Abstract 摘要
Tumor progression is accompanied by fibrosis, a condition of excessive extracellular matrix accumulation, which is associated with diminished antitumor immune infiltration. Here we demonstrate that tumor-associated macrophages (TAMs) respond to the stiffened fibrotic tumor microenvironment (TME) by initiating a collagen biosynthesis program directed by transforming growth factor-β. A collateral effect of this programming is an untenable metabolic milieu for productive CD8+ T cell antitumor responses, as collagen-synthesizing macrophages consume environmental arginine, synthesize proline and secrete ornithine that compromises CD8+ T cell function in female breast cancer. Thus, a stiff and fibrotic TME may impede antitumor immunity not only by direct physical exclusion of CD8+ T cells but also through secondary effects of a mechano-metabolic programming of TAMs, which creates an inhospitable metabolic milieu for CD8+ T cells to respond to anticancer immunotherapies.
Similar content being viewed by others
他人正在浏览的类似内容
Main 主页
Cancer immunotherapy has revolutionized treatment strategies. However, most patients with cancer do not respond sufficiently to immunotherapy for it to be broadly curative. Poor responsiveness to immune checkpoint blockade (ICB) is linked to a failure of immune cells, specifically cytotoxic T lymphocytes (CTLs), to infiltrate the tumor microenvironment (TME) of solid tumors1,2. Pan-cancer transcriptomic analysis has identified a distinct TME subtype characterized by enhanced fibrosis (that is, excessive fibrillar collagen accumulation) and poor immune infiltration that correlates with a poor prognosis and failure to respond to immunotherapy3. Fibrotic tissues and tumors are composed of a stiff cross-linked fibrillar collagen extracellular matrix (ECM). Therapeutics that reduce the stiffness of the tumor-associated fibrosis improve antitumor immune infiltration and ICB response4,5. However, it remains unclear how fibrillar collagen or ECM stiffness limits antitumor immunity and responsiveness to cancer immunotherapies.
癌症免疫疗法彻底改变了治疗策略。然而,大多数癌症患者对免疫疗法的反应并不充分,因而无法广泛治愈癌症。对免疫检查点阻断疗法(ICB)反应不佳与免疫细胞(特别是细胞毒性T淋巴细胞(CTL))未能浸润实体瘤的肿瘤微环境(TME)有关 1,2 。泛癌症转录组分析发现了一种独特的肿瘤微环境亚型,其特点是纤维化(即纤维胶原过度堆积)和免疫浸润不良,与预后不良和对免疫疗法无效相关 3 。纤维化组织和肿瘤由僵硬的交联纤维胶原细胞外基质(ECM)组成。降低肿瘤相关纤维化僵硬度的治疗方法可改善抗肿瘤免疫浸润和 ICB 反应 4,5 。 然而,目前仍不清楚纤维胶原或 ECM 僵硬度如何限制抗肿瘤免疫和对癌症免疫疗法的反应。
Breast cancer progression is accompanied by fibrosis, macrophage infiltration and transforming growth factor-β (TGFβ) signaling6,7. TGFβ is a key profibrosis factor that suppresses anti-programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) therapies in colorectal and urothelial cancers8,9. This has led to studies on the role of fibroblasts in TGFβ-mediated ECM remodeling10. However, fibroblast-targeting therapies, such as sonic hedgehog inhibition, have failed to show the anticipated antitumor efficacy and, in some patients, apparently worsened tumor progression11. This led us to hypothesize that alternative mechanisms may contribute to the fibrosis-associated CTL dysfunction in tumors.
乳腺癌的进展伴随着纤维化、巨噬细胞浸润和转化生长因子-β(TGFβ)信号 6,7 。TGFβ是一种关键的坏死因子,可抑制结直肠癌和尿道癌中的抗程序性死亡1(PD-1)/程序性死亡配体1(PD-L1)疗法 8,9 。这引发了对成纤维细胞在 TGFβ 介导的 ECM 重塑中的作用的研究 10 。 然而,成纤维细胞靶向疗法,如声波刺猬抑制剂,未能显示出预期的抗肿瘤疗效,而且在一些患者中,肿瘤进展明显恶化 11 。这使我们假设,另一种机制可能导致肿瘤纤维化相关的 CTL 功能障碍。
It is now appreciated that metabolism is critical for T cell function and may be a key driver of dysfunction in tumors12. Tumors are metabolically demanding environments in which CTLs compete for critical nutrients13. T cells require glucose and amino acids, such as arginine, for their expansion and antitumor activity14,15,16,17. Historically, the primary metabolic competition for CTLs in the TME was thought to be cancer cells; however, recent studies indicate that tumor-infiltrating myeloid cells are the greatest competitors for glucose in the TME18. Beyond glucose utilization, tumor-infiltrating myeloid cells consume arginine19 through facilitative (solute carrier family 7 member 1 (SLC7A1)/SLC7A2) or antiport (SLC7A6/SLC7A7) uptake. Arginine is then metabolized by arginase, inducible nitric oxide synthase or laccase domain-containing 1 (refs. 20,21). Interestingly, the previous pan-cancer transcriptomic analysis showed correlations between fibrosis and myeloid-derived cells that metabolize arginine3. Based on these findings, we postulated that myeloid metabolism associated with fibrosis may be a determinant of ICB efficacy.
