Abstract 抽象的
Ferritinophagy, a process involving selective autophagy of ferritin facilitated by nuclear receptor coactivator 4 (NCOA4), entails the recognition of ferritin by NCOA4 and subsequent delivery to the autophagosome. Within the autophagosome, ferritin undergoes degradation, leading to the release of iron in the lysosome. It is worth noting that excessive iron levels can trigger cell death. Recent evidence has elucidated the significant roles played by ferritinophagy and ferroptosis in regulation the initiation and progression of cancer. Given the crucial role of ferritinophagy in tumor biology, it may serve as a potential target for future anti-tumor therapeutic interventions. In this study, we have provided the distinctive features of ferritinophagy and its distinctions from ferroptosis. Moreover, we have briefly examined the fundamental regulatory mechanisms of ferritinophagy, encompassing the involvement of the specific receptor NCOA4, the Nrf2/HO-1 signaling and other pathways. Subsequently, we have synthesized the current understanding of the impact of ferritinophagy on cancer progression and its potential therapeutic applications, with a particular emphasis on the utilization of chemotherapy, nanomaterials, and immunotherapy to target the ferritinophagy pathway for anti-tumor purposes.
铁蛋白自噬是一个涉及核受体共激活因子 4 (NCOA4) 促进的铁蛋白选择性自噬的过程,需要 NCOA4 识别铁蛋白并随后递送至自噬体。在自噬体内,铁蛋白经历降解,导致溶酶体中铁的释放。值得注意的是,铁含量过高会引发细胞死亡。最近的证据阐明了铁蛋白自噬和铁死亡在调节癌症的发生和进展中发挥的重要作用。鉴于铁蛋白自噬在肿瘤生物学中的关键作用,它可能作为未来抗肿瘤治疗干预的潜在靶点。在这项研究中,我们提供了铁蛋白自噬的独特特征及其与铁死亡的区别。此外,我们还简要研究了铁蛋白自噬的基本调节机制,包括特定受体 NCOA4、Nrf2/HO-1 信号传导和其他途径的参与。随后,我们总结了目前对铁蛋白自噬对癌症进展的影响及其潜在治疗应用的理解,特别强调利用化疗、纳米材料和免疫疗法来靶向铁蛋白自噬途径以达到抗肿瘤目的。
Subject terms: Cancer metabolism, Cancer therapy
主题术语:癌症代谢、癌症治疗
Facts 事实
Ferritinophagy plays a crucial role in the maintenance of intracellular iron homeostasis.
铁蛋白自噬在维持细胞内铁稳态中起着至关重要的作用。Ferritinophagy is involved in the occurrence and development of cancer.
铁蛋白自噬参与癌症的发生和发展。Ferritinophagy may be a target for anti-cancer intervention in the future.
铁蛋白吞噬可能是未来抗癌干预的目标。
Open questions 开放式问题
What are the pathways regulating ferritinophagy in different cancers?
不同癌症中调节铁蛋白自噬的途径有哪些?How to exert anti-tumor effects by combining ferritinophagy with cancer immunity or drugs?
如何将铁蛋白自噬与癌症免疫或药物相结合发挥抗肿瘤作用?How does ferritinophagy apply to the clinic?
铁蛋白自噬如何应用于临床?
Introduction 介绍
Ferritinophagy is a selective form of autophagy that specifically targets intracellular ferritin for degradation. Ferritin is a protein complex composed of ferritin heavy chain (FTH1) and ferritin light chain (FTL) subunits, which functions to bind and store excess iron within cells [1]. Ferritinophagy plays a crucial role in maintaining intracellular iron homeostasis by facilitating the degradation and recycling of stored iron, thereby enabling its utilization in cellular processes, while concurrently mitigating iron-induced oxidative damage resulting from excessive iron accumulation [2]. Mancias et al. first identified and named the process of ferritinophagy by a discovering nuclear receptor coactivator 4 (NCOA4), a cargo receptor for iron autophagic degradation, through quantitative proteomics, and its mediated ferritin turnover contributes to elevated intracellular iron levels and ferroptosis [3]. The process of ferritinophagy is initiated under conditions when the levels of intracellular iron become low. When iron levels are low, the nuclear receptor corepressor of RE1-silencing transcription factor binds to the ferritin promoter region, leading to the transcriptional activation of the ferritin heavy and light chains [4], which resulting in an increasing of ferritin level and formation of ferritin nanoparticles within the cell. When iron levels rise again, autophagy receptors such as NCOA4 bind to ferritin nanoparticles and target them for degradation through the autophagy-lysosome pathway. The process of ferritinophagy ultimately leads to the release of iron ions from degraded ferritin molecules into the cytoplasm, allowing them to be utilized for cellular functions such as heme synthesis or the generation of ROS [5]. Ferritinophagy has been shown to play an important role in a variety of physiological processes, including cellular differentiation, erythropoiesis, and immune response [2]. Furthermore, dysregulation of ferritinophagy has been implicated in the pathophysiology of a variety of diseases, including cancer [6], neurodegenerative diseases [7], and iron overload disorders such as hemochromatosis [8]. Therefore, understanding the potential mechanisms of ferritinophagy may provide effective therapeutic strategies for the treatment of cancer. In this review, we summarize the recent progresses in ferritinophagy research and discuss the applications of ferritinophagy in cancer therapy.
铁蛋白自噬是自噬的一种选择性形式,专门针对细胞内铁蛋白进行降解。铁蛋白是一种由铁蛋白重链 (FTH1) 和铁蛋白轻链 (FTL) 亚基组成的蛋白质复合物,其功能是结合和储存细胞内多余的铁。 1 ]。铁蛋白吞噬在维持细胞内铁稳态方面发挥着至关重要的作用,通过促进储存的铁的降解和回收,从而使其能够在细胞过程中得到利用,同时减轻因铁积累过多而引起的铁诱导的氧化损伤。 2 ]。曼西亚斯等人。首先通过定量蛋白质组学发现核受体辅激活因子 4 (NCOA4)(一种铁自噬降解的货物受体),确定并命名了铁蛋白自噬过程,其介导的铁蛋白周转导致细胞内铁水平升高和铁死亡。 3 ]。当细胞内铁水平变低时,铁蛋白自噬过程就会启动。当铁水平较低时,RE1沉默转录因子的核受体辅阻遏物与铁蛋白启动子区域结合,导致铁蛋白重链和轻链的转录激活。 4 ],导致铁蛋白水平增加并在细胞内形成铁蛋白纳米颗粒。当铁水平再次升高时,NCOA4 等自噬受体会与铁蛋白纳米颗粒结合,并通过自噬-溶酶体途径将其靶向降解。铁蛋白自噬过程最终导致铁离子从降解的铁蛋白分子释放到细胞质中,使它们能够用于细胞功能,例如血红素合成或ROS的产生。 5 ]。 铁蛋白吞噬已被证明在多种生理过程中发挥重要作用,包括细胞分化、红细胞生成和免疫反应。 2 ]。此外,铁蛋白吞噬的失调与多种疾病的病理生理学有关,包括癌症。 6 ]、神经退行性疾病[ 7 ],以及铁超载疾病,例如血色素沉着症[ 8 ]。因此,了解铁蛋白自噬的潜在机制可能为癌症的治疗提供有效的治疗策略。在这篇综述中,我们总结了铁蛋白自噬研究的最新进展,并讨论了铁蛋白自噬在癌症治疗中的应用。
Main text 正文
Characteristics of ferritinophagy
铁蛋白自噬的特征
Ferritinophagy and autophagy
铁蛋白自噬和自噬
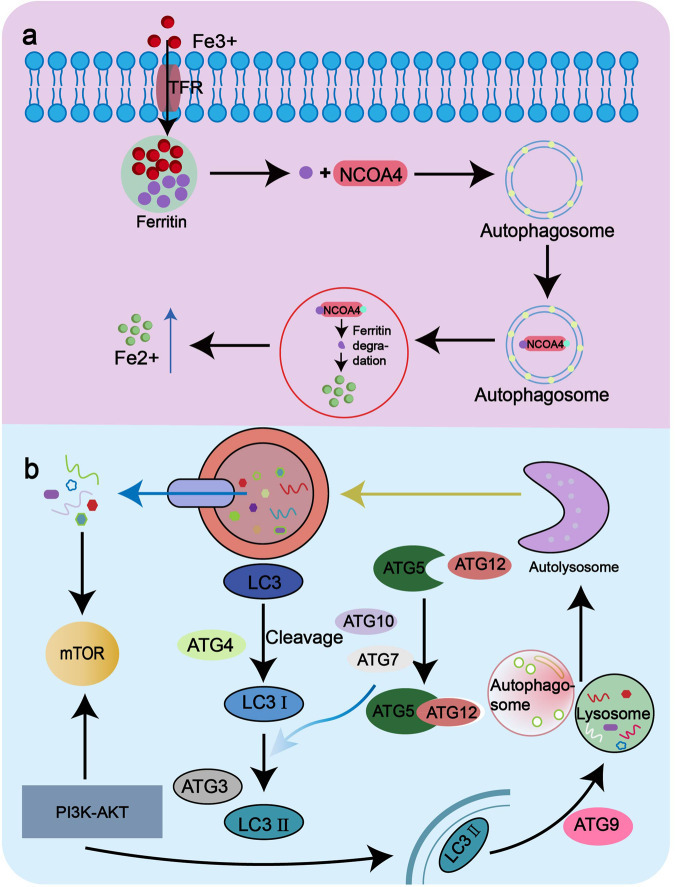
Ferritinophagy is a process by which cells degrade and recycle ferritin. And this process is mediated by autophagy, a cellular process in which damaged or unwanted cellular components are engulfed by double-membrane structures called autophagosomes and targeted for degradation by lysosomes. Ferritinophagy is a selective form of autophagy, and excess intracellular ferritin can induce iron-mediated apoptosis through autophagy by the selective autophagy receptor NCOA4 [9] which is the special molecule distinguished from other forms of autophagy (Fig. 1a). Furthermore, the process of ferritinophagy-induced cell death leads to the release of the proteoglycan decorin. This release serves as a signaling mechanism to activate both innate and adaptive immune responses, as well as the release of inflammatory factors including tumor necrosis factor and interleukin-6 (IL-6) [10, 11]. Inducing immunogenic necrosis of cancer cells can also activate anti-tumor immune response in vivo. ATP and HMGB1 are damage related substances of immunogenic cell death, which have been shown to be released during Ferroptosis and act as an immunogenic signal associated with the immunogenicity of iron-dead cancer cells [12]. Consequently, ferritinophagy exhibits distinct immune response characteristics. Moreover, ferritinophagy also has the characteristics of general autophagy, such as the altered expression content of autophagy-related genes autophagy related 5 (ATG5) and autophagy related 7 (ATG7) [13], increased LC3II/LC3I ratio [14] and genomic instability and mutations (Fig. 1b). It has been demonstrated that ferritinophagy in diffuse B-cell lymphoma cells triggered by eprenetapopt (APR-246) is characterized by tumor protein p53 (TP53) mutations [15]. Macroautophagy, endosomal microautophagy and ferritinophagy all also require the participation of Tax1 binding protein 1 (TAX1BP1) [16].
铁蛋白自噬是细胞降解和回收铁蛋白的过程。这个过程是由自噬介导的,自噬是一种细胞过程,其中受损或不需要的细胞成分被称为自噬体的双膜结构吞噬,并被溶酶体靶向降解。铁蛋白自噬是自噬的一种选择性形式,过量的细胞内铁蛋白可以通过选择性自噬受体 NCOA4 的自噬诱导铁介导的细胞凋亡。 9 ]是区别于其他形式自噬的特殊分子(图1)。 1a )。此外,铁蛋白吞噬诱导的细胞死亡过程导致蛋白聚糖核心蛋白聚糖的释放。这种释放作为一种信号机制来激活先天性和适应性免疫反应,以及炎症因子的释放,包括肿瘤坏死因子和白细胞介素 6 (IL-6)。 10 , 11 ]。诱导癌细胞的免疫原性坏死还可以激活体内抗肿瘤免疫反应。 ATP 和 HMGB1 是免疫原性细胞死亡的损伤相关物质,已被证明在铁死亡过程中释放,并充当与铁死亡癌细胞的免疫原性相关的免疫原性信号。 12 ]。因此,铁蛋白自噬表现出独特的免疫反应特征。此外,铁蛋白自噬还具有一般自噬的特征,如自噬相关基因自噬相关5(ATG5)和自噬相关7(ATG7)表达内容的改变。 13 ],增加 LC3II/LC3I 比率 [ 14 ]以及基因组不稳定性和突变(图1)。 1b )。 已证明 eprenetapopt (APR-246) 引发的弥漫性 B 细胞淋巴瘤细胞中的铁蛋白自噬以肿瘤蛋白 p53 (TP53) 突变为特征。 15 ]。巨自噬、内体微自噬和铁蛋白自噬也都需要 Tax1 结合蛋白 1 (TAX1BP1) 的参与 [ 16 ]。
Fig. 1. Autophagy pathway and ferritinophagy pathway.
图1.自噬途径和铁蛋白自噬途径。
a Ferritinophagy pathway. Transferrin receptor 1 (TfR1) interacts with Fe to transport iron into the cell where it is stored in the form of a ferritin complex. Subsequently, NCOA4 binds directly to FTH1 of the ferritin complex and is transported to the autophagosome. It binds to LC3 and is then transported to the autolysosome, where the ferritin complex is degraded to release ferrous. b Autophagy pathway. The substances to be degraded in the cell are first encapsulated by autophagy vesicles and then degraded by lysosomes. In the process of autophagy formation, cytoplasmic LC3-I generates lipidized LC3-II through the participation of ATG7 and ATG3. It attaches to the autophagosome membrane to form autophagosome membrane type LC3-II. LC3-II in the membrane was degraded by lysosomes along with the encapsulated contents.
