By Aimee Raleigh, Principal at Atlas Venture, as part of the From The Trenches feature of LifeSciVC
作者:Atlas Venture 负责人 Aimee Raleigh,作为 LifeSciVC 的 "From The Trenches "专题的一部分
Ever wondered what goes into diligencing a new idea, program, company, or platform? While each diligence is unique and every investor will have their own approach, below are some considerations that may be more “typical” in a diligence. I will emphasize the obvious disclaimer before diving in – this framework is merely meant to be exemplary and there are always nuances and exceptions unique to each diligence. Additionally, for illustrative reasons this is geared towards a single target / product focus vs. broader platform diligence, though many of these mental models will apply for selecting targets and indications for a platform. Sometimes people are disappointed to learn there is no official checklist investors use during a diligence, but that’s the beauty of working in the complex space of early-stage therapeutics!
有没有想过对一个新创意、新项目、新公司或新平台进行尽职调查的过程是怎样的?虽然每项尽职调查都是独一无二的,每个投资者也都有自己的方法,但以下是一些在尽职调查中可能比较 "典型 "的考虑因素。在进入正题之前,我想强调一个显而易见的免责声明--这个框架只是为了起到示范作用,每项尽职调查总会有细微差别和例外情况。此外,出于说明的原因,本文针对的是单一目标/产品重点,而不是更广泛的平台调查,尽管其中的许多思维模式也适用于为平台选择目标和适应症。有时,人们会失望地发现,投资者在尽职调查过程中并没有正式的清单,但这正是在复杂的早期治疗领域工作的魅力所在!
How does one think about therapeutic relevance for a new target? This will be an exemplary look at things I typically consider when diligencing a new opportunity for company creation, including key concepts and mental models.
如何考虑新靶点的治疗相关性?这将是一个范例,介绍我在为创建公司寻找新机会时通常会考虑的问题,包括关键概念和思维模式。
Target validation: Do we believe the target(s) plays a central role in disease biology and that modulation will modify disease?
目标验证:我们是否认为该靶点在疾病生物学中起着核心作用,并且调节该靶点会改变疾病?
There is a continuum of evidence for a given target – at one end are novel targets with some evidence of importance in disease, and at the other end are “de-risked” targets where the biology is precedented with an approved product or late-stage clinical asset(s). I will note that “de-risked” is never fully risk-free in therapeutics investing, as even precedented mechanisms hit stumbling blocks in preclinical or clinical development. The level of comfort with novelty of a target hypothesis might vary for an investor depending on the stage at which they typically invest. In the early-stage setting, we diligence and conceive of our own newco ideas across the spectrum of validation. There is nothing more exciting than digging into a new target and trying to develop a thesis on whether modulation may be impactful in disease. Is a novel target at the inflection point where enough evidence is available to suggest it may prove to be a compelling drug? In the absence of a clinical trial result or FDA label to point to, how does one create the case and target product profile (TPP) around a new target?
特定靶点的证据有一个连续统一体--一端是有一些证据表明对疾病有重要作用的新靶点,另一端是 "去风险 "靶点,其生物学特性已经有了获批产品或后期临床资产的先例。我要指出的是,在治疗投资中,"去风险 "从来都不是完全无风险的,因为即使是已有先例的机制也会在临床前或临床开发中遇到绊脚石。投资者对目标假说新颖性的放心程度可能因其通常投资的阶段而异。在早期阶段,我们会勤奋工作,构思我们自己的新想法,并进行全方位的验证。最令人兴奋的事情莫过于深入研究一个新的靶点,并试图就调节是否会对疾病产生影响提出论点。一个新靶点是否正处于拐点,有足够的证据表明它可能是一种令人信服的药物?在没有临床试验结果或 FDA 标签的情况下,如何围绕一个新靶点创建案例和靶点产品简介(TPP)?