Myeloid metabolism that depletes environmental arginine is associated with impaired T cell receptor signaling, T cell proliferation, proinflammatory mediator production, antitumor immunity and ICB efficacy17,20,22. Exogenous supplementation with arginine can improve ICB efficacy, T cell proliferation and T cell survival14,15,17. However, because T cells import arginine through multifunctional transporters that competitively import a number of structurally similar molecules (for example, lysine, ornithine and arginine), the abundance of these competing molecules can dictate whether arginine supplementation improves T cell survival14. Tumor-infiltrating myeloid cells display an amino acid metabolism signature marked by SLC25A15 (ref. 23), the mitochondrial ornithine translocase, which acts downstream of arginase 1 (ARG1) for an undetermined purpose. As tumor-infiltrating myeloid cells may outcompete T cells for environmental arginine that is requisite for antitumor immunity, it remains to be determined what purpose this metabolic program serves and why it is associated with fibrosis. Here we provide evidence that mechanistically links these independent observations. We demonstrate that breast cancer-associated fibrosis drives a collagen ECM synthesis program in tumor-associated macrophages (TAMs) that is tightly regulated by TGFβ. We show that this metabolic reprogramming of macrophages is associated with enhanced utilization of arginine and secretion of ornithine into the TME, creating an inhospitable milieu for antitumor T cells and compromising ICB responsiveness.
Results 成果
Fibrosis promotes ECM-synthetic programming in myeloid cells
纤维化促进髓系细胞的 ECM 合成编程
To explore the relationship between fibrosis, stromal stiffening and myeloid cell phenotypes in the TME, we used the MMTV-PyMT (mouse mammary tumor virus–polyomavirus middle T antigen) model of mammary tumorigenesis. MMTV-PyMT mice develop mammary tumors by 6–8 weeks, accompanied by the development of a progressively stiff and fibrotic stroma as the mice age from 8 to 11 weeks (Fig. 1a,b and Extended Data Fig. 1a,b)7,24. Our previous work7 revealed that depletion of TAMs is sufficient to prevent fibrosis in 11-week-old PyMT tumors (Extended Data Fig. 1c). To explore this further, we used flow cytometry to measure the abundance of TAMs in 8- and 11-week-old PyMT tumors (Fig. 1c). As there were no differences in the total number of TAMs (CD45+Ly6C−Ly6G−F4/80+MHCII+CD24+), we carried out flow-based phenotyping25, which indicated that the ratio of TAMs expressing CD11b/CD11c changed with the onset of fibrosis (Fig. 1d). Bulk RNA sequencing (RNAseq) of TAMs (Fig. 1e) revealed that fibrotic tumors showed increased expression of genes associated with interleukin-4 (IL-4) signaling (for example, Arg1, Vegfa and Mrc1) (Extended Data Fig. 1d), collagen ECM synthesis and remodeling pathways (Fig. 1f).
图 1:肿瘤纤维化和 ECM 硬化会促进髓系细胞的胶原 ECM 合成表型。
a, Representative birefringence of collagen fibers through polarized light microscopy of PS-red-stained 8- and 11-week-old FVB/N PyMT mammary tumors; related quantification in b (scale bar, 100 µm). b, Percentage area of the PyMT tumor determined to be fibrillar collagen with PS-red staining (n = 13 or 15 mice). c, Quantification of TAMs found within PyMT mammary tumors from 8- or 11-week-old FVB/N mice, using a FACS gating strategy (Supplementary Fig. 1) (n = 9 mice). d, Quantitation of the percentage of CD11clow/CD11bhigh and CD11chigh/CD11blow tumor-infiltrating CD45+ cell types in PyMT mammary tumors from 8- or 11-week-old FVB/N mice (n = 9 mice). e, Graphical representation of the experimental setup for f. f, GO enriched in TAMs derived from 11-week-old FVB/N PyMT mammary tumors relative to TAMs derived from 8-week-old tumors (n = 5 mice). g, Relative gene expression comparing the top GO category identified in f for TAMs derived from mammary tumors from 11-week-old FVB/N PyMT mice treated with or without LOXi in the drinking water (~3 mg kg−1 per day) (n = 5 mice). Data shown represent ±s.e.m. by two-tailed unpaired Student t test (b–d), Benjamini–Hochberg corrected one-tailed t test (f) or edgeR-determined differential expression of transcript abundance (g).
We tested how a stiff and fibrotic ECM affects TAM phenotypes by inhibiting lysyl oxidase (LOX)-mediated collagen cross-linking with β-aminopropionitrile (BAPN; LOX inhibition (LOXi))5,24. RNAseq revealed that the TAMs isolated from BAPN-treated tumors expressed lower levels of collagen ECM genes (Fig. 1g). Immunofluorescence microscopy confirmed that a number of the TAM-expressed26,27,28 collagen proteins (for example, collagen VI and XII) were less abundant in BAPN-treated mice, especially at the tumor–stroma border (Extended Data Fig. 1e). Consistent with this, TAMs were found in collagen VI-rich TME regions (Extended Data Fig. 1f). We validated this in vivo finding by generating mammary fat pad-derived fibroblast ECM with and without alternatively activated bone marrow-derived macrophages (BMDMs). We observed collagen VI deposition only when BMDMs were present (Extended Data Fig. 1g). These results suggest that a population of TAMs may contribute to tumor-associated fibrosis by synthesizing molecules (for example, collagen VI or growth factors7).
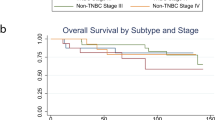
To determine whether myeloid cells in human tumors display similar transcriptional programs, we correlated the expression profiles of myeloid cells isolated from 364 human tumors across 12 cancer types with previously identified tumor immune ‘archetypes’ (Fig. 2a)29. Our analysis revealed that collagen transcripts in tumor-infiltrating myeloid cells were lowest in ‘immune-rich’ and highest in ‘immune stromal’ and ‘immune desert’ tumor immune archetypes. This suggests that collagen ECM expression in infiltrating myeloid cells may be anticorrelated with productive immune responses and patient survival29. Consistent with this, transcriptomes of 2,506 human breast tumor samples (The Cancer Genome Atlas (TCGA) repository) showed a collagen ECM signature correlated with IL-4-mediated alternative activation (‘protumor-like’) (Fig. 2b). The expression of this collagen signature in stage II and stage IIIA untreated tumor biopsy samples also correlated with reduced survival in patients with breast cancer (Extended Data Fig. 2). These findings imply that myeloid-specific expression of collagen ECM proteins is associated with compromised antitumor immunity and poor patient outcome.
a, Bubble plot of collagen gene expression of the tumor-associated myeloid compartment from the Immunoprofiler cohort (IPI) containing 12 different solid tumor types, grouped by cluster/archetype identified in the IPI cohort36. Mo, monocyte; Mp, macrophage; DC dendritic cell; DC2, type 2 dendritic cell. b, Spearman rank correlation of the gene set variation analysis of the top GO category identified in Fig. 1f and macrophage polarization signatures of tumor transcriptomes collected from TCGA repository (TCGA projects: pancreatic adenocarcinoma, lung adenocarcinoma, glioblastoma multiforme, breast invasive carcinoma, kidney renal clear cell carcinoma). LPS, lipopolysaccharide.