铁蛋白自噬途径。转铁蛋白受体 1 (TfR1) 与 Fe 相互作用,将铁转运到细胞中,并以铁蛋白复合物的形式储存。随后,NCOA4 直接与铁蛋白复合物的 FTH1 结合,并被转运至自噬体。它与 LC3 结合,然后被转运至自溶酶体,在那里铁蛋白复合物被降解以释放亚铁。 b自噬途径。细胞内待降解的物质首先被自噬小泡包裹,然后被溶酶体降解。在自噬形成过程中,细胞质LC3-I通过ATG7和ATG3的参与生成脂质化LC3-II。它附着在自噬体膜上形成自噬体膜类型LC3-II。膜中的 LC3-II 与封装的内容物一起被溶酶体降解。
Ferritinophagy and ferroptosis
铁蛋白自噬和铁死亡
Ferritinophagy is the apoptotic pathway of ferroptosis, which has common characteristics with ferroptosis but different from ferroptosis (Table 1).
铁蛋白自噬是铁死亡的凋亡途径,与铁死亡有共同特征,但又不同于铁死亡(表 1 )。
Table 1. 表 1.
Comparison of ferroptosis and iron ferritinophagy autophagy characteristics.
铁死亡和铁铁蛋白自噬自噬特征的比较。
| ferritinophagy 铁蛋白自噬 | References 参考 | ferroptosis 铁死亡 | References 参考 | |
|---|---|---|---|---|
| mode of cell death 细胞死亡方式 | selective autophagy induced apoptosis 选择性自噬诱导细胞凋亡 |
[9] [ 9 ] | iron-dependent programmed cell death 铁依赖性程序性细胞死亡 |
[17] [ 17 ] |
| cellular morphology 细胞形态 | autophagosome 自噬体 | [35] [ 35 ] | cell swelling and plasma membrane rupture 细胞肿胀和质膜破裂 |
[40] [ 40 ] |
| autophagy receptor 自噬受体 | NCOA4 | [3] [ 3 ] | HPCAL1 | [27] [ 27 ] |
| organelle characteristics 细胞器特征 |
phagocytosis and digestion in lysozyme 溶菌酶的吞噬作用和消化作用 |
[3] [ 3 ] | Mitochondrial shrinkage, mitochondrial membrane density concentration, mitochondrial crest reduction or disappearance, mitochondrial membrane rupture 线粒体收缩、线粒体膜密度浓缩、线粒体嵴减少或消失、线粒体膜破裂 |
[21–25] [ 21 – 25 ] |
| molecular marker 分子标记 | ATG5, ATG7, LC3II/LC3Iratio, TP53 mutation ATG5、ATG7、LC3II/LC3I 比率、TP53 突变 |
[14–16] [ 14 – 16 ] | SSBP1, PTGS2, SLC7A11, ACSL4, RGS4 SSBP1、PTGS2、SLC7A11、ACSL4、RGS4 |
[28] [ 28 ] |
| immunological markers 免疫学标志物 | DCN | [10, 11] [ 10 , 11 ] | ATP, HMGB1 ATP、HMGB1 | [12] [ 12 ] |
| epigenetic regulation 表观遗传调控 | miRNA, Methylation and acetylation miRNA、甲基化和乙酰化 |
[29] [ 29 ] | Histone ubiquitination, methylation, miRNA, and the immune microenvironment 组蛋白泛素化、甲基化、miRNA 和免疫微环境 |
[30–33] [ 30 – 33 ] |
| cellular metabolism 细胞代谢 | protein degradation 蛋白质降解 | [3] [ 3 ] | Antioxidant system imbalance, amino acid metabolism disorder, nucleotide metabolism disorder 抗氧化系统失衡、氨基酸代谢紊乱、核苷酸代谢紊乱 |
[26] [ 26 ] |
Ferroptosis is an iron-dependent form of programmed cell death characterized by the accumulation of LPOs [17], which is generated by the iron-dependent oxidation of polyunsaturated fatty acids [18]. The accumulation of LPOs in the cell membrane leads to membrane damage and ultimately, cell death. Ferroptosis is regulated by a variety of factors, including iron, glutathione peroxidase 4 (GPX4), and other antioxidant enzymes [19, 20]. Additionally, ferroptosis disrupts the equilibrium between redox reactions and promotes oxidative damage to cellular organelles. This oxidative damage manifests in morphological changes such as smaller mitochondria, reduced or absent mitochondrial cristae, and concentrated rupture of the outer mitochondrial membrane [21–25]. In addition to the imbalance of antioxidant system caused by excessive accumulation of LPOs, the disorder of amino acid metabolism and nucleotide metabolism is also related to ferroptosis [26]. In contrast to the autophagy receptor NCOA4, which is unique to ferritinophagy, a new ferroptosis receptor, Hippocalcin like 1, is also recently identified [27]. Compared to the ferroptosis specific molecular markers GPX4, prostaglandin-endoperoxidesynthase2, solute carrier family 7 member 11 (SLC7A11), acyl-CoA synthetase long-chain family member 4, regulator of G-protein signaling 4, single stranded DNA binding protein 1 (SSBP1) [28], it has been observed that ferritinophagy controls disturbed protein mechanisms encoding and regulating iron transcripts, including differences in miRNA, methylation and acetylation [29]. Histone ubiquitination, methylation, miRNA, and the immune microenvironment also modulate ferroptosis in cancer cells [30–33].
铁死亡是一种铁依赖形式的程序性细胞死亡,其特征是 LPO 的积累。 17 ],它是由多不饱和脂肪酸的铁依赖性氧化产生的[ 18 ]。 LPO 在细胞膜中的积累会导致膜损伤,并最终导致细胞死亡。铁死亡受多种因素调节,包括铁、谷胱甘肽过氧化物酶 4 (GPX4) 和其他抗氧化酶。 19 , 20 ]。此外,铁死亡会破坏氧化还原反应之间的平衡,并促进细胞器的氧化损伤。这种氧化损伤表现为形态变化,例如线粒体变小、线粒体嵴减少或缺失以及线粒体外膜集中破裂。 21 – 25 ]。除了LPO过度积累导致抗氧化系统失衡外,氨基酸代谢和核苷酸代谢紊乱也与铁死亡有关。 26 ]。与铁蛋白自噬所特有的自噬受体 NCOA4 相比,最近还发现了一种新的铁死亡受体 Hippocalcin like 1。 27 ]。与铁死亡特异性分子标记 GPX4、前列腺素内过氧化物合酶 2、溶质载体家族 7 成员 11 (SLC7A11)、酰基辅酶 A 合成酶长链家族成员 4、G 蛋白信号调节因子 4、单链 DNA 结合蛋白 1 (SSBP1) 相比)[ 28 ],据观察,铁蛋白自噬控制编码和调节铁转录本的蛋白质机制受到干扰,包括 miRNA、甲基化和乙酰化的差异[ 29 ]。 组蛋白泛素化、甲基化、miRNA 和免疫微环境也调节癌细胞的铁死亡。 30 – 33 ]。
Although ferroptosis and ferritinophagy are distinct processes, they are both involved in the regulation of iron metabolism in cells. Ferroptosis can lead to the release of iron from cells, while ferritinophagy can increase the availability of iron for cellular use. The precise relationship between these two processes and their role in iron metabolism is an active area of research [34].
尽管铁死亡和铁蛋白自噬是不同的过程,但它们都参与细胞内铁代谢的调节。铁死亡可导致细胞释放铁,而铁蛋白自噬可增加细胞使用铁的可用性。这两个过程之间的精确关系及其在铁代谢中的作用是一个活跃的研究领域。 34 ]。
Ferritinophagy regulatory mechanism
铁蛋白自噬调控机制
Ferritinophagy is regulated by several mechanisms that control both synthesis and degradation of ferritin. One of the key regulatory mechanisms is the control of intracellular iron levels, which can trigger or inhibit the process of ferritinophagy in response to the changes in cellular iron homeostasis. First, ferritin binds to NCOA4 via the FTH1 subunit to form a complex. Next, the double walled proto-autophagosome membrane contains the ferritin-NCOA4 complex to form a completely closed structure. Finally, the autophagosomes transfer to the lysosomes and fuse with each other, and the ferritin degrades and releases iron in the lysosomes [35]. Among them, intracellular proteins involved in the regulation of iron homeostasis also affect the sensitivity of cells to ferritinophagy.
铁蛋白吞噬受到多种控制铁蛋白合成和降解的机制的调节。关键的调节机制之一是细胞内铁水平的控制,它可以响应细胞铁稳态的变化而触发或抑制铁蛋白自噬过程。首先,铁蛋白通过 FTH1 亚基与 NCOA4 结合形成复合物。接下来,双壁原自噬体膜含有铁蛋白-NCOA4复合物,形成完全封闭的结构。最后,自噬体转移到溶酶体并相互融合,铁蛋白在溶酶体中降解并释放铁。 35 ]。其中,参与铁稳态调节的细胞内蛋白也影响细胞对铁蛋白自噬的敏感性。
Ferritinophagy is dependent on the selective ferritin degradation of iron as it is sensitive to iron ions and the iron transport proteins expression. The intracellular iron atom has a complete iron regulatory network. Once the iron uptake increase, a decrease in iron export or stores due to autophagic degradation of ferritin can result in the development and progression of ferroptosis promoted [36, 37].
铁蛋白吞噬依赖于铁的选择性铁蛋白降解,因为它对铁离子和铁转运蛋白表达敏感。细胞内铁原子具有完整的铁调节网络。一旦铁摄入增加,铁蛋白自噬降解导致铁输出或储存减少,可导致铁死亡的发生和进展。 36 , 37 ]。
Regulation of ferritinophagy through NCOA4
通过 NCOA4 调节铁蛋白吞噬
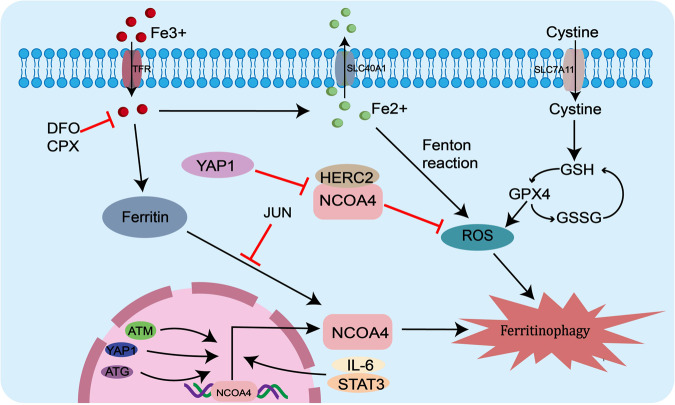
Ferritin is a cellular iron storage complex that can undergo lysosomal degradation via the selective autophagic adapter NCOA4, releasing iron for cellular use. Current studies have shown that alterations in many molecules can regulate the occurrence of iron autophagy by modulating the expression of NCOA4. For example, autophagy-associated genes (ATG) can activate NCOA4, and overexpression of NCOA4 promotes intracellular iron ion transportation and cellular ferritinophagy, thereby inducing ferroptosis [38, 39]. While knockdown of NCOA4 or ATG significantly reverses the reduction of transferrin, attenuates iron overload and lipid peroxidation, and alleviates cellular ferroptosis [40]. DNA double-strand break-related genes ataxia telangiectasia mutated (ATM) is indispensable in ferroptosis, and ATM can promote NCOA4-ferritin interactions to maintain ferritinophagy by phosphorylating NCOA4 [41]. Not only can changes at the molecular level affect NCOA4 expression, but signaling pathways can also regulate NCOA4 expression. In cells, NCOA4 binds to FTH1 and then connects to LC3II in the lysosome, releasing ferrous iron and enhancing the Fenton reaction to promote ferritinophagy, thus inducing the onset of ferroptosis [10, 42, 43]. The interaction between NCOA4 and FTH1 can be blocked by yes-associated protein 1, a key regulator of the Hippo signaling pathway, who can down-regulates GPX4, FTH1 and SLC7A11, and up-regulates siderofexin (SFXN1) and NCOA4 to reduce ROS production to inhibit hepatocyte ferroptosis [44]. NCOA4 is also regulated by the IL-6/STAT3 signaling pathway, which protects cardiomyocytes from ferritinophagy mediated ferroptosis by inhibiting STAT3 [45]. SUN et al. demonstrated that NCOA4 is dependent on the regulation of the JNK-JUN signaling pathway where JUN can bind to the promoter of NCOA4 to inhibit the interaction between NCOA4 and ferritin, thereby increasing ferritin autophagic degradation and promoting ferroptosis in chondrocytes [46]. In addition, heat damage or ionizing radiation can also cause cellular damage through NCOA4-mediated ferritinophagy [47–50]. All of above suggest that NCOA4 expression is associated with ferritinophagy in cancer cells [51, 52] (Fig. 2).