In order to start building a case for or against a target, I like to start with genetics – first human and then mouse. Are there known genetic diseases associated with the target? If so, are these Mendelian or complex polygenic diseases, and if the former is the inheritance pattern autosomal recessive or autosomal dominant? Is the functionality of mutations known? How heterogeneous are the observed phenotypes? Answers to the above questions help to develop a thesis around any allelic “dose response” – for example if heterozygotes exist with a mild phenotype and homozygous or compound heterozygous individuals have more severe disease. In addition to Mendelian genetics, what do we know about any genetic modifiers – any compelling findings from GWAS, WES, etc.? Are the phenotypes associated with mutations discordant or do they point towards a potential underlying biology with compelling effect size? Do mouse genetics line up with the human story? If any confounding data, is there a plausible rationale for the divergent phenotypes?
为了开始建立一个支持或反对目标的案例,我喜欢从遗传学入手--首先是人类,然后是小鼠。是否存在与目标相关的已知遗传疾病?如果有,是孟德尔遗传病还是复杂的多基因遗传病?如果是前者,遗传模式是常染色体隐性遗传还是常染色体显性遗传?突变的功能是否已知?观察到的表型的异质性如何?对上述问题的回答有助于围绕等位基因的 "剂量反应 "展开论述--例如,如果存在表型轻微的杂合子,而同源或复合杂合子个体的疾病则更为严重。除了孟德尔遗传学外,我们对任何遗传修饰因子了解多少--GWAS、WES 等是否有令人信服的发现?与突变相关的表型是不一致的,还是指向潜在的生物学基础,并具有令人信服的效应大小?小鼠遗传学是否与人类故事一致?如果有任何干扰数据,是否有合理的理由解释不同的表型?
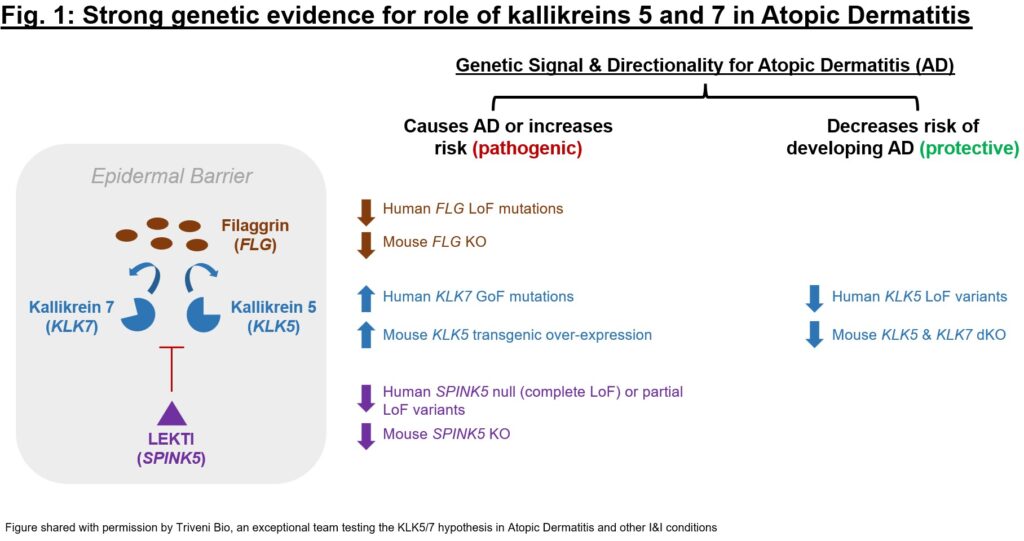
Once an individual target is evaluated, I might expand to a broader pathway. Are there nearby targets up- or down-stream from the novel target of interest that have genetics validation? Overall, are the “signals” from this screen suggestive of plausible biology? Genetic data for the target can offer a first glimpse at whether there is a tractable hypothesis to dig into. One example of such a genetics exercise is represented in Fig. 1, which depicts the sizeable amount of genetics evidence for kallikreins 5 and 7 (KLK5 and 7), two serine protease targets with a role in barrier dysfunction and immune dysregulation. For KLK5 and KLK7 as well as their endogenous regulator (LEKTI, encoded by SPINK5) and one of their substrates (filaggrin, FLG) there is evidence that KLK5 and 7 up-regulation is pathogenic and down-regulation protective in epidermal barrier dysfunction (especially for Atopic Dermatitis). The cumulative genetics evidence was so compelling that the exceptional team at Atlas portfolio company Triveni Bio is targeting both kallikreins in a dual antagonist antibody currently in IND-enabling studies.