Stiffness and TGFβ1 affect TAM ECM synthesis
Promoter motif analysis of the genes comprising the top Gene Ontology (GO) category (Fig. 1f) predicted regulation through suppressors of mothers against decapentaplegic (SMADs) and specificity protein 1 (SP1), a transcriptional response associated with TGFβ signaling (Fig. 3a). Because TAMs secrete TGFβ1 (ref. 7) and autocrine TGFβ signaling establishes the transcriptional identity of certain myeloid cells30, we predicted that autocrine TGFβ signaling may contribute to the ECM-synthetic phenotype of TAMs. IL-4 and TGFβ1 induced the expression of collagen VI when BMDMs were plated on stiff (but not soft) fibrillar collagen-conjugated polyacrylamide gels (Fig. 3b). Stiffness and TGFβ1 induced the expression of Arg1 and suppressed restin-like molecule α (Relma/Retnla/Fizz1) in IL-4-treated BMDMs (Extended Data Fig. 3a,b). Consistent with this, TAMs isolated from 11-week-old PyMT mammary tumors expressed lower levels of Retnla, higher levels of Arg1 and higher levels of the genes associated with TGFβ signaling (for example, collagen ECM genes) (Extended Data Fig. 1c). Additionally, blocking TGFβ1 signaling restored RETNLα levels on stiff surfaces without exogenous TGFβ1 (Extended Data Fig. 3b).
a, An xy plot of relative genes comprising the GO category ‘collagen-containing ECM’ in TAMs derived from 11-week-old FVB/N PyMT mammary tumors relative to TAMs derived from 8-week-old PyMT mammary tumors. TRRUST (transcriptional regulatory relationships unraveled by sentence-based text mining) transcription factor enrichment analysis: P value—SMAD1 (10−6) and SP1 (10−2.8); combined scores—SMAD1 (127.699827), SMAD4 (90.48497202), SMAD3 (48.37819162), SMAD2 (46.66904886) and SP1 (6.551517203). b, Representative immunofluorescence microscopy of collagen VI (ColVI, white) and DNA (blue) in IL-4-polarized BMDMs cultured on soft (400 Pa) or stiff (60,000 Pa) collagen I-coated polyacrylamide hydrogel surfaces treated with 1 ng ml−1 TGFβ1 for 24 h; representative effect of every replicate experiment (n = >10 independent experiments) (scale bar, 20 µm). c, Graphical representation of the experimental setup for d–j. d, Representative immunofluorescence microscopy of collagen VI or XII (white) and DNA (blue) in stiff-collagen orthotopic C57BL/6J PyMT mammary tumors after 3 weeks of growth in Tgfbr2MyeKO or control animals; related quantification in e (scale bar, 100 µm). e, Quantitation of immunofluorescence microscopy of collagen VI (top) or XII (bottom) in stiff-collagen orthotopic C57BL/6J PyMT mammary tumors after 3 weeks of growth in Tgfbr2MyeKO or control animals; each trace represents the mean fluorescence intensity (MFI) of collagen VI or XII across four transverse sections of the tumor–stromal border (starting ~50 µm into the stroma and 125–300 µm into the tumor) (n = 5 mice averaged in each trace). f, Quantification of tumor masses accumulated after 3 weeks of in vivo growth of stiff-collagen orthotopic C57BL/6J PyMT mammary tumors in Tgfbr2MyeKO or control animals (n = 13 or 11 mice). g, Representative immunofluorescence microscopy of cleaved caspase-3 (green) and DNA (blue) in stiff-collagen orthotopic C57BL/6J PyMT mammary tumors after 3 weeks of growth in Tgfbr2MyeKO or control animals; related quantification in h (scale bar, 100 µm). h, Quantitation of cleaved caspase-3 percentage area per field view in the representative image in g (n = 5 mice). i, Quantification of tumor-infiltrating CD8+ T cells as a percentage of CD45+ cell types found in stiff-collagen C57BL/6J orthotopic PyMT mammary tumors after 3 weeks of growth in Tgfbr2MyeKO or control animals (n = 8 or 6 mice). j, Ratio of tumor-infiltrating CD8+ to CD4+ cell types in stiff-collagen orthotopic C57BL/6J PyMT mammary tumors after 3 weeks of growth in Tgfbr2MyeKO or control animals (n = 8 or 6 mice). Data shown represent ±s.e.m. by two-tailed unpaired Student t test (f, h–j).
We hypothesized that either TAMs in stiff and fibrotic tumors may engage an autocrine TGFβ1 signaling circuit or that stiffness potentiates TGFβ signaling, as other growth factor signaling circuits are mechanosensitive31. To test these possibilities, we analyzed whether TGFβ-induced SMAD signaling is affected by the stiffness of the fibrillar collagen the cells interact with and then tested whether stiffness affects active TGFβ production. We found that stiffness enhanced SMAD signaling downstream of TGFβ (Extended Data Fig. 3c) and BMDMs on stiff fibrillar collagen produced higher levels of active TGFβ1 (Extended Data Fig. 3d,e). These data indicate that TAMs in stiff and fibrotic tumors may establish autocrine TGFβ1 circuits with enhanced sensitivity that reinforce the expression of an ECM-synthetic, Arg1-high and Retnla-low TAM phenotype that we observed in the 11-week-old stiff and fibrotic PyMT mammary tumors.