铁蛋白是一种细胞铁储存复合物,可以通过选择性自噬接头 NCOA4 进行溶酶体降解,释放铁供细胞使用。目前的研究表明,许多分子的改变可以通过调节NCOA4的表达来调节铁自噬的发生。例如,自噬相关基因(ATG)可以激活NCOA4,NCOA4的过度表达促进细胞内铁离子运输和细胞铁蛋白自噬,从而诱导铁死亡。 38 , 39 ]。虽然 NCOA4 或 ATG 的敲低可显着逆转转铁蛋白的减少,减轻铁过载和脂质过氧化,并减轻细胞铁死亡。 40 ]。 DNA双链断裂相关基因共济失调毛细血管扩张突变(ATM)在铁死亡中必不可少,ATM可以通过磷酸化NCOA4促进NCOA4-铁蛋白相互作用以维持铁蛋白自噬。 41 ]。不仅分子水平的变化可以影响NCOA4的表达,信号通路也可以调节NCOA4的表达。在细胞中,NCOA4与FTH1结合,然后在溶酶体中与LC3II连接,释放亚铁并增强Fenton反应,促进铁蛋白自噬,从而诱导铁死亡的发生。 10 , 42 , 43 ]。 NCOA4 和 FTH1 之间的相互作用可以被 yes 相关蛋白 1 阻断,yes 相关蛋白 1 是 Hippo 信号通路的关键调节因子,它可以下调 GPX4、FTH1 和 SLC7A11,上调 siderofexin (SFXN1) 和 NCOA4,从而减少 ROS 产生抑制肝细胞铁死亡[ 44 ]。 NCOA4 还受 IL-6/STAT3 信号通路的调节,通过抑制 STAT3 来保护心肌细胞免受铁蛋白自噬介导的铁死亡。 45 ]。孙等人。 证明NCOA4依赖于JNK-JUN信号通路的调节,其中JUN可以与NCOA4的启动子结合,抑制NCOA4与铁蛋白之间的相互作用,从而增加铁蛋白自噬降解并促进软骨细胞铁死亡。 46 ]。此外,热损伤或电离辐射也会通过 NCOA4 介导的铁蛋白自噬造成细胞损伤。 47 – 50 ]。所有这些都表明 NCOA4 表达与癌细胞中的铁蛋白自噬相关。 51 , 52 ] (如图。 2 )。
Fig. 2. The ferritinophagy pathway map is regulated by NCOA4.
图 2. 铁蛋白自噬途径图谱受 NCOA4 调节。
Molecules such as ATG, ATM, YAP1 and signaling pathways such as JNK-JUN can regulate the occurrence of intracellular ferritinophagy by regulating the expression of NCOA4. YAP1 can also inhibit cell death by blocking the interaction of NCOA4 with FTH1 and reducing the production of reactive oxygen species.
ATG、ATM、YAP1等分子和JNK-JUN等信号通路可以通过调节NCOA4的表达来调控细胞内铁蛋白自噬的发生。 YAP1 还可以通过阻断 NCOA4 与 FTH1 的相互作用并减少活性氧的产生来抑制细胞死亡。
Regulation of ferritinophagy through the Nrf2/HO-1 signaling pathway
通过 Nrf2/HO-1 信号通路调节铁蛋白自噬
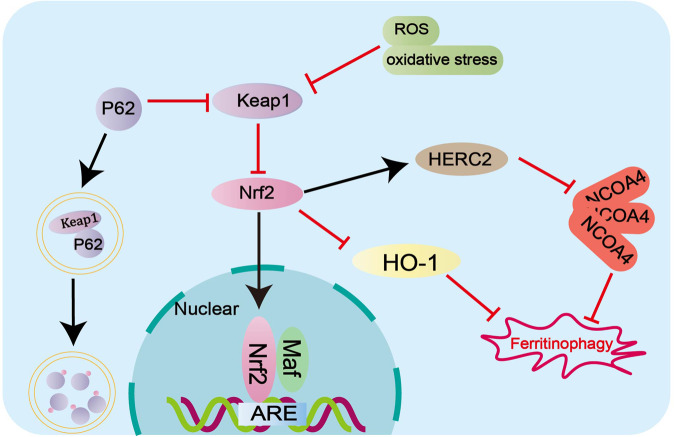
Heme oxygenase-1 (HO-1) is a rate-limiting enzyme in heme degradation that catalyzes the oxidative degradation of heme and the release of free iron, mediating cellular ferritinophagy and ferroptosis [53]. HO-1 is regulated by nuclear factor red lineage 2-related factor 2 (Nrf2), a key transcription factor that plays a protective role in ferroptosis. It has been demonstrated inhibiting the Nrf2/HO-1 signaling pathway is the feasibility of killing cancer cells [54, 55]. Also, Nrf2 controls the E3 ubiquitin protein ligase antibody (HERC2), which mediates NCOA4 turnover through the ubiquitin-proteasome system, reducing NCOA4 levels and resulting in blocked ferritinophagy [56]. Meanwhile, under conditions of oxidative stress, Nrf2 dissociates from Kelch-like ECH-associated protein 1 (Keap1), transfers Nrf2 to nucleus after accumulation and activates transcription of targeted anti-oxidative stress genes, thereby promoting cellular oxidative stress [57]. Moreover, Nrf2 can inhibit ferroptosis in colorectal cancer by binding with Keap1 to down-regulate Nrf2 [58]. It has been shown in previous studies that the p62-Keap1-Nrf2 pathway regulates protein conversion from cytoplasmic LC3, who is a characteristic protein in autophagy process, to membrane LC3II [59]. Therefore, Nrf2 has an effect on ferritinophagy-dependent ferroptosis through autophagy protein activation (Fig. 3).
血红素加氧酶-1 (HO-1) 是血红素降解中的限速酶,可催化血红素的氧化降解和游离铁的释放,介导细胞铁蛋白自噬和铁死亡。 53 ]。 HO-1 受核因子红色谱系 2 相关因子 2 (Nrf2) 的调节,Nrf2 是一种在铁死亡中发挥保护作用的关键转录因子。现已证明抑制Nrf2/HO-1信号通路具有杀死癌细胞的可行性[ 54 , 55 ]。此外,Nrf2 控制 E3 泛素蛋白连接酶抗体 (HERC2),该抗体通过泛素-蛋白酶体系统介导 NCOA4 周转,降低 NCOA4 水平并导致铁蛋白自噬受阻。 56 ]。同时,在氧化应激条件下,Nrf2与Kelch样ECH相关蛋白1(Keap1)解离,积累后将Nrf2转移至细胞核,并激活靶向抗氧化应激基因的转录,从而促进细胞氧化应激。 57 ]。此外,Nrf2 可以通过与 Keap1 结合下调 Nrf2 来抑制结直肠癌中的铁死亡。 58 ]。既往研究表明,p62-Keap1-Nrf2通路调节蛋白质从细胞质LC3(自噬过程中的特征蛋白)到膜LC3II的转化。 59 ]。因此,Nrf2通过自噬蛋白激活对铁蛋白自噬依赖性铁死亡产生影响(图1)。 3 )。
Fig. 3. Nrf2 signaling pathway regulates ferritinophagy.
图 3. Nrf2 信号通路调节铁蛋白自噬。
Not only can Nrf2/HO-1 signaling pathway and p62-Keap1-Nrf2 pathway regulate ferritinophagy, Nrf2 also controls E3 ubiquitin protein ligase antibody (HERC2), mediates NCOA4 turnover through the ubiquitin-proteasome system, and inhibits the occurrence of ferritinophagy.
Nrf2/HO-1信号通路和p62-Keap1-Nrf2通路不仅可以调节铁蛋白自噬,Nrf2还控制E3泛素蛋白连接酶抗体(HERC2),通过泛素-蛋白酶体系统介导NCOA4周转,抑制铁蛋白自噬的发生。
Other pathways modulate ferritinophagy
其他途径调节铁蛋白自噬
The regulation of ferritinophagy involves not only its characteristic markers, but also the coordination with other pathways. One such example is the association of the oncogene RAS with ferritinophagy, where its related KRAS-MAPK pathway enhances cell viability through the synthesis of mitochondrial sulfur-cluster proteins [60]. Additionally, the activation of ferritinophagy by the AMPK-mTOR-ULK1 axis is crucial for ferroptosis, as indicated by the increased levels of LC3 and MDA, and the decreased levels of FTH [61]. Moreover, the regulation of the SLC38A9-mTOR axis and cholesterol also play a role in the dependence of ferritinophagy [62]. A mutant of isocitrate dehydrogenase 1 (IDH1R132H) has been shown to enhance ferritinophagy in gliomas through the inhibition of the PRMT1-PTX3 axis signaling pathway [63]. Tripartite motif-containing protiens (TRIMs), a family of E3 ubiquitin ligases, play a crucial role in the degradation of endogenous proteins via the ubiquitin-proteasome system. Specifically, TRIM11 has been identified as an inhibitor of intracellular ferritinophagy by modulating NCOA4, ferritin, and Fe2+ levels through UBE2N/TAX1BP1 signaling [64]. Furthermore, circRNAs in non-coding RNAs have also been implicated in the regulation of ferritinophagy, exerting a positive regulatory effect on ferroptosis in hepatocellular carcinoma cells by suppressing ALKBH5-mediated autophagy inhibition [65]. Related studies have demonstrated that SLC7A11/GPX4 expression and ferritinophagy can not only direct the susceptibility to ferroptosis, respectively [62], but also the antioxidant SLC7A11/system xc-/xCT or GPX4 can over-activate ferritinophagy leading to ferroptosis [66, 67]. Therefore, GPX4 can regulate ferroptosis not only directly but also indirectly by regulating ferritinophagy [62, 68].
铁蛋白自噬的调控不仅涉及其特征标记,还涉及与其他途径的协调。其中一个例子是癌基因 RAS 与铁蛋白自噬的关联,其相关的 KRAS-MAPK 途径通过合成线粒体硫簇蛋白来增强细胞活力。 60 ]。此外,AMPK-mTOR-ULK1 轴激活铁蛋白吞噬对于铁死亡至关重要,LC3 和 MDA 水平升高以及 FTH 水平降低表明了这一点。 61 ]。此外,SLC38A9-mTOR轴和胆固醇的调节也在铁蛋白自噬的依赖性中发挥作用。 62 ]。异柠檬酸脱氢酶 1 (IDH1R132H) 的突变体已被证明可以通过抑制 PRMT1-PTX3 轴信号通路来增强神经胶质瘤中的铁蛋白自噬。 63 ]。含三联基序的蛋白质 (TRIM) 是 E3 泛素连接酶家族,在通过泛素-蛋白酶体系统降解内源蛋白方面发挥着至关重要的作用。具体来说,TRIM11 已被确定为细胞内铁蛋白自噬的抑制剂,通过 UBE2N/TAX1BP1 信号传导调节 NCOA4、铁蛋白和 Fe2+ 水平。 64 ]。此外,非编码RNA中的circRNA也参与铁蛋白自噬的调节,通过抑制ALKBH5介导的自噬抑制,对肝细胞癌细胞的铁死亡发挥积极的调节作用。 65 ]。相关研究表明,SLC7A11/GPX4表达和铁蛋白自噬不仅可以分别指导铁死亡的易感性。 62 ],而且抗氧化剂 SLC7A11/system xc-/xCT 或 GPX4 也可以过度激活铁蛋白自噬,导致铁死亡 [ 66 , 67 ]。 因此,GPX4不仅可以直接调节铁死亡,还可以通过调节铁蛋白自噬间接调节铁死亡。 62 , 68 ]。
Application of ferritinophagy in cancers
铁蛋白自噬在癌症中的应用
The process of cancer development involves alteration of many markers, and intracellular iron concentration and ferritin imbalance play an important role in ferritinophagy-mediated cancer cell growth. Ferritinophagy, an inducible pathway in the onset of ferroptosis, can induce ferroptosis by targeting ferritin, which provides a new strategy for cancer therapy. Dysfunction of the enzyme body autophagy pathway and impaired iron metabolism are associated with many diseases in humans [69]. Previous studies have confirmed that decreased iron transporter protein (FPN) and increased transferrin receptor (TFR1) are associated with prognosis in ovarian cancer [70]. In addition, TFR1 expression is also associated with prognosis in lung cancer [71], cervical cancer [72], and hepatocellular carcinoma [73]. A growing body of research evidence suggests the involvement of ferritinophagy in cancer evolution.
癌症发展过程涉及许多标志物的改变,细胞内铁浓度和铁蛋白失衡在铁蛋白自噬介导的癌细胞生长中起着重要作用。铁蛋白自噬是铁死亡发生的一种诱导途径,它可以通过靶向铁蛋白来诱导铁死亡,这为癌症治疗提供了新策略。酶体自噬途径的功能障碍和铁代谢受损与人类的许多疾病有关。 69 ]。先前的研究已证实铁转运蛋白(FPN)减少和转铁蛋白受体(TFR1)增加与卵巢癌的预后相关。 70 ]。此外,TFR1表达还与肺癌的预后相关。 71 ]、宫颈癌[ 72 ]和肝细胞癌[ 73 ]。越来越多的研究证据表明,铁蛋白吞噬参与了癌症的进化。
Application of ferritinophagy in anti-cancer
铁蛋白自噬在抗癌中的应用
Ferritinophagy promotes cell death and inhibit Epithelial-Mesenchymal Transition (EMT) by affecting the lipid peroxidation pathway
铁蛋白自噬通过影响脂质过氧化途径促进细胞死亡并抑制上皮间质转化(EMT)
Unrestricted cellular proliferation is a notable pathological characteristic observed in cancer. The process of ferritinophagy initiates lipid peroxidation, resulting in membrane impairment and cellular demise by degrading ferritin, all of which are closely linked to the progression of cancer. Genes or pathways that regulate the lipid peroxidation system have an impact on the growth and development of cancer cells [74, 75]. Genes or pathways that regulate the lipid peroxidation system have an impact on the growth and development of cancer cells. For instance, iron chelator 2-pyridylhydrazone dithiocarbamate s-acetate acid (PdtaA) has been found to enhance the production of reactive oxygen species (ROS) and induce cell cycle arrest in HepG2 cells through ferritinophagy [76]. Additionally, PdtaA down-regulates GPX4 and xCT, leading to ferroptosis. Another research team has demonstrated the chelator’s ability to inhibit epithelial-mesenchymal transition (EMT) in gastric cancer cells through ferritinophagy mediated the ROS/p53 pathway [77] (Fig. 4a). The resulting increased lipid peroxide production can promote autophagy-mediated ferroptosis in cancer cells.
