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miravitlles M., Ferrer M., Pont A., et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59(5):387–395. doi: 10.1136/thx.2003.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bourbeau J., Ford G., Zackon H., et al. Impact on patients’ health status following early identification of a COPD exacerbation. Eur Respir J. 2007;30(5):907. doi: 10.1183/09031936.00166606. [DOI] [PubMed] [Google Scholar]
- 135.Laurin C., Moullec G., Bacon S.L., et al. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918–923. doi: 10.1164/rccm.201105-0939PP. [DOI] [PubMed] [Google Scholar]
- 136.Garcia-Aymerich J., Farrero E., Felez M.A., et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–105. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baghai-Ravary R., Quint J.K., Goldring J.J., et al. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med. 2009;103(2):216–223. doi: 10.1016/j.rmed.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 138.Donaldson G.C., Wilkinson T.M.A., Hurst J.R., et al. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(5):446–452. doi: 10.1164/rccm.200408-1054OC. [DOI] [PubMed] [Google Scholar]
- 139.Spruit M.A., Gosselink R., Troosters T., et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58(9):752–756. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Donaldson G.C., Goldring J.J., Wedzicha J.A. Influence of season on exacerbation characteristics in patients with COPD. Chest. 2012;141(1):94–100. doi: 10.1378/chest.11-0281. [DOI] [PubMed] [Google Scholar]
- 141.Wise R.A., Calverley P.M., Carter K., et al. Seasonal variations in exacerbations and deaths in patients with COPD during the TIOSPIR((R)) trial. Int J Chron Obstruct Pulmon Dis. 2018;13:605–616. doi: 10.2147/COPD.S148393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Quint J.K., Donaldson G.C., Goldring J.J., et al. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest. 2010;137(4):812–822. doi: 10.1378/chest.09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mallia P., Message S.D., Kebadze T., et al. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;7:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mallia P., Message S.D., Contoli M., et al. Lymphocyte subsets in experimental rhinovirus infection in chronic obstructive pulmonary disease. Respir Med. 2014;108(1):78–85. doi: 10.1016/j.rmed.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Y.-W.R., Leung J.M., Sin D.D. A systematic review of diagnostic biomarkers of COPD exacerbation. PLoS One. 2016;11(7):e0158843. doi: 10.1371/journal.pone.0158843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gallego M., Pomares X., Capilla S., et al. C-reactive protein in outpatients with acute exacerbation of COPD: its relationship with microbial etiology and severity. Int J Chron Obstruct Pulmon Dis. 2016;11:2633–2640. doi: 10.2147/COPD.S117129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kawamatawong T., Apiwattanaporn A., Siricharoonwong W. Serum inflammatory biomarkers and clinical outcomes of COPD exacerbation caused by different pathogens. Int J Chron Obstruct Pulmon Dis. 2017;12:1625–1630. doi: 10.2147/COPD.S132132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mathioudakis A.G., Chatzimavridou-Grigoriadou V., Corlateanu A., et al. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017;26(143):160073. doi: 10.1183/16000617.0073-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Inoue Y., Kawayama T., Iwanaga T., et al. High plasma brain natriuretic peptide levels in stable COPD without pulmonary hypertension or cor pulmonale. Intern Med. 2009;48(7):503–512. doi: 10.2169/internalmedicine.48.1701. [DOI] [PubMed] [Google Scholar]
- 150.European Respiratory Society SS, Calistru P.I. vol. 42. ERS Journals; 2013. (European respiratory journal). [Google Scholar]
- 151.Adrish M., Nannaka V.B., Cano E.J., et al. Significance of NT-pro-BNP in acute exacerbation of COPD patients without underlying left ventricular dysfunction. Int J Chron Obstruct Pulmon Dis. 2017;12:1183–1189. doi: 10.2147/COPD.S134953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wedzicha J.A., Seemungal T.A., MacCallum P.K., et al. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost. 2000;84(2):210–215. [PubMed] [Google Scholar]
- 153.Celli B.R., Locantore N., Yates J., et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 154.Adamko D.J., Nair P., Mayers I., et al. Metabolomic profiling of asthma and chronic obstructive pulmonary disease: a pilot study differentiating diseases. J Allergy Clin Immunol. 2015;136(3):571–580.e3. doi: 10.1016/j.jaci.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 155.Kolsum U., Donaldson G.C., Singh R., et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir Res. 2017;18(1):88. doi: 10.1186/s12931-017-0570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Malerba M., Radaeli A., Olivini A., et al. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. Biomed Res Int. 2014;2014:271918. doi: 10.1155/2014/271918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bhowmik A., Seemungal T.A.R., Donaldson G.C., et al. Effects of exacerbations and seasonality on exhaled nitric oxide in COPD. Eur Respir J. 2005;26(6):1009–1015. doi: 10.1183/09031936.05.00047305. [DOI] [PubMed] [Google Scholar]
- 158.Millares L., Ferrari R., Galleg M., et al. Bronchial microbiome of severe COPD patients colonised by Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2014;33(7):1101–1111. doi: 10.1007/s10096-013-2044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]