To test the TGFβ dependence of the ECM-synthetic TAM phenotype, we generated a mouse model lacking TGFβ signaling in the myeloid cells (LysM-Cre × Tgfbr2flox/flox, Tgfbr2MyeKO). We then injected PyMT tumor cells in ribose-stiffened collagen into the mammary fat pads of mice and analyzed ECM accumulation, tumor mass and the immune infiltrate (Fig. 3c). We observed a reduction in collagen VI at the tumor–myeloid–stromal boundary and collagen XII throughout the Tgfbr2MyeKO tumors (Fig. 3d,e). Tumor mass was reduced (Fig. 3f) and cell death was increased in the tumor–stromal border zones, indicated by increased levels of cleaved caspase-3 (apoptosis) in Tgfbr2MyeKO tumors (Fig. 3g,h). Because we observed similar numbers of TAMs where differences in apoptosis were apparent (Extended Data Fig. 3f), we hypothesized that tumor cell apoptosis could be affected by TAM-mediated suppression of antitumor T cell responses32,33. Using multiparameter flow cytometry, we observed a significant increase in the proportion of CD8+ T cells and an enhanced ratio of CD8+/CD4+ T cells within the Tgfbr2MyeKO tumors (Fig. 3i,j), suggesting a more productive antitumor response in Tgfbr2MyeKO tumors.
Stiffness and TGFβ1 synergize to tune TAM metabolism
Thus far, we have shown that tumor progression is accompanied by a TGFβ-driven ECM-synthetic program in TAMs that correlates with lower CTL infiltration. We next investigated how the ECM-synthetic phenotype restricts CTL-mediated antitumor immunity. T cells have a unique metabolism supporting their effector functions and survival in the TME14. Specific metabolic programs are essential for immune cells to achieve discrete phenotypes or functions34,35,36, and available metabolite concentrations can affect these programs14,37,38,39. As ECM stiffness exerts important effects on cellular metabolism40, we profiled polar metabolites in spontaneous PyMT tumors in which stromal stiffness and fibrosis were prevented by inhibiting LOX cross-linking of collagens. Our objective was to determine whether fibrosis affects metabolite levels and test whether that could affect infiltrating immune cells. We found that preventing collagen cross-linking and stromal stiffening through LOXi caused an approximately fourfold increase in the arginine concentration in tumors (Fig. 4a).
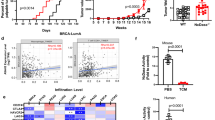
a, Relative arginine concentration in mammary tumors from 11-week-old C57BL/6J PyMT mice treated with or without LOXi in the drinking water (~3 mg kg−1 per day); LC–MS analysis (n = 3 mice). b, Relative ornithine and proline concentrations in mammary tumors from 11-week-old C57BL/6J PyMT mice treated with or without LOXi in the drinking water (~3 mg kg−1 per day); LC–MS analysis (n = 3 mice). c, Relative ornithine and proline concentrations in stiff-collagen orthotopic C57BL/6J PyMT mammary tumors after 3 weeks of growth in Tgfbr2MyeKO or control animals; LC–MS analysis (n = 7 or 6 mice). d, Relative medium concentrations of arginine and ornithine from a culture with IL-4-polarized BMDMs treated with or without 1 ng ml−1 TGFβ1 for 22 h, swapped for fresh medium for 2 h, which was measured by LC–MS analysis (n = 3 independent experiments). e, Graphical depiction of the observed relationship between TGFβ1 signaling and ECM stiffness in the context of collagen synthesis and proline metabolism. f, Graphical representation (top) of the experimental setup for g–j and Kaplan–Meier survival curve (bottom) of Arg1MyeKO or LysM-Cre tumor-bearing C57BL/6J mice (time until the tumor reached 2 cm in diameter) (n = 16 mice, two separate cohorts of 8 mice per genotype). Mantel–Cox test: χ2 = 25.30, degree of freedom (d.f.) = 1 and P < 0.0001. g, Representative immunofluorescence microscopy of collagen VI (white) and DNA (blue) in Arg1MyeKO or LysM-Cre C57BL/6J tumors; related quantification in h (scale bar, 100 µm). h, Quantitation of collagen VI percentage area per field view in the representative image in g (n = 5 animals and two images of each). i, Representative immunofluorescence microscopy of cleaved caspase-3 (red), CD8 (white) and DNA (blue) in Arg1MyeKO or LysM-Cre C57BL/6J tumors; related quantification in j (scale bar, 100 µm). j, Quantitation of cleaved caspase-3 percentage area per field view in the representative image in i (n = 5 animals and two images of each). Data shown represent ±s.e.m. by two-tailed unpaired Student t test (a–d and h–j) or Mantel–Cox (f).
Tumor interstitial fluid (TIF) analysis indicated that solid tumors have lower TME arginine and human breast tumors contain significantly lower concentrations of arginine relative to normal tissues41,42. Myeloid cells expressing high levels of Arg1 have been implicated as major consumers of arginine in the TME43,44. Collagen comprises a disproportionally high abundance of proline (approximately eightfold higher than the proteome median), which can be synthesized from arginine or glutamine. Tumor metabolomics revealed that, corresponding to the increased concentration of arginine, there was a commensurate decrease in two products of arginine metabolism—proline and ornithine—in the PyMT mice, in which collagen cross-linking and stromal stiffness were reduced (Fig. 4b). There is evidence of a direct relationship between arginine depletion and ornithine enrichment in the TME41,42, and TGFβ signaling enhances proline synthesis45 through glutamine and arginine metabolism46. Therefore, we sought to determine whether myeloid TGFβ signaling affects the proportion of arginine/ornithine/proline in tumors. Using mass spectrometry (MS)-based metabolomics, we profiled Tgfbr2MyeKO tumors and their corresponding controls. We found that, while levels of arginine were unaffected (Extended Data Fig. 4a), ornithine and proline levels were significantly reduced in Tgfbr2MyeKO tumors (Fig. 4c). These findings implicate TAM TGFβ signaling as a regulator of these metabolites in tumors.