不受限制的细胞增殖是癌症中观察到的显着病理特征。铁蛋白吞噬过程会引发脂质过氧化,通过铁蛋白降解导致膜损伤和细胞死亡,所有这些都与癌症的进展密切相关。调节脂质过氧化系统的基因或途径对癌细胞的生长和发育有影响。 74 , 75 ]。调节脂质过氧化系统的基因或途径对癌细胞的生长和发育有影响。例如,铁螯合剂 2-吡啶腙二硫代氨基甲酸酯 s-乙酸 (PdtaA) 已被发现可增强活性氧 (ROS) 的产生,并通过铁蛋白自噬诱导 HepG2 细胞的细胞周期停滞。 76 ]。此外,PdtaA 下调 GPX4 和 xCT,导致铁死亡。另一个研究小组证明了螯合剂能够通过铁蛋白自噬介导的 ROS/p53 途径抑制胃癌细胞的上皮间质转化 (EMT)。 77 ] (如图。 4a )。由此产生的脂质过氧化物产量增加可以促进癌细胞中自噬介导的铁死亡。
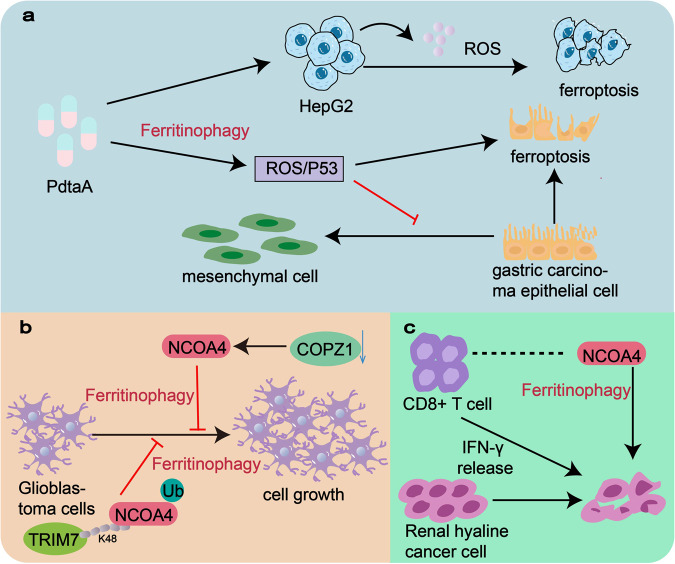
Fig. 4. Application of ferritinophagy in anti-cancer.
图4.铁蛋白自噬在抗癌中的应用。
a Iron chelator 2-pyridylhydrazone dithiocarbamate s-acetate acid (PdtaA) promotes ROS production and induces ferroptosis in HepG2 cells through ferritinophagy. Moreover, it can inhibit epithelial-mesenchymal transformation (EMT) of gastric cancer cells through the ROS/p53 pathway mediated by ferritinophagy. b Knockdown of coatomer protein complex subunit zeta 1 (COPZ1) inhibited glioblastoma (GBM) cell proliferation by increasing NCOA4. The K48-linked chain in TRIM7 binds directly to NCOA4 and ubiquitinates it, inhibiting the growth of human glioblastoma cells mediated by NCOA4 by promoting ferritinophagy. c Low expression of NCOA4 gene in renal clear cell carcinoma is associated with reduced immune cell infiltration and impaired IFN-γ receptor signaling pathways. Moreover, CD8+ T cells can induce cell death to eliminate cancer by specifically enhancing lipid peroxidation of cancer cells.
a铁螯合剂 2-吡啶腙二硫代氨基甲酸酯 s-乙酸 (PdtaA) 可促进 ROS 产生,并通过铁蛋白自噬诱导 HepG2 细胞铁死亡。此外,它还可以通过铁蛋白自噬介导的ROS/p53途径抑制胃癌细胞的上皮间质转化(EMT)。 b外壳蛋白复合物亚基 zeta 1 (COPZ1) 的敲低通过增加 NCOA4 抑制胶质母细胞瘤 (GBM) 细胞增殖。 TRIM7 中的 K48 连接链直接与 NCOA4 结合并将其泛素化,通过促进铁蛋白自噬来抑制 NCOA4 介导的人胶质母细胞瘤细胞的生长。 c肾透明细胞癌中 NCOA4 基因的低表达与免疫细胞浸润减少和 IFN-γ 受体信号通路受损有关。此外,CD8+ T细胞可以通过特异性增强癌细胞的脂质过氧化来诱导细胞死亡以消除癌症。
Ferritinophagy inhibits cancer cell growth through the mediation of NCOA4
铁蛋白自噬通过 NCOA4 的介导抑制癌细胞生长
Cellular autophagic processes inhibit cancer cell activity mediated by ferritin. The main mode of iron released from ferritin is selective autophagy mediated by NCOA4, therefore, NCOA4-mediated ferritinophagy has a key role in cancer progression. For instance, the inhibition of coatomer protein complex subunit zeta 1 (COPZ1) leads to a decrease in glioblastoma (GBM) cell proliferation through the upregulation of NCOA4 and the facilitation of ferritin degradation [78]. TRIM7’s K48-linked chain directly binds to NCOA4 and ubiquitinates it, thereby impeding the growth of human GBM cells mediated by NCOA4 and promoting ferritinophagy [79] (Fig. 4b).
细胞自噬过程抑制铁蛋白介导的癌细胞活性。铁蛋白释放铁的主要方式是NCOA4介导的选择性自噬,因此NCOA4介导的铁蛋白自噬在癌症进展中具有关键作用。例如,抑制外壳蛋白复合物亚基 zeta 1 (COPZ1) 通过上调 NCOA4 和促进铁蛋白降解,导致胶质母细胞瘤 (GBM) 细胞增殖减少。 78 ]。 TRIM7的K48连接链直接与NCOA4结合并将其泛素化,从而阻碍NCOA4介导的人GBM细胞的生长并促进铁蛋白自噬。 79 ] (如图。 4b )。
Ferritinophagy and immune response coordinate anti-cancer
铁蛋白自噬和免疫反应协调抗癌
The body is susceptible to interference by foreign pathogens when its immune function is reduced, and cancers are prone to occur at this time. Cancer cells that die from ferritinophagy release certain molecules to stimulate the immune system to fight against cancers, and enhanced ferritinophagy and immune activation can synergistically reinforce each other in inducing cancer cell death. NCOA4 not only facilitates cellular ferritinophagy but also plays a role in cancer cell-mediated immune responses. For instance, the diminished expression of NCOA4 in renal clear cell carcinoma has been found to govern unfavorable cancer prognosis and impaired infiltration of immune cells. In the present study, it was observed that CD8+ T cells activated through immunotherapy can effectively induce cancer cell death by augmenting lipid peroxidation in cancer cells [51] (Fig. 4c). Furthermore, NCOA4 can act as a target for macrophages to regulate iron overload and as a signal for inflammatory molecules [80].
人体免疫功能降低时,容易受到外来病原体的干扰,此时就容易发生癌症。死于铁蛋白自噬的癌细胞会释放某些分子来刺激免疫系统对抗癌症,增强的铁蛋白自噬和免疫激活可以协同增强彼此诱导癌细胞死亡的作用。 NCOA4 不仅促进细胞铁蛋白自噬,还在癌细胞介导的免疫反应中发挥作用。例如,已发现肾透明细胞癌中 NCOA4 表达的减少会导致癌症预后不良和免疫细胞浸润受损。在本研究中,观察到通过免疫疗法激活的CD8+ T细胞可以通过增强癌细胞中的脂质过氧化来有效诱导癌细胞死亡。 51 ] (如图。 4c )。此外,NCOA4 可以作为巨噬细胞的靶标来调节铁过载,并作为炎症分子的信号。 80 ]。
Ferritinophagy in non-cancer
非癌症中的铁蛋白自噬
Currently, ferritinophagy primarily exhibits an anti-cancer effect in cancers, although there is evidence suggesting that the regulation of ferritinophagy-related pathways does not impact cell function. For example, the silencing of NCOA4 in colon cancer cells did not yield any noteworthy disparity in the expression of ferritin and TFR1, thereby suggesting that the growth of colon cancer cells does not necessitate the participation of ferritinophagy [81]. During the investigation into the process of lipid peroxide formation in ferritinophagy, a research team conducted experiments that revealed the ability of the ferroptosis inducer erastin to promote ferritinophagy in HeLa cells expressing NCOA4. Conversely, another ferroptosis inducer, RAS-selective lethal 3, was found to delay the death of HeLa cells overexpressing NCOA4 [82]. This demonstrates that whether ferritinophagy can regulate the onset of ferroptosis depended on the inducing compounds and the downstream pathways activated by cell death.
目前,铁蛋白自噬主要在癌症中表现出抗癌作用,尽管有证据表明铁蛋白自噬相关途径的调节不会影响细胞功能。例如,结肠癌细胞中 NCOA4 的沉默并未导致铁蛋白和 TFR1 的表达出现任何显着差异,从而表明结肠癌细胞的生长不需要铁蛋白自噬的参与。 81 ]。在研究铁蛋白自噬中脂质过氧化物形成过程的过程中,一个研究小组进行的实验揭示了铁死亡诱导剂erastin在表达NCOA4的HeLa细胞中促进铁蛋白自噬的能力。相反,另一种铁死亡诱导剂 RAS 选择性致死 3 被发现可以延迟过度表达 NCOA4 的 HeLa 细胞的死亡。 82 ]。这表明铁蛋白自噬是否可以调节铁死亡的发生取决于诱导化合物和细胞死亡激活的下游途径。
Application of targeting ferritinophagy in cancer treatment
靶向铁蛋白自噬在癌症治疗中的应用
Chemotherapy exert anti-cancer effect by targeting ferritinophagy
化疗通过靶向铁蛋白吞噬发挥抗癌作用
Autophagy is an adaptive response to metabolic and therapeutic stress in a manner that can be applied to clinical therapeutic targets and prognostic monitoring. Regulation of any process in the ferritinophagy pathway can lead to cellular alterations. In the first place, NCOA4 plays a role in cancer therapy. Studies have shown that lipopolysaccharide (LPS) can regulate ferritin conversion by inhibiting NCOA4 as a therapeutic target for cancer [83]. Powdered tetrandrine citrate (TetC), a novel highly water-soluble powdered cycloheximide salt, has strong anticancer activity not only in chronic myeloid leukemia, but also in breast cancer cells by inhibiting GPX4 expression and activation of NCOA4 to promote ferritinophagy in cancer cells inducing ferrogenic cell death [80]. In addition, other pathways have been validated in ferritinophagy-mediated cancer therapy. For example, the process of iron transportation into mitochondria by lysosomes during ferritinophagy can serve as a potential target for enhancing pancreatic cancer survival rates and overcoming treatment resistance [84]. The reactive oxygen species (ROS) generated through ferritinophagy can activate the p53 and PHD2/HIF-1α signaling pathways, while also inhibiting the iron chelator 2,2’-di-pyridylketone hydrazone dithiocarbamate s-butyric acid (DpdtbA), thereby inducing EMT in gastric cancer cells [85]. The administration of the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells reliant on ferritinophagy triggers the ferroptosis process. Consequently, cancer cells treated with JQ1 exhibit an increased LC3II/LC3I ratio, reduced FTH1 expression, and decreased expression of autophagy-related genes ATG5 and ATG7 [86]. Above demonstrating that more and more ferritinophagy-related molecules are becoming targets in cancer therapy.
自噬是对代谢和治疗应激的适应性反应,可应用于临床治疗目标和预后监测。调节铁蛋白自噬途径中的任何过程都可能导致细胞改变。首先,NCOA4 在癌症治疗中发挥作用。研究表明,脂多糖(LPS)可以通过抑制NCOA4来调节铁蛋白转化,作为癌症的治疗靶点。 83 ]。粉防己碱柠檬酸盐(TetC)是一种新型高水溶性粉状放线菌酮盐,不仅对慢性粒细胞白血病具有很强的抗癌活性,而且对乳腺癌细胞也具有很强的抗癌活性,通过抑制GPX4的表达和NCOA4的激活,促进癌细胞中铁蛋白的自噬,从而诱导铁生成细胞死亡[ 80 ]。此外,其他途径已在铁蛋白自噬介导的癌症治疗中得到验证。例如,铁蛋白吞噬过程中铁通过溶酶体转运到线粒体的过程可以作为提高胰腺癌存活率和克服治疗耐药性的潜在靶标。 84 ]。铁蛋白吞噬产生的活性氧(ROS)可以激活p53和PHD2/HIF-1α信号通路,同时抑制铁螯合剂2,2'-二吡啶酮腙二硫代氨基甲酸酯s-丁酸(DpdtbA),从而诱导EMT在胃癌细胞中[ 85 ]。在依赖铁蛋白自噬的癌细胞中施用溴结构域蛋白 BRD4 抑制剂 (+)-JQ1 会触发铁死亡过程。因此,用 JQ1 处理的癌细胞表现出 LC3II/LC3I 比率增加、FTH1 表达减少以及自噬相关基因 ATG5 和 ATG7 表达减少。 86 ]。 以上表明越来越多的铁蛋白自噬相关分子正在成为癌症治疗的靶标。
Therapeutic mechanisms for common anticancer drugs have also been found in the ferritinophagy pathway. Low-dose cisplatin combined with ursolic acid inhibits cancer cell growth by activating autophagic degradation of ferritin and overloading intracellular iron ions [87]. Heavy floor saponin formosaninC (FC) induces ROS formation and GPX4 damage, promoting ferroptosis and ferritinophagy in triple-negative breast cancer MDA-MB-231 cells and increasing their chemosensitivity to cisplatin [88]. The combination of artesunate and hepatocellular carcinoma advanced first-line drug sorafenib induces oxidative stress and ferritinophagy in hepatocellular carcinoma cells and promotes lysosome-dependent FTL degradation to improve the efficacy of single drug [89]. Clinically, we need to know not only the drug efficacy, but also the adverse effects and the conditions of application of each drug to avoid additional serious damage to patients body. Rifampicin, the most common causative agent of antituberculosis drug-induced liver injury (AT-DILI), can reduce the hepatotoxicity of rifampicin by activating the autophagic pathway to reduce ferritinophagy and ferroptosis [90]. Additionally, the anticancer antibiotic Adriamycin was observed to enhance ferritinophagy by affecting the SPATA2/CYLD pathway, leading to NCOA4 depletion and ferroptosis induction in cardiomyocytes [91]. Carboplatin, a first-line drug for the treatment of human retinoblastoma (RB), develops acquired multidrug resistance (MDR) after long-term treatment, and its resistant mechanism can be eliminated by inducing autophagy-dependent ferroptosis [92]. In addition, it has also been recognized that the same drug efficacy can be exerted through multiple pathways. Dihydroartemisinin, a derivative of artemisinin, has been shown to have cytotoxic effects on a variety of malignant cancer cells of human origin, which can induce ferritinophagy and lysosomal degradation resulting in cell death in an autophagy-independent manner [93].