对单个靶点进行评估后,我可能会扩展到更广泛的途径。在感兴趣的新靶点的上游或下游,附近是否有经过遗传学验证的靶点?总体而言,这一筛选的 "信号 "是否暗示了合理的生物学原理?靶点的遗传学数据可以让我们初步了解是否存在可以深入研究的假说。图 1 是这种遗传学研究的一个例子,描述了 Kallikreins 5 和 7(KLK5 和 7)的大量遗传学证据,这两种丝氨酸蛋白酶靶标在屏障功能障碍和免疫失调中发挥作用。对于 KLK5 和 KLK7 以及它们的内源调节因子(LEKTI,由 SPINK5 编码)和它们的底物之一(filaggrin,FLG),有证据表明 KLK5 和 7 的上调对表皮屏障功能障碍(尤其是特应性皮炎)具有致病作用,而下调则具有保护作用。累积的遗传学证据是如此令人信服,以至于 Atlas 投资组合公司 Triveni Bio 的杰出团队正在针对这两种 Kallikreins 开发双拮抗剂抗体,目前正在进行 IND 许可研究。
Human and mouse genetics can inform not only efficacy but also safety. The ideal target is one where modulation will not drive adverse phenotypes in healthy individuals, which is important if one intends on trialing in healthy volunteers in Phase 1 but also gives comfort for chronic target modulation. If adverse events are anticipated, it is important to understand gene dosage for such an effect (e.g., adverse profile seen in homozygous null individuals but not in heterozygous carriers?) and whether a molecule’s pharmacology can help to mitigate safety risk. Finally, as part of a broader pathway analysis, consider potential implications for selectivity. Especially for oligo or small molecule discovery and development, it’s important to understand whether there are highly homologous sequences or proteins that may be impacted by a given therapeutic approach.
人类遗传学和小鼠遗传学不仅可以提供疗效信息,还可以提供安全性信息。理想的靶点是对其进行调控不会导致健康个体出现不良表型,如果打算在第一阶段对健康志愿者进行试验,这一点非常重要,同时也能为长期靶点调控提供依据。如果预期会出现不良事件,那么了解这种效应的基因剂量(例如,在同基因空位个体中会出现不良反应,而在异基因携带者中则不会?最后,作为更广泛的途径分析的一部分,要考虑对选择性的潜在影响。特别是对于寡核苷酸或小分子化合物的发现和开发,了解是否存在可能受特定治疗方法影响的高度同源序列或蛋白质非常重要。
While supportive genetics aren’t absolutely required to move forward with a novel target, they certainly help to drive conviction in potential clinical relevance. Suggested resources for this first pass genetics diligence include OMIM, GWAS Catalog, DisGeNET, OpenTargets, Genebass, and the International Mouse Phenotyping Consortium.
虽然支持性遗传学并非推进新靶点的绝对必要条件,但它们肯定有助于推动对潜在临床相关性的信念。建议用于第一轮遗传学调查的资源包括 OMIM、GWAS Catalog、DisGeNET、OpenTargets、Genebass 和国际小鼠表型协会。
Directionality and Druggability: Does the proposed “direction” of insult and therapeutic intervention make sense, and can we drug our novel target with a compelling modality?
方向性和可药用性:所提出的损伤和治疗干预 "方向 "是否合理,我们能否用一种令人信服的方式对我们的新靶点进行药物治疗?
From the above genetics exploration, is the functional impact known? In most simplistic terms this can usually be reduced to “gain of function” (GoF) vs. “loss of function” (LoF) effect, but can also be quite nuanced (e.g., some phenotypes can be driven by both LoF or GoF mutations, or functionality can be difficult to characterize for variants). Once a direction of pathology is determined, how might one intervene?
通过上述遗传学探索,是否知道功能影响?用最简单的术语来说,这通常可以简化为 "功能增益"(GoF)与 "功能丧失"(LoF)效应,但也可能有相当细微的差别(例如,某些表型可能同时受 LoF 或 GoF 突变的驱动,或者变异的功能性可能难以定性)。一旦确定了病理方向,该如何干预呢?
- For inhibition, is a small molecule or antibody-based approach best, or is the etiology tissue-centric so that an oligo, gene editing, or other strategy might address (e.g., in liver, in CNS)?