To determine whether TGFβ stimulates the production of ornithine and proline, we analyzed IL-4-stimulated BMDMs interacting with a stiff matrix in vitro that were treated with or without TGFβ. The analysis revealed that TGFβ triggered enhanced arginine uptake and ornithine efflux into the culture medium (Fig. 4d). These results suggest that TGFβ promoted the conversion of arginine to proline, and the intermediate (ornithine) was produced in excess such that it was secreted into the medium (Fig. 4e)47,48. As ARG1 is an enzyme that converts arginine to ornithine, and we found that it was enriched in late-stage tumor TAMs in a TGFβ- and ECM stiffness-dependent fashion, we next sought to test whether myeloid-specific deletion of Arg1 affects tumor growth, myeloid-derived ECM protein levels (using collagen VI abundance as a proxy) and apoptosis in the TME. We generated myeloid-specific Arg1 knockout (Arg1MyeKO) mice with orthotopic PyMT tumors in ribose-stiffened collagen gels (Fig. 4f) and determined that the median survival was 22.5% longer for the heterozygous Arg1MyeKO mice compared with controls (Fig. 4f). Collagen VI levels were lower (Fig. 4g,h) and apoptosis markers were higher in Arg1MyeKO tumors (Fig. 4i,j). While CTLs were localized to the periphery of the control tumors, Arg1MyeKO tumors contained CTLs that were dispersed throughout (Fig. 4i).
We next sought to confirm that arginine metabolism contributes to proline synthesis and ECM production. IL-4-polarized BMDMs without arginine or homozygous Arg1KO BMDMs with arginine did not produce collagen VI (a proxy for ECM production) in vitro (Extended Data Fig. 4b), indicating a requirement for arginine and Arg1. We supplemented Arg1+ BMDMs with [13C6]arginine and fate traced its metabolism to proline (m + 5) (Fig. 5a,b). ECM stiffness and TGFβ synergistically enhanced the utilization of arginine for proline synthesis, consistent with Arg1 expression. Intracellular hydroxyproline levels (Extended Data Fig. 4c) were also enhanced by TGFβ and ECM stiffness, whereas intracellular ornithine levels (Fig. 5c) were increased only by ECM stiffness and proline levels were elevated by TGFβ (Fig. 5d). Because de novo proline synthesis can also be fed by glutamine metabolism, which is also mechanosensitive49, we assessed whether ECM stiffness influences glutamine conversion into proline. We found that stiff fibrillar collagen caused approximately fivefold higher incorporation of 13C into proline from [13C5]glutamine (Extended Data Fig. 4d) that was dependent on TGFβ signaling (Extended Data Fig. 4e). These findings indicate that the two primary pathways implicated in de novo proline synthesis are both mechanosensitive and TGFβ sensitive.
a, Graphical representation of the experimental setup for b–d. b, 13C tracing of [13C6]arginine metabolism into [13C5]proline in BMDMs cultured on soft (400 Pa) or stiff (60,000 Pa) collagen I-coated polyacrylamide hydrogel surfaces in a medium containing [12C6]arginine, treated with or without 1 ng ml−1 TGFβ1 for 20 h then swapped for fresh medium containing [13C6]arginine for 4 h; BMDMs were harvested and measured by LC–MS (n = 3 independent experiments). c, Relative ornithine concentration in BMDMs cultured on soft (400 Pa) or stiff (60,000 Pa) collagen I-coated polyacrylamide hydrogel surfaces with or without 1 ng ml−1 TGFβ1 for 24 h, measured by LC–MS (n = 3 independent experiments). d, Relative proline concentration in BMDMs cultured on soft (400 Pa) or stiff (60,000 Pa) collagen I-coated polyacrylamide hydrogel surfaces with or without 1 ng ml−1 TGFβ1 for 24 h, measured by LC–MS (n = 3 independent experiments). e, Relative concentrations of metabolites in the culture medium of IL-4-polarized BMDMs cultured on soft (400 Pa) or stiff (60,000 Pa) collagen I-coated polyacrylamide hydrogel surfaces treated with or without 1 ng ml−1 TGFβ1 for 24 h and then swapped for fresh serum-free medium for 24 h, measured by LC–MS analysis (n = 3 independent experiments). f, Graphical representation of the experimental setup for g. g, Relative concentrations of ornithine in the culture medium of IL-4-polarized BMDMs cultured on stiff (60,000 Pa) collagen I-coated polyacrylamide hydrogel surfaces treated with 1 ng ml−1 TGFβ1 for 24 h with or without 1 µM CB-1158, measured by LC–MS analysis (n = 3 independent experiments). h, Graphical representation of the experimental setup for i. i, Relative concentrations of ornithine in the culture medium of IL-4-polarized Arg1KO BMDMs cultured on soft (400 Pa) or stiff (60,000 Pa) collagen I-coated polyacrylamide hydrogel surfaces treated with 1 ng ml−1 TGFβ1 for 24 h, measured by LC–MS analysis (n = 3 independent experiments). Data shown represent ±s.e.m. by one-way analysis of variance (ANOVA) with Tukey test for multiple comparisons (b–e) or two-tailed unpaired Student t test (g and i).
To assess whether ECM stiffness influences the utilization of exogenous arginine and glutamine, we analyzed IL-4-stimulated BMDMs interacting with a soft or stiff matrix that were treated with or without TGFβ and quantified the metabolite composition of the medium. We observed that the primary metabolite consumed was arginine, and the metabolites that accumulated were proline and ornithine (Fig. 5e). These findings indicate that stiffness potentiates the accumulation of ornithine intracellularly and that the presence of TGFβ enhances its secretion into the medium. We verified that this effect was ARG1 dependent with the arginase inhibitor CB-1158 (Fig. 5f,g)50 and homozygous Arg1KO BMDMs (Fig. 5h,i). It has been proposed that proline synthesis serves as a vent for excess mitochondrial reducing equivalents (for example, NADH and NADPH) when mitochondrial respiration is suppressed. We found that TGFβ suppresses mitochondrial respiration (Extended Data Fig. 4f) and NADH levels are synergistically enhanced by TGFβ and ECM stiffness (Extended Data Fig. 4g). This implies that stiffness increases proline synthesis as a compensatory response to increased NADH, similar to what has been found in fibroblasts45.