常见抗癌药物的治疗机制也在铁蛋白自噬途径中被发现。小剂量顺铂联合熊果酸通过激活铁蛋白自噬降解和细胞内铁离子超载抑制癌细胞生长。 87 ]。重地板皂苷 formosaninC (FC) 诱导 ROS 形成和 GPX4 损伤,促进三阴性乳腺癌 MDA-MB-231 细胞的铁死亡和铁蛋白自噬,并增加其对顺铂的化学敏感性。 88 ]。青蒿琥酯与肝细胞癌先进一线药物索拉非尼联用,诱导肝细胞癌细胞氧化应激和铁蛋白自噬,促进溶酶体依赖性FTL降解,提高单药疗效。 89 ]。临床上,我们不仅需要了解药物的疗效,还需要了解每种药物的不良反应以及使用条件,避免对患者身体造成额外的严重损害。利福平是抗结核药物性肝损伤(AT-DILI)最常见的致病因子,可通过激活自噬途径减少铁蛋白自噬和铁死亡,从而降低利福平的肝毒性。 90 ]。此外,抗癌抗生素阿霉素被观察到通过影响 SPATA2/CYLD 通路来增强铁蛋白自噬,导致心肌细胞中 NCOA4 耗竭和铁死亡诱导。 91 ]。卡铂是治疗人视网膜母细胞瘤(RB)的一线药物,长期治疗后会产生获得性多药耐药(MDR),其耐药机制可通过诱导自噬依赖性铁死亡来消除。 92 ]。此外,人们还认识到,相同的药物功效可以通过多种途径发挥出来。 双氢青蒿素是青蒿素的衍生物,已被证明对多种人类来源的恶性癌细胞具有细胞毒作用,可以诱导铁蛋白自噬和溶酶体降解,从而以不依赖自噬的方式导致细胞死亡。 93 ]。
Nanomaterials and photodynamic therapy acts anti-cancer effect by targeting ferritinophagy
纳米材料和光动力疗法通过靶向铁蛋白吞噬发挥抗癌作用
Nanomaterials have emerged as a valuable tool in the field of cancer treatment, particularly through their ability to modulate the ferritinophagy pathway. For instance, MoS2 nanosheets have been found to induce ferroptosis by degrading ferritin within the lysosome and inhibiting the function of recombinant FPN via NCOA4-dependent [94]. Moreover, nanomaterial-based systems have also been shown to connect ferritinophagy with the anticancer effects of the immune system. In this context, Zinc oxide nanoparticles exhibit promising biomedical applications by leveraging microautophagy/autophagy to induce ferroptosis in human umbilical vein endothelial cells, leading to vascular inflammation and ferritinophagy [95].
纳米材料已成为癌症治疗领域的宝贵工具,特别是通过其调节铁蛋白吞噬途径的能力。例如,MoS2 纳米片被发现可通过降解溶酶体内的铁蛋白并通过 NCOA4 依赖性抑制重组 FPN 的功能来诱导铁死亡。 94 ]。此外,基于纳米材料的系统也被证明可以将铁蛋白自噬与免疫系统的抗癌作用联系起来。在这种背景下,氧化锌纳米粒子通过利用微自噬/自噬诱导人脐静脉内皮细胞铁死亡,导致血管炎症和铁蛋白自噬,从而展现出有前景的生物医学应用。 95 ]。
In addition to common pharmacological treatments, photodynamic therapy (PDT) has established a new targeted therapeutic approach for osteosarcoma. PDT can promote ferroptosis in human osteosarcoma cells through NCOA4-mediated ferritinophagy and GPX4 inactivation, synergistic over-accumulation of lipid peroxides (LPOs), and significant induction of cytochrome c-activated mitochondrial apoptosis [96]. Immunity is closely related to cancers and activation of the immune system can also co-mediate cancer therapy with activation of ferritinophagy [97]. PDT-mediated activation of T cells and ferroptosis activation are connected, suggesting a theoretical basis for a new paradigm of cancer therapy [98]. Related studies have confirmed that increased ferroptosis can also promote the anticancer effects of immunotherapy. For instance, testosterone has been found to have a significant impact on prostate cancer, as supraphysiological levels of testosterone can impede the growth of prostate cells through ferritinophagy and nuclear autophagy. Additionally, it activates immune signaling pathways driven by nucleic acid sensors, leading to an increased migration of cytotoxic immune cells to the cancer site [99].
除了常见的药物治疗外,光动力疗法(PDT)为骨肉瘤建立了新的靶向治疗方法。 PDT 可通过 NCOA4 介导的铁蛋白自噬和 GPX4 失活、脂质过氧化物 (LPO) 的协同过度积累以及显着诱导细胞色素 c 激活的线粒体凋亡来促进人骨肉瘤细胞的铁死亡。 96 ]。免疫与癌症密切相关,免疫系统的激活也可以与铁蛋白自噬的激活共同介导癌症治疗。 97 ]。 PDT 介导的 T 细胞激活和铁死亡激活是相关的,这为癌症治疗的新范式提供了理论基础。 98 ]。相关研究证实,铁死亡增加也能促进免疫疗法的抗癌作用。例如,睾酮已被发现对前列腺癌有显着影响,因为超生理水平的睾酮可通过铁蛋白自噬和核自噬阻碍前列腺细胞的生长。此外,它还激活由核酸传感器驱动的免疫信号通路,导致细胞毒性免疫细胞向癌症部位的迁移增加。 99 ]。
Immunity plays an anti-cancer role by targeting ferritinophagy
免疫通过靶向铁蛋白吞噬发挥抗癌作用
The human immune system has the role of immune surveillance, defense and regulation, which is closely related to the occurrence and development of cancers. When the immune function of body decreases, the cancer is easy to invade, so the cancer immunity can be used to study the occurrence, development and regression of cancers [100]. Furthermore, the search for cancer markers remains an ongoing and extensive endeavor. The emergence of new antigenic markers for cancer cells is a prominent immunological feature of cancer cells [101], and the release of inflammatory factors by the immune system stimulated by ferritinophagy death cells [7] provides us with good direction. Immune checkpoint blockade is a powerful oncologi inflammatory factors treatment modality for a wide range of human malignancies [102], and targeting the cancer ferritinophagy pathway might be an immune checkpoint blockade therapy. Preclinical data suggest that immune checkpoint blockade can act synergistically with radiotherapy through agonism of innate immune sensing pathways activated by DNA damage [103]. Notably, two separate studies have concurrently demonstrated that immunotherapy-activated CD8+ T cells augment lipid peroxidation in ferroptosis cancer cells. Furthermore, it has been observed that heightened ferroptosis contributes to the effectiveness of immunotherapy in combating cancer [104, 105]. In addition, the reduction in iron and FTH due to ferroptosis inhibition impedes dendritic cell-mediated anticancer immunity and therefore may interfere with immunotherapy [106, 107]. How the induction of ferroptosis through ferritinophagy modulates immune efficacy is still unknown, and the mechanisms controlling the immune response to specific ferritinophagy remain poorly defined. A current challenge in oncology is to effectively integrate immunotherapy with conventional therapies, involved radiotherapy. Recent years have provided insights into the molecular mechanisms [108], pharmacological regulation [109] and functional significance of ferritinophagy in health and disease [110]. However, the mediators of its immune response elicitation remain poorly defined. Immunogenic cell death is an important factor in the success of anticancer therapy [111]. Although it is promising that the application of the ferritinophagy pathway to therapy, how ferritinophagy works with the immune system to fight cancer cell processes remains unclear.
人体免疫系统具有免疫监视、防御和调节作用,与癌症的发生、发展密切相关。当机体免疫功能下降时,癌症就容易侵袭,因此可以利用癌症免疫来研究癌症的发生、发展和消退。 100 ]。此外,对癌症标志物的寻找仍然是一项持续且广泛的努力。癌细胞新抗原标记的出现是癌细胞的一个突出的免疫学特征。 101 ],以及铁蛋白吞噬死亡细胞刺激的免疫系统释放炎症因子[ 7 ] 为我们提供了良好的方向。免疫检查点阻断是一种针对多种人类恶性肿瘤的强大的肿瘤炎症因子治疗方式。 102 ],针对癌症铁蛋白吞噬途径可能是一种免疫检查点阻断疗法。临床前数据表明,免疫检查点阻断可以通过 DNA 损伤激活的先天免疫传感途径的激动作用与放射治疗产生协同作用。 103 ]。值得注意的是,两项独立的研究同时证明,免疫疗法激活的 CD8+ T 细胞会增强铁死亡癌细胞中的脂质过氧化作用。此外,据观察,铁死亡的加剧有助于免疫疗法对抗癌症的有效性。 104 , 105 ]。此外,铁死亡抑制导致铁和 FTH 的减少会阻碍树突状细胞介导的抗癌免疫,因此可能会干扰免疫治疗。 106 , 107 ]。 通过铁蛋白自噬诱导铁死亡如何调节免疫功效仍不清楚,并且控制对特定铁蛋白自噬的免疫反应的机制仍不清楚。肿瘤学当前的挑战是将免疫疗法与传统疗法(包括放射疗法)有效整合。近年来,人们对分子机制有了深入的了解[ 108 ]、药理调节[ 109 ] 铁蛋白自噬在健康和疾病中的功能意义 [ 110 ]。然而,其免疫反应引发的介质仍然不明确。免疫原性细胞死亡是抗癌治疗成功的重要因素[ 111 ]。尽管铁蛋白自噬途径在治疗中的应用前景广阔,但铁蛋白自噬如何与免疫系统一起对抗癌细胞过程仍不清楚。
Conclusion 结论
Great progress has been made in the discovery of ferritinophagy research. Many studies have shown that ferritinophagy is closely related to the occurrence and development of cancer. Therefore, exploring the characteristics and regulatory mechanisms of ferritinophagy will help us to better understand its role in cancer. In the previous sections we have learned that ferroptosis is often accompanied by the occurrence of cell swelling and plasma membrane rupture, whereas apoptotic cells often exhibit cell shrinkage and blistering of the plasma membrane. The autophagic degradation pathway usually protects cells from apoptosis, but selective ferritinophagy promotes iron cell apoptosis [40], which enlightens us whether ferritinophagy has different features from ferroptosis in terms of cell morphological changes.
铁蛋白自噬研究的发现取得了巨大进展。许多研究表明,铁蛋白自噬与癌症的发生、发展密切相关。因此,探索铁蛋白自噬的特征和调控机制将有助于我们更好地了解其在癌症中的作用。在前面的章节中我们了解到,铁死亡常常伴随着细胞肿胀和质膜破裂的发生,而凋亡细胞则常常表现出细胞皱缩和质膜起泡。自噬降解途径通常可以保护细胞免于凋亡,但选择性铁蛋白自噬会促进铁细胞凋亡。 40 ],这揭示了铁蛋白自噬在细胞形态变化方面是否具有与铁死亡不同的特征。
At present, there are teams that have linked iron autophagy with clinical drugs to anti-cancer in vitro, which greatly accelerates the application of iron autophagy in preclinical. Currently, targeted therapy is a widely used therapeutic approach, but still generates resistance to drug-induced autophagy and death in cancer cells [112]. Ferritinophagy, the death of non-apoptotic cells, is an emerging cancer therapeutic target, which has the potential to resist the therapeutic effect of cancer therapy without apoptosis [113]. It still has very important clinical significance to target ferritinophagy against cancer as there are no clinically relevant drugs for cancer treatment, but the application of nanomaterials in iron autophagy brings us new hope in the treatment of cancer. However, a lot of efforts are needed to move from preclinical research to clinical application. We should not only explore the application of single drugs in cancers, but also urgently search for combination drugs to improve the efficacy of drug therapy. Exploring the synergistic effect of immunotherapy and biotherapy combined in ferritinophagy provides us with a good way forward.
目前已有团队将铁自噬与临床药物联系起来,进行体外抗癌,大大加速了铁自噬在临床前的应用。目前,靶向治疗是一种广泛使用的治疗方法,但仍然会产生对药物诱导的自噬和癌细胞死亡的抵抗力。 112 ]。铁蛋白自噬,即非凋亡细胞的死亡,是一种新兴的癌症治疗靶点,有可能在不发生细胞凋亡的情况下抵抗癌症治疗的治疗效果。 113 ]。由于目前还没有临床相关的癌症治疗药物,靶向铁蛋白自噬对抗癌症仍然具有非常重要的临床意义,但纳米材料在铁自噬中的应用给我们带来了癌症治疗的新希望。然而,从临床前研究到临床应用还需要付出很多努力。我们不仅要探索单一药物在癌症中的应用,还迫切需要寻找联合药物以提高药物治疗的疗效。探索免疫治疗和生物治疗联合在铁蛋白自噬中的协同作用为我们提供了一个很好的前进方向。
Acknowledgements 致谢
We wish to thank lab members for their valuable and enthusiastic work and scientific discussion.