对于抑制剂,小分子或抗体是最好的方法,还是病因以组织为中心,因此寡聚物、基因编辑或其他策略可以解决(如在肝脏、中枢神经系统中)? - To impart “gain-of-function” pharmacology, consider inducing or up-regulating expression (e.g., with gene editing or gene therapy, enzyme replacement therapy), agonism (e.g., with antibodies), or correction (e.g., in the case of CFTR for Trikafta). Consider whether there is enough (partially functional) target that remaining to agonize or correct – if not, can wild-type up-regulation address the pharmacology, or does the target protein need to be entirely replaced?
要赋予 "功能增益 "药理作用,可考虑诱导或上调表达(如基因编辑或基因治疗、酶替代疗法)、激动(如抗体)或校正(如针对 CFTR 的 Trikafta)。考虑是否还有足够的(部分功能性)靶点可以激动或校正--如果没有,野生型上调是否可以解决药理学问题,还是需要完全替换靶蛋白?
The modality will need to be paired with the “geography” in which disease modification is required. Factoring in both efficacy and safety, is this a systemic or localized approach? Are there delivery approaches to help achieve more localized delivery? While delivery approaches have rapidly advanced over the past few years (e.g., TfR1 brain or muscle “shuttles” enabling higher tissue exposures and activity for antibodies, enzymes, and / or oligos), there are still modalities and tissues for which delivery is the key challenge for successful therapeutic intervention.
治疗方式需要与需要改变疾病的 "地理位置 "相匹配。考虑到疗效和安全性,这是一种系统性方法还是局部性方法?是否有递送方法可以帮助实现更局部的递送?在过去几年中,递送方法迅速发展(例如,TfR1 大脑或肌肉 "穿梭器 "使抗体、酶和/或寡核苷酸具有更高的组织暴露度和活性),但仍有一些模式和组织的递送是成功治疗干预的关键挑战。
Pharmacology: Is this the “right” molecule, does it get to the desired location in the body for the intended amount of time, and does the effect of the molecule on the body make sense?
药理学:这是 "正确的 "分子吗?它是否能在预期时间内到达体内的预期位置?
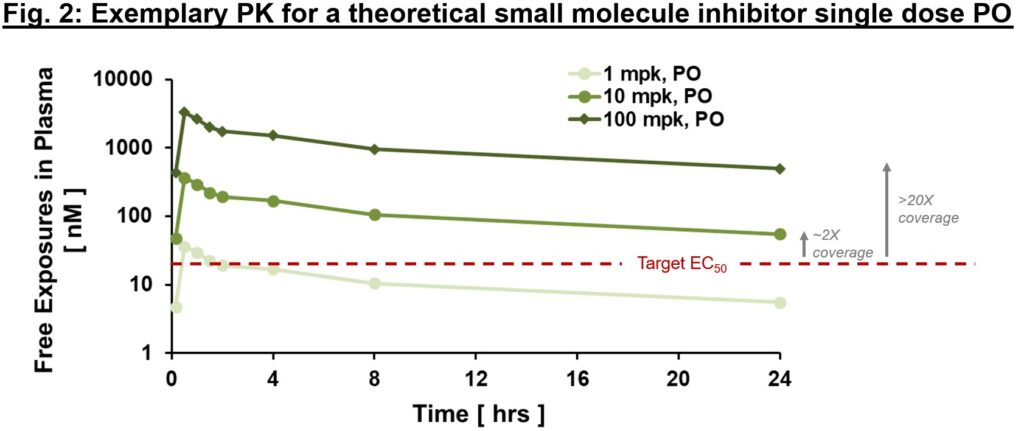
To achieve the desired biology, we must have conviction that a therapy can (1) get to the desired target with sufficient exposures over a certain period of time (pharmacokinetics/PK readout), (2) interact with the target in the desired fashion, enacting proximal and distal “downstream” biology (pharmacodynamics/PD readout), and (3) modify the disease in a way to alter the clinical course (efficacy readout). When diligencing a new target, if there are “tool” molecules with data in the literature, gaining confidence in an exposure/PD/efficacy relationship can help build confidence in the target and mechanism. In the best-case scenario, one can build a thesis on exposure multiples over EC50 required to effect desired biology, as in Fig. 2., based on “tool” molecule precedent.