Ornithine impairs CD8+ CTL metabolism and antitumor activity
Commensurate with the arginine consumption, we observed an increase in environmental ornithine in the TME of the PyMT tumors, as well as in the culture medium of TGFβ-stimulated Arg1+ BMDMs interacting with a stiff ECM. Arginine is an essential metabolite for activated lymphocyte survival and antitumor responses14. Recent studies have shown that conditioning tumors with arginine-synthetic bacteria, which consume ammonia to synthesize arginine, potentiates cancer immunotherapies reliant on activated CTLs15. Due to the nature of the metabolic pathway introduced into these bacteria, we hypothesized that these engineered bacteria might use ornithine as a substrate to synthesize arginine, thereby correcting the arginine–ornithine imbalance in the TME. To test this prediction, we assessed whether the engineered arginine-synthetic strain of Escherichia coli Nissle 1917 (Arg-ECN) could synthesize arginine from a medium devoid of ammonia but replete with ornithine. We found that Arg-ECN avidly synthesized arginine from the ornithine supplied in the medium even without exogenous ammonia (Extended Data Fig. 5a). This indicates that the ornithine accumulation we had observed may also be a co-occurring factor, along with arginine depletion, driving immunosuppression in the TME.
The clinical relevance of arginine/ornithine/proline metabolism is difficult to determine because human tumor samples are collected from fasted individuals, and fasting has a marked effect on the levels of these metabolites in the tissues and circulation51. However, it has been documented that human breast tumors have lower arginine levels and higher proline levels than normal tissues (Extended Data Fig. 5b)42, and ornithine is a critical intermediate of this process. To test whether elevated ornithine levels influence antitumor immune responses in the TME, we applied gavage-based supplementation of water, glycine, ornithine or arginine to the MMTV-PyMT transgenic mammary tumor model (Extended Data Fig. 5c). Glycine was included as an isocaloric control. Dietary supplementation with ornithine, arginine or glycine did not affect the size of orthotopic PyMT primary tumors (Extended Data Fig. 5d), but ornithine supplementation increased the concentration of plasma ornithine by ~12-fold (Extended Data Fig. 5e). Supplementation with arginine also increased circulating ornithine levels by approximately threefold; however, the relationship between arginine and ornithine concentrations was biased toward increased arginine.
Using multiparameter flow cytometry, we assessed the number of CTLs in the TME. We found that none of the dietary supplements affected the percentage of CD8+ CTLs among the total infiltrating immune cells (Fig. 6a,b). However, we found that ornithine supplementation reduced the proportion of activated CD44+PD-1+ tumor-infiltrating CD8+ CTLs (Fig. 6c) and the expression levels of PD-1 and CD69 (Fig. 6d,e), whereas arginine supplementation did not, despite also increasing the circulating ornithine levels (Extended Data Fig. 5e). Restimulated CD8+ CTLs isolated from ornithine-supplemented tumors showed lower Ki-67 positivity (Fig. 6f), indicating that ornithine impairs proliferation. Immunofluorescence revealed that arginine supplementation increased the number of apoptotic cells at tumor–stroma border zones (Extended Data Fig. 5f) and CD8+ CTLs in the tumor core (Extended Data Fig. 5g,h). Because Tgfbr2MyeKO PyMT tumors were smaller after 3 weeks of growth and had less ornithine but not more arginine, we tested whether ornithine supplementation in Tgfbr2MyeKO mice could normalize these tumor growth differences. We found that ornithine supplementation abolished tumor mass differences (Fig. 6g,h), and few CD8+ CTLs were detected within the tumor core (Extended Data Fig. 5i).
a, Graphical description of the experimental setup for b–f. b, Percentage of CD8+ CTLs among CD45+ cells isolated from stiff-collagen PyMT tumors after 3 weeks of growth in C57BL/6J mice gavaged daily with 2 g kg−1 glycine, ornithine, arginine or water (100 µl) (n = 8 mice). c, Percentage of CD44+PD-1+ cells among CD8+ CTLs isolated from stiff-collagen PyMT tumors after 3 weeks of growth in C57BL/6J mice gavaged daily with 2 g kg−1 glycine, ornithine, arginine or water (100 µl) (n = 8 mice). d, MFI of PD-1 staining of endogenous CD8+ CTLs isolated from stiff-collagen PyMT tumors after 3 weeks of growth in C57BL/6J mice gavaged daily with 2 g kg−1 glycine, ornithine, arginine or water (100 µl) (n = 8 mice). gMFI, geometric MFI. e, MFI of CD69 staining of endogenous CD8+ CTLs isolated from stiff-collagen PyMT tumors after 3 weeks of growth in C57BL/6J mice gavaged daily with 2 g kg−1 glycine, ornithine, arginine or water (100 µl) (n = 8 mice). f, Quantification of Ki-67 incorporation into endogenous CD8+ CTLs isolated from stiff-collagen PyMT tumors after 3 weeks of growth in C57BL/6J mice gavaged daily with 2 g kg−1 glycine, ornithine, arginine or water (100 µl) and stimulated ex vivo with PMA (50 ng ml−1) and ionomycin (500 ng ml−1) (n = 8 mice). g, Graphical description of the experimental setup for h. h, Quantification of tumor masses accumulated after 3 weeks of in vivo growth of soft- or stiff-collagen orthotopic PyMT mammary tumors in Tgfbr2MyeKO or control mice gavaged daily with 2 g kg−1 ornithine (n = 9 or 10 mice). i, Heat map of relative metabolite levels in CD3/CD28-activated CD8+ CTLs cultured for 72 h in a medium containing a molar ratio of 3:1 ornithine/arginine; the medium was refreshed every 24 h, and samples were analyzed by LC–MS (n = 3 independent experiments). GSSG, oxidized glutathione; UDP-GlcNAc, uridine diphosphate N-acetylglucosamine; GABA, γ-aminobutyric acid; AMP, adenosine monophosphate; NADPH, nicotinamide adenine dinucleotide phosphate. j, Representative immunofluorescence microscopy of stiff-collagen OVA-PyMT tumor organoids challenged with GFP+ OT-I CTLs (green) for 24 h in a medium containing a molar ratio of 1:1 (0.5 mM) or 3:1 (1.5 mM) ornithine/arginine, cleaved caspase-3 (red) and DNA (blue), representative of the effect observed in four independent experiments (scale bar, 40 µm). Data shown represent ±s.e.m. by one-way ANOVA with Tukey test for multiple comparisons (b–f) or two-tailed unpaired Student t test (h).