我们要感谢实验室成员的宝贵而热情的工作和科学讨论。
Author contributions 作者贡献
JW and NW reviewed the literature and made original draft preparation; MP, LO, XJ, QP and YZ reviewed the literature; ZH and QL designed the outline and revised the manuscript. All authors read and approved the final manuscript.
JW和NW审阅文献并做出初稿准备; MP、LO、XJ、QP 和 YZ 审阅了文献; ZH和QL设计了大纲并修改了手稿。所有作者阅读并批准了最终手稿。
Funding 资金
This work was supported in part by grants from the following sources: the National Natural Science Foundation of China (82203233, 82202966, 82173142, 81972636), the Natural Science Foundation of Hunan Province (2023JJ60469, 2023JJ40413, 2023JJ30372, 2023JJ30375, 2022JJ80078, 2020JJ5336), the Research Project of Health Commission of Hunan Province (202203034978, 202109031837, 20201020), Hunan Provincial Science and Technology Department (2020TP1018), Ascend Foundation of National cancer center (NCC201909B06), and by Hunan Cancer Hospital Climb Plan (ZX2020001-3, YF2020002).
这项工作得到了以下来源的部分资助:国家自然科学基金(82203233、82202966、82173142、81972636)、湖南省自然科学基金(2023JJ60469、2023JJ40413、2023JJ30372、 2023JJ30375、2022JJ80078、2020JJ5336)、湖南省卫健委科研项目(202203034978、202109031837、20201020)、湖南省科技厅项目(2020TP1018)、国家癌症中心登高基金(NCC201909B06),以及湖南省肿瘤医院攀登计划(ZX2020001-3,YF2020002)。
Competing interests 利益竞争
The authors declare no competing interests.
作者声明没有竞争利益。
Footnotes 脚注
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
出版商说明施普林格·自然对于已出版地图和机构隶属关系中的管辖权主张保持中立。
These authors contributed equally: Jiewen Wang, Nayiyuan Wu.
这些作者做出了同等贡献:Jiewen Wang、Nayiyuan Wu。
Contributor Information 贡献者信息
Zuping He, Email: zupinghe@hunnu.edu.cn.
何祖平,Email:zupinghe@hunnu.edu.cn。
Qianjin Liao, Email: march-on@126.com.
廖前进,电子邮件:march-on@126.com。
References 参考
-
1.Dai Y, Zhu C, Xiao W, Chen X, Cai Y. Mycobacterium tuberculosis induces host autophagic ferritin degradation for enhanced iron bioavailability and bacterial growth. Autophagy. 2023:1–3. [DOI] [PMC free article] [PubMed]
1.戴Y,朱C,肖W,陈X,蔡Y。结核分枝杆菌诱导宿主自噬铁蛋白降解,以增强铁的生物利用度和细菌生长。自噬。 2023:1–3。 [ DOI ] [ PMC free article ] [ PubMed ] -
2.Jin X, Jiang C, Zou Z, Huang H, Li X, Xu S, et al. Ferritinophagy in the etiopathogenic mechanism of related diseases. J Nutr Biochem. 2023;117:109339. doi: 10.1016/j.jnutbio.2023.109339IF: 4.8 Q1 . [DOI] [PubMed] [Google Scholar]
2.金晓,蒋超,邹Z,黄华,李晓,徐S,等。铁蛋白自噬在相关疾病的发病机制中的作用。 J 营养生物化学。 2023;117:109339。 DOI:10.1016/j.jnutbio.2023.109339如果:4.8 Q1 。 [ DOI ] [ PubMed ] [ Google Scholar ] -
3.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–9. doi: 10.1038/nature13148IF: 50.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
3.曼西亚斯JD、王X、吉吉SP、哈珀JW、金梅尔曼AC。定量蛋白质组学将 NCOA4 确定为介导铁蛋白自噬的货物受体。自然。 2014;509:105–9。 doi:10.1038/nature13148如果:50.5 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
4.Maksour S, Ooi L, Dottori M. More than a Corepressor: The role of CoREST proteins in neurodevelopment. eNeuro. 2020;7. [DOI] [PMC free article] [PubMed]
4. Maksour S、Ooi L、Dottori M。不仅仅是辅阻遏物:CoREST 蛋白在神经发育中的作用。电子神经。 2020;7。 [ DOI ] [ PMC free article ] [ PubMed ] -
5.Rochette L, Dogon G, Rigal E, Zeller M, Cottin Y, Vergely C. Lipid peroxidation and iron metabolism: two corner stones in the homeostasis control of ferroptosis. Int J Mol Sci. 2022;24:449. doi: 10.3390/ijms24010449IF: 4.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
5. Rochette L、Dogon G、Rigal E、Zeller M、Cottin Y、Vergely C。脂质过氧化和铁代谢:铁死亡稳态控制的两个基石。国际分子科学杂志。 2022 年;24:449。号码:10.3390/ijms24010449如果:4.9 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
6.Santana-Codina N, Del Rey MQ, Kapner KS, Zhang H, Gikandi A, Malcolm C, et al. NCOA4-mediated ferritinophagy is a pancreatic cancer dependency via maintenance of iron bioavailability for iron-sulfur cluster proteins. Cancer Discov. 2022;12:2180–97. doi: 10.1158/2159-8290.CD-22-0043IF: 29.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
6. Santana-Codina N、Del Rey MQ、Kapner KS、Zhang H、Gikandi A、Malcolm C 等。 NCOA4 介导的铁蛋白自噬是通过维持铁硫簇蛋白的铁生物利用度而产生的胰腺癌依赖性。癌症发现。 2022;12:2180–97。号码:10.1158/2159-8290.CD-22-0043如果:29.7 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
7.Lee J, Hyun DH. The interplay between intracellular iron homeostasis and neuroinflammation in neurodegenerative diseases. Antioxid (Basel, Switzerland) 2023;12:918. doi: 10.3390/antiox12040918IF: 6.0 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
7.李J,玄DH。神经退行性疾病中细胞内铁稳态与神经炎症之间的相互作用。抗氧化剂(瑞士巴塞尔)2023;12:918。 DOI:10.3390/antiox12040918如果:6.0 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
8.Kotla NK, Dutta P, Parimi S, Das NK. The role of ferritin in health and disease: recent advances and understandings. Metabolites. 2022;12:609. doi: 10.3390/metabo12070609IF: 3.4 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
8.科特拉·NK、杜塔·P、帕里米·S、达斯·NK。铁蛋白在健康和疾病中的作用:最新进展和理解。代谢物。 2022;12:609。 doi:10.3390/metabo12070609如果:3.4 Q2 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
9.Zhu ZH, Xu XT, Shen CJ, Yuan JT, Lou SY, Ma XL, et al. A novel sesquiterpene lactone fraction from Eupatorium Chinense L. suppresses hepatocellular carcinoma growth by triggering ferritinophagy and mitochondrial damage. Phytomedicine. 2023;112:154671. doi: 10.1016/j.phymed.2023.154671IF: 6.7 Q1 . [DOI] [PubMed] [Google Scholar]
9.朱振华,徐晓霞,沉成杰,袁建涛,楼思义,马晓龙,等。一种来自紫茎泽兰的新型倍半萜内酯成分通过引发铁蛋白自噬和线粒体损伤来抑制肝细胞癌的生长。植物药。 2023;112:154671。 doi:10.1016/j.phymed.2023.154671如果:6.7 Q1 。 [ DOI ] [ PubMed ] [ Google Scholar ] -
10.Lee J, You JH, Roh JL. Poly(rC)-binding protein 1 represses ferritinophagy-mediated ferroptosis in head and neck cancer. Redox Biol. 2022;51:102276. doi: 10.1016/j.redox.2022.102276IF: 10.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
10.李杰,尤杰华,卢杰林。 Poly(rC) 结合蛋白 1 抑制头颈癌中铁蛋白自噬介导的铁死亡。氧化还原生物。 2022;51:102276。 doi:10.1016/j.redox.2022.102276如果:10.7 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
11.Liu J, Zhu S, Zeng L, Li J, Klionsky DJ, Kroemer G, et al. DCN released from ferroptotic cells ignites AGER-dependent immune responses. Autophagy. 2022;18:2036–49. doi: 10.1080/15548627.2021.2008692IF: 14.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
11.刘杰,朱松,曾立,李杰,Klionsky DJ,Kroemer G,等。铁死亡细胞释放的 DCN 会引发 AGER 依赖性免疫反应。自噬。 2022;18:2036–49。号码:10.1080/15548627.2021.2008692如果:14.6 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
12.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8:e001369. doi: 10.1136/jitc-2020-001369IF: 10.3 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
12. Efimova I、Catanzaro E、Van der Meeren L、Turubanova VD、Hammad H、Mishchenko TA 等。早期铁死亡癌细胞的疫苗接种可诱导有效的抗肿瘤免疫。 J 免疫疗法癌症。 2020;8:e001369。 DOI:10.1136/jitc-2020-001369如果:10.3 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
13.Yamamoto H, Zhang S, Mizushima N. Autophagy genes in biology and disease. Nat Rev Genet. 2023;24:382–400. doi: 10.1038/s41576-022-00562-wIF: 39.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
13. Yamamoto H,Zhang S,Mizushima N。生物学和疾病中的自噬基因。纳特·吉内特 (Nat Rev Genet)。 2023;24:382–400。 DOI:10.1038/s41576-022-00562-w如果:39.1 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
14.Mancias JD, Kimmelman AC. Mechanisms of selective autophagy in normal physiology and cancer. J Mol Biol. 2016;428:1659–80. doi: 10.1016/j.jmb.2016.02.027IF: 4.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
14.曼西亚斯 JD,金梅尔曼 AC。正常生理和癌症中选择性自噬的机制。分子生物学杂志。 2016;428:1659–80。 doi:10.1016/j.jmb.2016.02.027如果:4.7 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
15.Hong Y, Ren T, Wang X, Liu X, Fei Y, Meng S, et al. APR-246 triggers ferritinophagy and ferroptosis of diffuse large B-cell lymphoma cells with distinct TP53 mutations. Leukemia. 2022;36:2269–80. doi: 10.1038/s41375-022-01634-wIF: 12.8 Q1 . [DOI] [PubMed] [Google Scholar]
15.洪Y,任涛,王X,刘X,费Y,孟S,等。 APR-246 触发具有独特 TP53 突变的弥漫性大 B 细胞淋巴瘤细胞的铁蛋白自噬和铁死亡。白血病。 2022;36:2269–80。 DOI:10.1038/s41375-022-01634-w如果:12.8 Q1 。 [ DOI ] [ PubMed ] [ Google Scholar ] -
16.Ohshima T, Yamamoto H, Sakamaki Y, Saito C, Mizushima N. NCOA4 drives ferritin phase separation to facilitate macroferritinophagy and microferritinophagy. J Cell Biol. 2022;221:e202203102. doi: 10.1083/jcb.202203102IF: 7.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
16. Ohshima T、Yamamoto H、Sakamaki Y、Saito C、Mizushima N。NCOA4 驱动铁蛋白相分离,以促进大铁蛋白自噬和微铁蛋白自噬。 J 细胞生物学。 2022;221:e202203102。 DOI:10.1083/jcb.202203102如果:7.4 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
17.Soh J, Lim ZX, Lim EH, Kennedy BK, Goh J. Ironing out exercise on immuno-oncological outcomes. J Immunother Cancer. 2022;10:e002976. doi: 10.1136/jitc-2021-002976IF: 10.3 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
17. Soh J、Lim ZX、Lim EH、Kennedy BK、Goh J。消除运动对免疫肿瘤学结果的影响。 J 免疫疗法癌症。 2022;10:e002976。 DOI:10.1136/jitc-2021-002976如果:10.3 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
18.Fernández-García V, González-Ramos S, Martín-Sanz P, Castrillo A, Boscá L. Unraveling the interplay between iron homeostasis, ferroptosis and extramedullary hematopoiesis. Pharm Res. 2022;183:106386. doi: 10.1016/j.phrs.2022.106386IF: 9.1 Q1 . [DOI] [PubMed] [Google Scholar]
18. Fernández-García V、González-Ramos S、Martín-Sanz P、Castrilo A、Boscá L。揭示铁稳态、铁死亡和髓外造血之间的相互作用。医药研究中心。 2022;183:106386。 doi:10.1016/j.phrs.2022.106386如果:9.1 Q1 。 [ DOI ] [ PubMed ] [ Google Scholar ] -
19.Gao M, Fan K, Chen Y, Zhang G, Chen J, Zhang Y. Understanding the mechanistic regulation of ferroptosis in cancer: the gene matters. J Genet Genom. 2022;49:913–26. doi: 10.1016/j.jgg.2022.06.002IF: 6.6 Q1 . [DOI] [PubMed] [Google Scholar]
19.高敏,范凯,陈Y,张G,陈J,张Y。了解癌症铁死亡的机制调控:基因问题。 J 基因基因组。 2022;49:913–26。 doi:10.1016/j.jgg.2022.06.002如果:6.6 Q1 。 [ DOI ] [ PubMed ] [ Google Scholar ] -
20.Fujii J, Homma T, Kobayashi S. Ferroptosis caused by cysteine insufficiency and oxidative insult. Free Radic Res. 2020;54:969–80. doi: 10.1080/10715762.2019.1666983IF: 3.6 Q2 . [DOI] [PubMed] [Google Scholar]
20. Fujii J,Homma T,Kobayashi S。半胱氨酸不足和氧化损伤引起的铁死亡。自由基研究。 2020;54:969–80。号码:10.1080/10715762.2019.1666983如果:3.6 Q2 。 [ DOI ] [ PubMed ] [ Google Scholar ] -
21.Chen X, Kang R, Kroemer G, Tang D. Organelle-specific regulation of ferroptosis. Cell Death Differ. 2021;28:2843–56. doi: 10.1038/s41418-021-00859-zIF: 13.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
21. Chen X,Kang R,Kroemer G,Tang D。铁死亡的细胞器特异性调节。细胞死亡不同。 2021;28:2843–56。 DOI:10.1038/s41418-021-00859-z如果:13.7 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
22.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. doi: 10.1038/s41422-019-0164-5IF: 28.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
22. Tang D、Kang R、Berghe TV、Vandenabeele P、Kroemer G。调节细胞死亡的分子机制。细胞研究。 2019;29:347–64。 DOI:10.1038/s41422-019-0164-5如果:28.1 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
23.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–79. doi: 10.1038/cdd.2015.158IF: 13.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
23.谢Y,侯文,宋X,余Y,黄J,孙X,等。铁死亡:过程和功能。细胞死亡不同。 2016;23:369–79。 doi:10.1038/cdd.2015.158如果:13.7 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] -
24.Zhao C, Yu D, He Z, Bao L, Feng L, Chen L, et al. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic Biol Med. 2021;175:236–48. doi: 10.1016/j.freeradbiomed.2021.09.008IF: 7.1 Q1 . [DOI] [PubMed] [Google Scholar]
24.赵春,余东,何志,鲍丽,冯丽,陈丽,等。内质网应激介导的自噬激活参与镉诱导的肾小管上皮细胞铁死亡。自由基生物医学。 2021;175:236–48。 doi:10.1016/j.freeradbiomed.2021.09.008如果:7.1 Q1 。 [ DOI ] [ PubMed ] [ Google Scholar ] -