为了实现理想的生物学效果,我们必须确信一种疗法能够:(1) 在一定时间内以足够的暴露量到达理想的靶点(药代动力学/PK 读数);(2) 以理想的方式与靶点相互作用,产生近端和远端的 "下游 "生物学效果(药效学/PD 读数);(3) 以改变临床病程的方式改变疾病(疗效读数)。在对新靶点进行深入研究时,如果文献中有 "工具 "分子的数据,那么对暴露/PD/疗效关系的信心有助于建立对靶点和机制的信心。在最好的情况下,人们可以根据 "工具 "分子的先例,就影响所需的生物学效果所需的暴露倍数(如图 2 所示) 50 。
Given pharmacology can be a topic of its own, a colleague in the Atlas portfolio, Haojing Rong, has helped author a companion pharmacology blog post – if of interest in double clicking on this topic, please stay tuned for tomorrow’s post!
鉴于药理学是一个独立的话题,Atlas 组合中的一位同事 Haojing Rong 帮助撰写了一篇配套的药理学博文--如果有兴趣双击这个话题,请继续关注明天的博文!
One point to emphasize in the pharmacology diligence is availability and suitability of biomarkers. These can range from “proximal” target engagement readout (e.g., PCSK9 transcript knockdown) to more distal pharmacodynamic measures (e.g., LDL-c lowering). Often for a new target, the biomarker thesis will need to be developed and validated with in vitro and in vivo assays. When diligencing a new mechanism, consider carefully the totality of biomarkers available as well as which will be prioritized for in vitro screening assays and in vivo PD readouts. Ideally multiple different PD markers can be leveraged to improve translational read-through and reduce likelihood of drug discovery due to an erroneous or assay-driven signal. If you are evaluating a novel target without a clear biomarker strategy, consider the heavier lift that will likely be involved in early discovery assays.
药理学研究需要强调的一点是生物标记物的可用性和适用性。这些生物标记物的范围可以从 "近端 "靶点参与读数(如 PCSK9 转录本敲除)到更远端的药效学测量(如降低 LDL-c)。对于一个新靶点,通常需要开发生物标志物论文,并通过体外和体内试验进行验证。在研究新机制时,应仔细考虑现有的全部生物标志物,以及哪些生物标志物将优先用于体外筛选试验和体内PD读数。理想情况下,可以利用多种不同的 PD 标志物来提高转化通读率,并降低因错误信号或检测驱动信号而导致药物发现失败的可能性。如果您正在评估一个没有明确生物标记物策略的新靶点,请考虑早期发现测定可能涉及的更多工作。
Path to clinical “Proof of Concept” (PoC): What does the path look like to fund discovery and development of a program through clinical efficacy readouts?
临床 "概念验证"(PoC)的路径:通过临床疗效读数资助项目的发现和开发的路径是怎样的?
Different investors will have comfort with varying “starting points” in the drug discovery funnel; company builders will likely have experience across the spectrum from target nomination to Phase 2 asset in-license, while other investors may prefer to invest at development candidate (DC) nomination stage or later. What tends to be unifying is investors’ desire to fund through key de-risking milestones in a given financing. Frequently, de-risking is perceived as “proof of concept” (PoC) in the clinic for the lead asset, which is typically a Phase 1b or Phase 2a trial in a desired patient population with a registration (or directionally translational to registration) endpoint readout. Certainly there are other inflection points that may be meaningful, and these vary depending on whether there is any clinical precedent for the mechanism or the extent of validation possible via genetics and pharmacology models. These alternative inflection points include IND clearance, Phase 1 MAD data (which, especially for indications such as obesity where healthy volunteer data is well-precedented as a benchmark, can drive substantial value), and Phase 1 safety datasets if adverse events are a concern for the mechanism.