Models of T cell activation using CD3/CD28 antibodies have demonstrated that activation triggers distinct metabolic programming14,38,52. To explore how ornithine affects CD3/CD28-activated CD8+ CTL metabolism, we titrated the concentration of ornithine relative to arginine (0:1, 1:1, 3:1 and 9:1) and measured the intracellular metabolome with MS-based metabolomics (the 9:1 concentration ratio mimics the concentration in the whole tumor, whereas 3:1 mimics the concentration in TIF). After 24 h of activation, increased concentrations of ornithine resulted in decreased ATP and increased arginine levels (Extended Data Fig. 6a). While it is not clear why arginine is a favored metabolic substrate in activated CTLs, suppression of arginine metabolism and a decrease in ATP levels could impede translation (high ATP demand), thereby reducing the synthesis of effector proteins and retarding antitumor programming. Peak utilization of arginine occurs ~72 h after activation14. Accordingly, we analyzed the metabolome of CD8+ CTLs after 3 days of culture in an environment containing a molar ratio of ~3:1 ornithine/arginine (Fig. 6i). The results revealed that nearly all metabolite pools were suppressed by environmental ornithine, except for oxidized glutathione, which negatively affects the survival and inflammatory programming of CTLs53,54.
Because arginine and ornithine are structurally similar and use permease-type importers of the cationic amino acid transporter/SLC7A family, we hypothesized that, due to overlapping import requirements, arginine and ornithine may competitively inhibit each other’s import, and uptake would be proportional to their environmental concentrations47,48. To test this, we assessed the intracellular metabolome of CD3/CD28-activated CD8+ CTLs after 3 days of activation with the last 24-h medium containing 1 mM [13C5]ornithine with or without 1 mM arginine (Extended Data Fig. 6b). Surprisingly, there was no difference in the total amount of ornithine between the two conditions. The relative amount of [13C5]ornithine indicated that when ornithine was present, it amounted to nearly 97.5% of endogenous ornithine even when equimolar concentrations of arginine were available. We had expected that ~50% of the ornithine pool would come from arginine (that is, [12C5]ornithine). However, the presence of arginine altered the fate of ornithine metabolism and increased the levels of argininosuccinate, citrate and asparagine, which was undetectable in cells cultured without arginine (Extended Data Fig. 6c).
To determine whether ornithine levels affect antigen-specific CD8+ CTL antitumor responses, we used a cell line derived from the PyMT-mCherryOVA model55 that expresses the model antigen chicken ovalbumin (OVA) and transgenic OT-I CD8+ CTLs that specifically recognize the OVA-derived SIINFEKL peptide. PyMT-OVA tumor organoids embedded within a ribose-stiffened three-dimensional (3D) collagen gel or cultured as a monolayer on top of glass coverslips were cocultured with green fluorescent protein-positive (GFP+) OT-I CD8+ CTLs in ornithine (Fig. 6j and Extended Data Fig. 6d). Tumor cells cultured with ornithine appeared to be resistant to OT-I-mediated apoptosis, and the only GFP+ OT-I cells we could find in ornithine-supplemented conditions were undergoing apoptosis or terminally fragmented (~1- to 3-μm fragments with no observable nuclei).
PD-1 expression on CD8+ CTLs typifies ongoing recognition of tumor antigens that may be responsive to checkpoint blockade-based immunotherapies56. We noticed that ornithine supplementation decreased PD-1 expression on CTLs isolated from PyMT tumors (Fig. 6c,d). Accordingly, we sought to test whether PyMT tumor responsiveness to PD-1 blockade is affected by elevated circulating levels of ornithine. Using gavage-based supplementation of ornithine or water with and without anti-PD-1 treatment in mice bearing PyMT tumors, we found that ornithine negatively affected ICB (Fig. 7a,b). Quantitation of amino acid levels in TIF revealed that ornithine supplementation increased the levels of ornithine (Fig. 7c) and arginine in mice that did not receive ICB (Fig. 7d). We observed similar trends for proline (Fig. 7e) but not glutamine (Fig. 7f). Overall, while ornithine supplementation impeded the efficacy of ICB in this tumor model, it had limited effects on the levels of other metabolites in TIF, suggesting that these effects were primarily driven by ornithine itself (Fig. 7g).