25.Jiang C, Zhang S, Li D, Chen L, Zhao Y, Mei G, et al. Impaired ferritinophagy flux induced by high fat diet mediates hepatic insulin resistance via endoplasmic reticulum stress. Food Chem Toxicol. 2020;140:111329. doi: 10.1016/j.fct.2020.111329IF: 3.9 Q1 . [DOI] [PubMed] [Google Scholar]
25.江成,张S,李东,陈L,赵Y,梅G,等。高脂肪饮食引起的铁蛋白吞噬通量受损通过内质网应激介导肝脏胰岛素抵抗。食品化学毒理学。 2020;140:111329。 DOI:10.1016/j.fct.2020.111329如果:3.9 Q1 。 [ DOI ] [ PubMed ] [ Google Scholar ] -
26.Wang K, Zhang Z, Tsai HI, Liu Y, Gao J, Wang M, et al. Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ. 2021;28:1222–36. doi: 10.1038/s41418-020-00644-4IF: 13.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
26.王凯,张志,蔡海伊,刘Y,高静,王明,等。支链氨基酸转氨酶 2 调节癌细胞中的铁死亡细胞。细胞死亡不同。 2021;28:1222–36。 DOI:10.1038/s41418-020-00644-4如果:13.7 Q1 。 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ] - 27.Chen X, Song X, Li J, Zhang R, Yu C, Zhou Z, et al. Identification of HPCAL1 as a specific autophagy receptor involved in ferroptosis. Autophagy. 2022;21:54–74. doi: 10.1080/15548627.2022.2059170IF: 14.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su J, Li Y, Liu Q, Peng G, Qin C, Li Y. Identification of SSBP1 as a ferroptosis-related biomarker of glioblastoma based on a novel mitochondria-related gene risk model and in vitro experiments. J Transl Med. 2022;20:440. doi: 10.1186/s12967-022-03657-4IF: 6.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manz DH, Blanchette NL, Paul BT, Torti FM, Torti SV. Iron and cancer: recent insights. Ann N. Y Acad Sci. 2016;1368:149–61. doi: 10.1111/nyas.13008IF: 4.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Zhou T, Su Y, He L, Wang Z. Involvement of histone methylation in the regulation of neuronal death. J Physiol Biochem. 2023;79:685–93. doi: 10.1007/s13105-023-00978-wIF: 3.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 31.Wu S, Pan R, Lu J, Wu X, Xie J, Tang H, et al. Development and verification of a prognostic ferroptosis-related gene model in triple-negative breast cancer. Front Oncol. 2022;12:896927. doi: 10.3389/fonc.2022.896927IF: 3.5 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou Y, Zheng S, Xie X, Ye F, Hu X, Tian Z, et al. N6-methyladenosine regulated FGFR4 attenuates ferroptotic cell death in recalcitrant HER2-positive breast cancer. Nat Commun. 2022;13:2672. doi: 10.1038/s41467-022-30217-7IF: 14.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao R, Wang H, Liu W, Wang J, Lu S, Tang H, et al. Establishment of a prognostic ferroptosis-related gene profile in acute myeloid leukaemia. J Cell Mol Med. 2021;25:10950–60. doi: 10.1111/jcmm.17013IF: 4.3 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Klionsky DJ, Shen HM. The emerging mechanisms and functions of microautophagy. Nat Rev Mol Cell Biol. 2023;24:186–203. doi: 10.1038/s41580-022-00529-zIF: 81.3 Q1 . [DOI] [PubMed] [Google Scholar]
- 35.Muhoberac BB, Vidal R. Iron, ferritin, hereditary ferritinopathy, and neurodegeneration. Front Neurosci. 2019;13:1195. doi: 10.3389/fnins.2019.01195IF: 3.2 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumneang N, Siri-Angkul N, Kumfu S, Chattipakorn SC, Chattipakorn N. The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch Biochem Biophys. 2020;680:108241. doi: 10.1016/j.abb.2019.108241IF: 3.8 Q1 . [DOI] [PubMed] [Google Scholar]
- 37.Yamada N, Karasawa T, Wakiya T, Sadatomo A, Ito H, Kamata R, et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: potential role of ferroptosis. Am J Transplant. 2020;20:1606–18. doi: 10.1111/ajt.15773IF: 8.9 Q1 . [DOI] [PubMed] [Google Scholar]
- 38.Li J, Yuan J, Li Y, Wang J, Xie Q, Ma R, et al. d-Borneol enhances cisplatin sensitivity via autophagy dependent EMT signaling and NCOA4-mediated ferritinophagy. Phytomedicine. 2022;106:154411. doi: 10.1016/j.phymed.2022.154411IF: 6.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 39.Nai A, Lidonnici MR, Federico G, Pettinato M, Olivari V, Carrillo F, et al. NCOA4-mediated ferritinophagy in macrophages is crucial to sustain erythropoiesis in mice. Haematologica. 2021;106:795–805. doi: 10.3324/haematol.2019.241232IF: 8.2 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–28. doi: 10.1080/15548627.2016.1187366IF: 14.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H, Liu Q, Shan X, Gao W, Chen Q. ATM orchestrates ferritinophagy and ferroptosis by phosphorylating NCOA4. Autophagy. 2023;19:2062–77. doi: 10.1080/15548627.2023.2170960IF: 14.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Man H, Zhou L, Zhu G, Zheng Y, Ye Z, Huang Z, et al. Super-resolution imaging of autophagy by a preferred pair of self-labeling protein tags and fluorescent ligands. Anal Chem. 2022;94:15057–66. doi: 10.1021/acs.analchem.2c03125IF: 6.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 43.Masaldan S, Clatworthy SAS, Gamell C, Meggyesy PM, Rigopoulos AT, Haupt S, et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018;14:100–15. doi: 10.1016/j.redox.2017.08.015IF: 10.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Zhu Q, Li R, Zhang J, Ye X, Li X. YAP1 protects against septic liver injury via ferroptosis resistance. Cell Biosci. 2022;12:163. doi: 10.1186/s13578-022-00902-7IF: 6.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu M, Peng L, Huo S, Peng D, Gou J, Shi W, et al. STAT3 signaling promotes cardiac injury by upregulating NCOA4-mediated ferritinophagy and ferroptosis in high-fat-diet fed mice. Free Radic Bio Med. 2023;201:111–25. doi: 10.1016/j.freeradbiomed.2023.03.003IF: 7.1 Q1 . [DOI] [PubMed] [Google Scholar]
- 46.Sun K, Hou L, Guo Z, Wang G, Guo J, Xu J, et al. JNK-JUN-NCOA4 axis contributes to chondrocyte ferroptosis and aggravates osteoarthritis via ferritinophagy. Free Radic Bio Med. 2023;200:87–101. doi: 10.1016/j.freeradbiomed.2023.03.008IF: 7.1 Q1 . [DOI] [PubMed] [Google Scholar]
- 47.Distéfano AM, Martin MV, Córdoba JP, Bellido AM, D’Ippólito S, Colman SL, et al. Heat stress induces ferroptosis-like cell death in plants. J Cell Biol. 2017;216:463–76. doi: 10.1083/jcb.201605110IF: 7.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS, et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem Biol. 2020;15:469–84. doi: 10.1021/acschembio.9b00939IF: 3.5 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou H, Zhou YL, Mao JA, Tang LF, Xu J, Wang ZX, et al. NCOA4-mediated ferritinophagy is involved in ionizing radiation-induced ferroptosis of intestinal epithelial cells. Redox Biol. 2022;55:102413. doi: 10.1016/j.redox.2022.102413IF: 10.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–60. doi: 10.1016/S1474-4422(14)70117-6IF: 46.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mou Y, Wu J, Zhang Y, Abdihamid O, Duan C, Li B. Low expression of ferritinophagy-related NCOA4 gene in relation to unfavorable outcome and defective immune cells infiltration in clear cell renal carcinoma. BMC Cancer. 2021;21:18. doi: 10.1186/s12885-020-07726-zIF: 3.4 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Zhang H. NCOA4: More than a receptor for ferritinophagy. J Cell Biol. 2022;221:e202209004. doi: 10.1083/jcb.202209004IF: 7.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Facchinetti MM. Heme-oxygenase-1. Antioxid Redox Signal. 2020;32:1239–42. doi: 10.1089/ars.2020.8065IF: 5.9 Q1 . [DOI] [PubMed] [Google Scholar]
- 54.Chen J, Zhou S, Zhang X, Zhao H. S-3’-hydroxy-7’, 2’, 4’-trimethoxyisoxane, a novel ferroptosis inducer, promotes NSCLC cell death through inhibiting Nrf2/HO-1 signaling pathway. Front Pharmacol. 2022;13:973611. doi: 10.3389/fphar.2022.973611IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anandhan A, Dodson M, Shakya A, Chen J, Liu P, Wei Y, et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci Adv. 2023;9:eade9585. doi: 10.1126/sciadv.ade9585IF: 11.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mancias JD, Pontano Vaites L, Nissim S, Biancur DE, Kim AJ, Wang X, et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. eLife. 2015;4:e10308. doi: 10.7554/eLife.10308IF: 6.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharm Sci. 2013;34:340–46. doi: 10.1016/j.tips.2013.04.005IF: 13.9 Q1 . [DOI] [PubMed] [Google Scholar]
- 58.Han Y, Gao X, Wu N, Jin Y, Zhou H, Wang W, et al. Long noncoding RNA LINC00239 inhibits ferroptosis in colorectal cancer by binding to Keap1 to stabilize Nrf2. Cell Death Dis. 2022;13:742. doi: 10.1038/s41419-022-05192-yIF: 8.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Xu B, Ren X, Wang L, Xu Y, Zhao Y, et al. Inhibition of CISD2 promotes ferroptosis through ferritinophagy-mediated ferritin turnover and regulation of p62-Keap1-NRF2 pathway. Cell Mol Biol Lett. 2022;27:81. doi: 10.1186/s11658-022-00383-zIF: 9.2 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ravichandran M, Hu J, Cai C, Ward NP, Venida A, Foakes C, et al. Coordinated transcriptional and catabolic programs support iron-dependent adaptation to RAS-MAPK pathway inhibition in pancreatic cancer. Cancer Discov. 2022;12:2198–219. doi: 10.1158/2159-8290.CD-22-0044IF: 29.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao L, Zhao C, Feng L, Zhao Y, Duan S, Qiu M, et al. Ferritinophagy is involved in Bisphenol A-induced ferroptosis of renal tubular epithelial cells through the activation of the AMPK-mTOR-ULK1 pathway. Food Chem Toxicol. 2022;163:112909. doi: 10.1016/j.fct.2022.112909IF: 3.9 Q1 . [DOI] [PubMed] [Google Scholar]
- 62.Liu C, Liao W, Chen J, Yu K, Wu Y, Zhang S, et al. Cholesterol confers ferroptosis resistance onto myeloid-biased hematopoietic stem cells and prevents irradiation-induced myelosuppression. Redox Biol. 2023;62:102661. doi: 10.1016/j.redox.2023.102661IF: 10.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lathoria K, Gowda P, Umdor SB, Patrick S, Suri V, Sen E. PRMT1 driven PTX3 regulates ferritinophagy in glioma. Autophagy. 2023;19:1997–2014. doi: 10.1080/15548627.2023.2165757IF: 14.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shang M, Weng L, Xu G, Wu S, Liu B, Yin X, et al. TRIM11 suppresses ferritinophagy and gemcitabine sensitivity through UBE2N/TAX1BP1 signaling in pancreatic ductal adenocarcinoma. J Cell Physiol. 2021;236:6868–83. doi: 10.1002/jcp.30346IF: 4.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 65.Liu Z, Wang Q, Wang X, Xu Z, Wei X, Li J. Circular RNA cIARS regulates ferroptosis in HCC cells through interacting with RNA binding protein ALKBH5. Cell Death Discov. 2020;6:72. doi: 10.1038/s41420-020-00306-xIF: 6.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. doi: 10.1016/j.cell.2013.12.010IF: 45.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042IF: 45.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhai X, Shan S, Wan J, Tian H, Wang J, Xin L. Silver nanoparticles induce a size-dependent neurotoxicity to SH-SY5Y neuroblastoma cells via ferritinophagy-mediated oxidative stress. Neurotox Res. 2022;40:1369–79. doi: 10.1007/s12640-022-00570-yIF: 2.9 Q2 . [DOI] [PubMed] [Google Scholar]
- 69.Biasiotto G, Di Lorenzo D, Archetti S, Zanella I. Iron and neurodegeneration: is ferritinophagy the link? Mol Neurobiol. 2016;53:5542–74. doi: 10.1007/s12035-015-9473-yIF: 4.6 Q1 . [DOI] [PubMed] [Google Scholar]