不同的投资者对药物发现漏斗中的不同 "起点 "会有不同的适应性;公司建立者很可能拥有从目标提名到第二阶段资产许可的全方位经验,而其他投资者可能更喜欢在候选开发(DC)提名阶段或更晚些时候进行投资。趋于统一的是,投资者希望在特定融资中通过关键的去风险里程碑来提供资金。通常情况下,去风险被认为是先导资产在临床上的 "概念验证"(PoC),这通常是在理想的患者群体中进行的 1b 期或 2a 期试验,其终点是注册(或向注册转化的方向)。当然,还有其他可能有意义的拐点,这些拐点因机制是否有临床先例或通过遗传学和药理学模型进行验证的程度而异。这些可供选择的拐点包括 IND 许可、第一阶段 MAD 数据(尤其是对于肥胖症等适应症,健康志愿者的数据作为基准是前所未有的,可以产生巨大的价值),以及第一阶段安全性数据集(如果不良事件是该机制的一个关注点)。
Ultimately investors will want to gain comfort that there is a well-conceived plan for de-risking the target thesis, and the plan can be financed with venture dollars. When considering a new target hypothesis, consider whether there are any opportunities for acceleration in the form of “tool” compounds or starting substrate (from the patent or other literature) for a more targeted medicinal chemistry, antibody discovery, etc. campaign. Alternatively, are there potential existing assets that may be available for in-licensing, especially if the potential licensor has pivoted strategic directions (i.e., asset has not been de-prioritized due to safety or efficacy)?
最终,投资者希望得到的安慰是,有一个周密的计划来降低目标论题的风险,而且该计划可以用风险资金来资助。在考虑新的目标假设时,要考虑是否有机会以 "工具 "化合物或起始底物(来自专利或其他文献)的形式加速进行更有针对性的药物化学、抗体发现等活动。另外,是否有潜在的现有资产可用于内许可,特别是如果潜在的许可方已经调整了战略方向(即资产并未因安全性或有效性而被取消优先级)?
Ultimately, having a clear view of what a PoC trial looks like (desired patient population and key inclusion/exclusion criteria, primary vs. secondary endpoints, length of trial, typical recruitment timelines, etc.) helps in planning, even in the early discovery stage. Trial considerations will ultimately inform nonclinical studies such as GLP tox, and will serve as the basis for timeline and budget discussions around a fundraise. When considering a novel target thesis, consider carefully the desired PoC inflection point through which you hope to fund through, and work backwards form there in developing the earlier nonclinical and clinical requirements to enable that PoC trial.
最终,即使在早期发现阶段,对 PoC 试验(所需患者人群和主要纳入/排除标准、主要终点与次要终点、试验长度、典型招募时间表等)有一个清晰的认识也有助于规划。试验考虑因素最终将为非临床研究(如 GLP tox)提供依据,并将作为围绕筹资讨论时间表和预算的基础。在考虑新的目标论文时,请仔细考虑您希望通过该论文获得资金的预期 PoC 拐点,并从该拐点出发,制定早期的非临床和临床要求,以实现 PoC 试验。
Product opportunity: If a drug is successfully discovered and developed, will it matter to patients and other stakeholders?
产品机会:如果成功发现并开发出一种药物,它对患者和其他利益相关者是否重要?
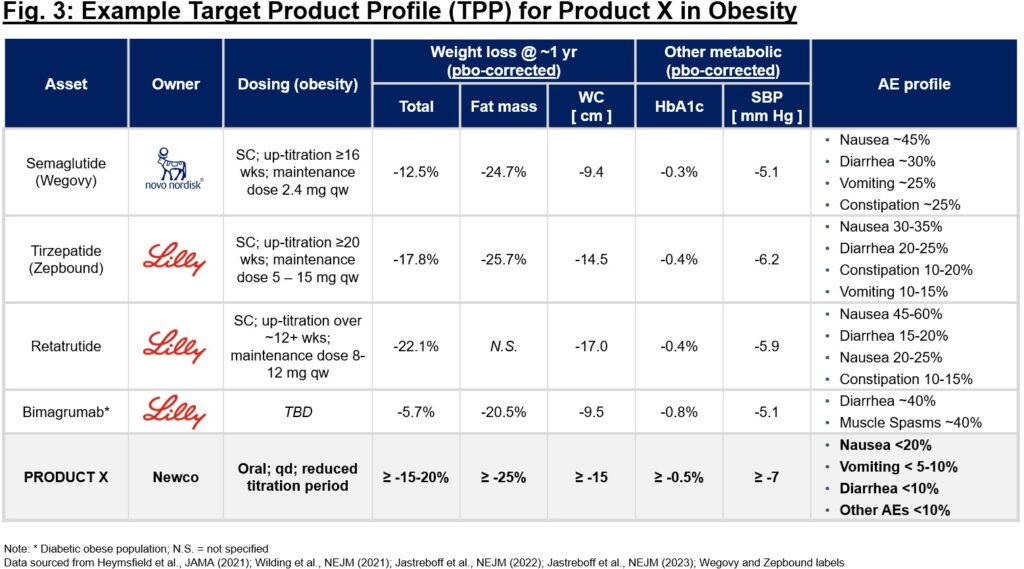
The product thesis is of great importance in the planning stage of any company (regardless of whether asset- or platform-centric). Consider anticipated time to market (not just time to the clinic), competitiveness of the mechanism itself and broader competition for the indication at large, and potential impact on patient lives should discovery and development be successful. It is essential to crystallize a target product profile (TPP) early in the process of diligencing a new target, even if completely theoretical. Using a “base case” vs. “upside” TPP, one can (in)validate the therapeutic benefit hypothesis and confirm whether it will be differentiating if drug discovery efforts are successful. This TPP can serve as substrate when pressure-testing a thesis with KOLs, patients, investors, and others. If you can’t garner excitement on the TPP alone, that might be a sign to reconsider the unmet need and differentiation thesis. Consider Fig. 3 – early benchmarking of competitive profiles can help elucidate the label characteristics that make a new profile attractive or not. In this case, given the crowded obesity market, confidence in a best-in-class profile that will offer a differentiated solution to patients and will garner eventual adoption by physicians and reimbursement by payers is critical before embarking on discovery and development.
在任何公司(无论以资产还是平台为中心)的规划阶段,产品论题都非常重要。要考虑预期的上市时间(不仅仅是进入临床的时间)、机制本身的竞争力和适应症的广泛竞争,以及如果发现和开发成功,对患者生活的潜在影响。在对新靶点进行深入研究的过程中,即使完全是理论性的,也必须尽早明确靶点产品概况(TPP)。利用 "基本情况 "与 "上升情况 "的 TPP,可以(不)验证治疗效益假设,并确认如果药物研发工作取得成功,该假设是否会产生差异。在与 KOL、患者、投资者和其他人一起对论文进行压力测试时,这种 TPP 可以作为基底。如果仅凭 TPP 无法获得兴奋点,这可能是一个信号,表明需要重新考虑未满足的需求和差异化论点。请看图 3--对竞争产品的早期基准分析有助于阐明新产品是否具有吸引力的标签特征。在这种情况下,考虑到肥胖症市场的拥挤程度,在开始探索和开发之前,关键是要对同类最佳概况充满信心,它将为患者提供差异化的解决方案,并最终获得医生的采用和支付方的报销。
To sum it up, every diligence usually involves some flavor of target validation, confidence the target can be drugged in the right direction, belief in the pharmacology (or clear path to proving or disproving the PK/PD/efficacy relationship), path to PoC that can be financed with venture dollars, and conviction that the product opportunity merits the time and investment required to bring a new medicine to patients. Of course, each target thesis has its own nuance and there is no one-size-fits-all checklist we go through as investors. If you are considering a new product concept or company idea, hope this piece helps in the brainstorming!
总而言之,每一次尽职调查通常都涉及某种程度的目标验证、对目标药物的正确方向的信心、对药理学的信念(或证明或反驳 PK/PD/ 药效关系的清晰路径)、可以用风险投资资金进行 PoC 的路径,以及对产品机会值得投入时间和投资为患者提供新药的信念。当然,每个目标论题都有其自身的细微差别,作为投资者,我们没有一个放之四海而皆准的清单。如果您正在考虑新的产品概念或公司理念,希望这篇文章能对您的头脑风暴有所帮助!
Acknowledgements: I would like to thing Haojing Rong for collaborating with me on this double-header blog post. Please check out the sister piece – a deep dive on pharmacology – which will post tomorrow!
致谢:我要感谢郝京荣与我合作撰写这篇双头博文。请关注明天发布的姊妹篇--药理学深度研究!