- 70.Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, Ning G, et al. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene. 2017;36:4089–99. doi: 10.1038/onc.2017.11IF: 6.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kondo K, Noguchi M, Mukai K, Matsuno Y, Sato Y, Shimosato Y, et al. Transferrin receptor expression in adenocarcinoma of the lung as a histopathologic indicator of prognosis. Chest. 1990;97:1367–71. doi: 10.1378/chest.97.6.1367IF: 9.5 Q1 . [DOI] [PubMed] [Google Scholar]
- 72.Xu X, Liu T, Wu J, Wang Y, Hong Y, Zhou H. Transferrin receptor-involved HIF-1 signaling pathway in cervical cancer. Cancer Gene Ther. 2019;26:356–65. doi: 10.1038/s41417-019-0078-xIF: 4.8 Q1 . [DOI] [PubMed] [Google Scholar]
- 73.Adachi M, Kai K, Yamaji K, Ide T, Noshiro H, Kawaguchi A, et al. Transferrin receptor 1 overexpression is associated with tumour de-differentiation and acts as a potential prognostic indicator of hepatocellular carcinoma. Histopathology. 2019;75:63–73. doi: 10.1111/his.13847IF: 3.9 Q1 . [DOI] [PubMed] [Google Scholar]
- 74.Ajoolabady A, Aslkhodapasandhokmabad H, Libby P, Tuomilehto J, Lip GYH, Penninger JM, et al. Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol Metab. 2021;32:444–62. doi: 10.1016/j.tem.2021.04.010IF: 11.4 Q1 . [DOI] [PubMed] [Google Scholar]
- 75.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2IF: 8.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li L, Li H, Li Y, Feng J, Guan D, Zhang Y, et al. Ferritinophagy-mediated ROS production contributed to proliferation inhibition, apoptosis, and ferroptosis induction in action of mechanism of 2-pyridylhydrazone dithiocarbamate acetate. Oxid Med Cell Longev. 2021;2021:5594059. doi: 10.1155/2021/5594059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng J, Li C, Xu R, Li Y, Hou Q, Feng R, et al. DpdtC-induced EMT inhibition in MGC-803 cells was partly through ferritinophagy-mediated ROS/p53 pathway. Oxid Med Cell Longev. 2020;2020:9762390. doi: 10.1155/2020/9762390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Kong Y, Ma Y, Ni S, Wikerholmen T, Xi K, et al. Loss of COPZ1 induces NCOA4 mediated autophagy and ferroptosis in glioblastoma cell lines. Oncogene. 2021;40:1425–39. doi: 10.1038/s41388-020-01622-3IF: 6.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li K, Chen B, Xu A, Shen J, Li K, Hao K, et al. TRIM7 modulates NCOA4-mediated ferritinophagy and ferroptosis in glioblastoma cells. Redox Biol. 2022;56:102451. doi: 10.1016/j.redox.2022.102451IF: 10.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin J, Lin Y, Fang W, Zhang X, Wei J, Hu G, et al. Tetrandrine citrate suppresses breast cancer via depletion of glutathione peroxidase 4 and activation of nuclear receptor coactivator 4-mediated ferritinophagy. Front Pharmacol. 2022;13:820593. doi: 10.3389/fphar.2022.820593IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hasan M, Reddy SM, Das NK. Ferritinophagy is not required for colon cancer cell growth. Cell Biol Int. 2020;44:2307–14. doi: 10.1002/cbin.11439IF: 3.3 Q3 . [DOI] [PubMed] [Google Scholar]
- 82.Gryzik M, Asperti M, Denardo A, Arosio P, Poli M. NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochim Biophys Acta Mol Cell Res. 2021;1868:118913. doi: 10.1016/j.bbamcr.2020.118913IF: 4.6 Q1 . [DOI] [PubMed] [Google Scholar]
- 83.Guggisberg CA, Kim J, Lee J, Chen X, Ryu MS. NCOA4 regulates iron recycling and responds to hepcidin activity and lipopolysaccharide in macrophages. Antioxid (Basel, Switzerland). 2022;11:1926. doi: 10.3390/antiox11101926IF: 6.0 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jain V, Amaravadi RK. Pumping iron: ferritinophagy promotes survival and therapy resistance in pancreatic cancer. Cancer Discov. 2022;12:2023–25. doi: 10.1158/2159-8290.CD-22-0734IF: 29.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guan D, Li C, Li Y, Li Y, Wang G, Gao F, et al. The DpdtbA induced EMT inhibition in gastric cancer cell lines was through ferritinophagy-mediated activation of p53 and PHD2/hif-1α pathway. J Inorg Biochem. 2021;218:111413. doi: 10.1016/j.jinorgbio.2021.111413IF: 3.8 Q1 . [DOI] [PubMed] [Google Scholar]
- 86.Sui S, Zhang J, Xu S, Wang Q, Wang P, Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 2019;10:331. doi: 10.1038/s41419-019-1564-7IF: 8.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang Z, Dong H, Li T, Wang N, Wei X, Wu H, et al. The synergistic reducing drug resistance effect of cisplatin and ursolic acid on osteosarcoma through a multistep mechanism involving ferritinophagy. Oxid Med Cell Longev. 2021;2021:5192271. doi: 10.1155/2021/5192271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen HC, Tang HH, Hsu WH, Wu SY, Cheng WH, Wang BY, et al. Vulnerability of triple-negative breast cancer to saponin formosanin c-induced ferroptosis. Antioxid (Basel, Switzerland). 2022;11:298. doi: 10.3390/antiox11020298IF: 6.0 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH, Wang ZX, et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharm Sin. 2021;42:301–10. doi: 10.1038/s41401-020-0478-3IF: 6.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou J, Tan Y, Hu L, Fu J, Li D, Chen J, et al. Inhibition of HSPA8 by rifampicin contributes to ferroptosis via enhancing autophagy. Liver Int. 2022;42:2889–99. doi: 10.1111/liv.15459IF: 6.0 Q1 . [DOI] [PubMed] [Google Scholar]
- 91.Zhou YJ, Duan DQ, Lu LQ, Tang LJ, Zhang XJ, Luo XJ, et al. The SPATA2/CYLD pathway contributes to doxorubicin-induced cardiomyocyte ferroptosis via enhancing ferritinophagy. Chem Biol Interact. 2022;368:110205. doi: 10.1016/j.cbi.2022.110205IF: 4.7 Q1 . [DOI] [PubMed] [Google Scholar]
- 92.Liu K, Huang J, Liu J, Klionsky DJ, Kang R, Tang D. Induction of autophagy-dependent ferroptosis to eliminate drug-tolerant human retinoblastoma cells. Cell Death Dis. 2022;13:521. doi: 10.1038/s41419-022-04974-8IF: 8.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grignano E, Cantero-Aguilar L, Tuerdi Z, Chabane T, Vazquez R, Johnson N, et al. Dihydroartemisinin-induced ferroptosis in acute myeloid leukemia: links to iron metabolism and metallothionein. Cell Death Discov. 2023;9:97. doi: 10.1038/s41420-023-01371-8IF: 6.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu B, Jiang W, Ye Y, Liu L, Wei X, Zhang Q, et al. 2D MoS(2) nanosheets induce ferroptosis by promoting NCOA4-dependent ferritinophagy and inhibiting ferroportin. Small (Weinh der Bergstr, Ger). 2023;19:e2208063. doi: 10.1002/smll.202208063IF: 13.0 Q1 . [DOI] [PubMed] [Google Scholar]
- 95.Qin X, Zhang J, Wang B, Xu G, Yang X, Zou Z, et al. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy. 2021;17:4266–85. doi: 10.1080/15548627.2021.1911016IF: 14.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Zhang L, Zhao G, Zhang Y, Zhan F, Chen Z, et al. Homologous targeting nanoparticles for enhanced PDT against osteosarcoma HOS cells and the related molecular mechanisms. J Nanobiotechnol. 2022;20:83. doi: 10.1186/s12951-021-01201-yIF: 10.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zuo T, Fang T, Zhang J, Yang J, Xu R, Wang Z, et al. pH-sensitive molecular-switch-containing polymer nanoparticle for breast cancer therapy with ferritinophagy-cascade ferroptosis and tumor immune activation. Adv Health Mater. 2021;10:e2100683. doi: 10.1002/adhm.202100683IF: 10.0 Q1 . [DOI] [PubMed] [Google Scholar]
- 98.He Z, Zhou H, Zhang Y, Du X, Liu S, Ji J, et al. Oxygen-boosted biomimetic nanoplatform for synergetic phototherapy/ferroptosis activation and reversal of immune-suppressed tumor microenvironment. Biomaterials. 2022;290:121832. doi: 10.1016/j.biomaterials.2022.121832IF: 12.8 Q1 . [DOI] [PubMed] [Google Scholar]
- 99.Kumar R, Mendonca J, Owoyemi O, Boyapati K, Thomas N, Kanacharoen S, et al. Supraphysiologic testosterone induces ferroptosis and activates immune pathways through nucleophagy in prostate cancer. Cancer Res. 2021;81:5948–62. doi: 10.1158/0008-5472.CAN-20-3607IF: 12.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He N, Mao XJ, Ding YM, Zuo T, Chen YY, Wang LL. New insights into the biological roles of immune cells in neural stem cells in post-traumatic injury of the central nervous system. Neural Regen Res. 2023;18:1908–16. doi: 10.4103/1673-5374.367836IF: 5.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li W, Zhao X, Zhang R, Liu X, Qi Z, Zhang Y, et al. Ferroptosis inhibition protects vascular endothelial cells and maintains integrity of the blood-spinal cord barrier after spinal cord injury. Neural Regen Res. 2023;18:2474–81. doi: 10.4103/1673-5374.371377IF: 5.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gebrael G, Sahu KK, Agarwal N, Maughan BL. Update on combined immunotherapy for the treatment of advanced renal cell carcinoma. Hum Vaccin Immunother. 2023;19:2193528. doi: 10.1080/21645515.2023.2193528IF: 4.1 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dibajnia P, Cardenas LM, Lalani AA. The emerging landscape of neo/adjuvant immunotherapy in renal cell carcinoma. Hum Vaccin Immunother. 2023;19:2178217. doi: 10.1080/21645515.2023.2178217IF: 4.1 Q2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–74. doi: 10.1038/s41586-019-1170-yIF: 50.5 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–85. doi: 10.1158/2159-8290.CD-19-0338IF: 29.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wiernicki B, Maschalidi S, Pinney J, Adjemian S, Vanden Berghe T, Ravichandran KS, et al. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat Commun. 2022;13:3676. doi: 10.1038/s41467-022-31218-2IF: 14.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sottile R, Federico G, Garofalo C, Tallerico R, Faniello MC, Quaresima B, et al. Iron and ferritin modulate MHC class I expression and NK cell recognition. Front Immunol. 2019;10:224. doi: 10.3389/fimmu.2019.00224IF: 5.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun K, Li C, Liao S, Yao X, Ouyang Y, Liu Y, et al. Ferritinophagy, a form of autophagic ferroptosis: new insights into cancer treatment. Front Pharmacol. 2022;13:1043344. doi: 10.3389/fphar.2022.1043344IF: 4.4 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boag MK, Roberts A, Uversky VN, Ma L, Richardson DR, Pountney DL. Ferritinophagy and α-Synuclein: pharmacological targeting of autophagy to restore iron regulation in Parkinson’s disease. Int J Mol Sci. 2022;23:2378. doi: 10.3390/ijms23042378IF: 4.9 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Y, Cheng J, Lin Q, Ni Z. Autophagy-dependent ferroptosis in kidney disease. Front Med. 2022;9:1071864. doi: 10.3389/fmed.2022.1071864IF: 3.1 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang JY, Gao WD, Lin JY, Xu S, Zhang LJ, Lu XC, et al. Nanotechnology-based photo-immunotherapy: a new hope for inhibition of melanoma growth and metastasis. J Drug Target. 2023;31:555–68. doi: 10.1080/1061186X.2023.2216402IF: 4.3 Q1 . [DOI] [PubMed] [Google Scholar]
- 112.Hu Q, Wei W, Wu D, Huang F, Li M, Li W, et al. Blockade of GCH1/BH4 axis activates ferritinophagy to mitigate the resistance of colorectal cancer to erastin-induced ferroptosis. Front Cell Dev Biol. 2022;10:810327. doi: 10.3389/fcell.2022.810327IF: 4.6 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang X, Hua P, He C, Chen M. Non-apoptotic cell death-based cancer therapy: molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharm Sin B. 2022;12:3567–93. doi: 10.1016/j.apsb.2022.03.020IF: 14.7 Q1 . [DOI] [PMC free article] [PubMed] [Google Scholar]