a, Kaplan–Meier survival curve of PyMT tumor-bearing C57BL/6J mice gavaged daily with 2 g kg−1 ornithine or water (100 µl) and treated with isotype control or anti-PD-1 blocking antibodies on days 5, 9 and 13 (time until the tumor reached 2 cm in diameter) (n = 8). Mantel–Cox test, isotype (water) versus anti-PD-1 (water): χ2 = 5.925, d.f. = 1 and P = 0.0149. Mantel–Cox test, isotype (water) versus anti-PD-1 (ornithine): χ2 = 2.282, d.f. = 1 and P = 0.1309. b, Tumor volume measurements of stiff-collagen PyMT tumors in C57BL/6J mice gavaged daily with 2 g kg−1 ornithine or water (100 µl) and treated with isotype control or anti-PD-1 blocking antibodies on days 5, 9 and 13 (n = 8 mice, error bars indicate the s.d.). c–f, Concentration of ornithine (c), arginine (d), proline (e) and glutamine (f) in TIF derived from PyMT tumors in C57BL/6J mice gavaged daily with 2 g kg−1 ornithine or water (100 µl) at the terminal endpoint; LC–MS analysis (n = 7 mice). g, Heat map of relative amino acid concentrations in TIF derived from PyMT tumors in C57BL/6J mice gavaged daily with 2 g kg−1 ornithine or water (100 µl) at the terminal endpoint, grayscale intensity indicates fold change; LC–MS analysis (n = 7 mice). h, Graphical description of the experimental setup for i and j. i, Tumor volume measurements of stiff-collagen PyMT tumors over 4 weeks of growth in C57BL/6J mice gavaged daily with 2 g kg−1 glycine, ornithine or arginine (100 µl) for the first 14 days and treated with isotype control or anti-PD-1 blocking antibodies on days 5, 9 and 13 (n = 8 mice, error bars indicate the s.d.). j, Kaplan–Meier survival curves for the experiment depicted in h. Mean survival of mice supplemented with glycine and treated with isotype control and mice supplemented with ornithine and treated with anti-PD-1 blockade: 21 days. Mean survival of mice supplemented with glycine and arginine and treated with anti-PD-1 blockade: 28 days. Mantel–Cox test, anti-PD-1 (arginine) versus anti-PD-1 (ornithine): χ2 = 5.644, d.f. = 1 and P = 0.0175. k, Graphical representation of the identified relationship between the TME, infiltrating myeloid cells, mechano-metabolic programming and CTLs in the TME. Data shown represent ±s.e.m. or s.d. (b and i) by Mantel–Cox (a and j) or one-way ANOVA with Tukey test for multiple comparisons (c, e and f) or two-tailed unpaired Student t test (b, d and i).
To test the effects of amino acids on ICB further, we assessed tumor growth in mice receiving glycine (approximately isocaloric amino acid supplement control), ornithine or arginine supplementation. While ornithine, arginine or glycine supplementation alone did not affect the growth of orthotopic PyMT tumors, tumor growth was affected in the mice treated with anti-PD-1 antibodies (Fig. 7i). Ornithine supplementation abolished any beneficial effects of the anti-PD-1 blockade such that tumors grew to the humane endpoint as quickly as in isotype-treated control animals (Fig. 7j). By contrast, checkpoint blockade in the context of arginine and glycine supplementation exerted a ~33% increase in the median survival time of the treated mice. These data suggest that fibrotic tumors, which are enriched in ornithine through ECM-synthetic Arg1+ TAMs, may impede antitumor CD8+ CTL responses by creating an inhospitable metabolic milieu for their antitumor activities (Fig. 7k).
Discussion
We found that the stiff fibrotic tumor stroma synergizes with TGFβ to induce a collagen ECM-synthetic phenotype in TAMs. Our analyses indicated that collagen ECM-synthetic TAMs condition the TME with ornithine, which alters CD8+ CTL function and limits ICB responsiveness. Our results expand upon the prevailing dogma that cancer fibrosis acts as a physical barrier that causes an ‘immunologically cold’ TME8,9. These observations offer a complementary explanation in which fibrosis triggers TAM phenotypes that adjust the metabolic characteristics of the TME, compromising CTL function. Indeed, while prior work has demonstrated that inhibiting LOX-dependent cross-linking and stromal stiffening increases CTL motility and ICB responsiveness, we show that this model reverts the collagen ECM-synthetic TAM phenotype and the associated metabolic effects to restore antitumor immunity4,5. Thus, a stiff and fibrotic TME may impede antitumor immunity not only by direct physical exclusion of CD8+ CTLs but also through secondary effects of a macrophage mechano-metabolic programming that creates an inhospitable metabolic milieu of productive antitumor immunity.
Early tumor-infiltrating TAMs promote the development of tumor-associated fibrosis by conditioning the TME with TGFβ1, which acts on stromal fibroblasts to synthesize fibrillar collagens and collagen cross-linking enzymes, leading to a stiffened TME7. In turn, the stiffened, fibrotic ECM recruits TAMs6,57, promotes tumor aggression58, and metabolically reprograms tumor cells40 and infiltrating immune cells. Our results demonstrate that the infiltrating TAMs themselves synthesize and secrete ECM proteins, including collagen VI and XII, which can exert pleiotropic effects on the organization and stiffness of the tumor epithelium and non-cellular and cellular stroma59,60,61,62. Thus, while cancer-associated fibroblasts are the primary source of fibrillar collagen in the TME, TAMs contribute specific collagens to the TME, such as the beaded filament collagen VI, which forms microfibrillar networks that entrain epithelial basement membranes to interstitial ECM and facilitate wound healing, scar tissue formation, re-epithelialization and metastatic progression61,62. The relative contributions of TAM-deposited ECM proteins to tumor fibrosis and their role in creating spatial ECM and cellular heterogeneity merit further investigation.
Therapeutic approaches intended to treat fibrosis in the TME have not yet yielded clinical success, possibly because they were not combined with ICB, targeted inappropriate stromal cell types or were based on inaccurate assumptions. For instance, cancer-associated fibrosis is thought to drive tumor aggression in part because it serves as a physical barrier to CTL infiltration4,5. Yet, T cells have been shown to migrate through submicron spaces when extravasating into inflamed tissues or through engineered pore sizes much smaller than the highly cross-linked fibrillar collagen matrices observed in tumors, implying that the dense collagen-rich ECM in tumors is unlikely to impede T cell infiltration considerably63,64,65. Our findings suggest that the fibrotic TME distorts antitumor immunity through alterations in myeloid metabolism. Thus, approaches designed to remediate the metabolic microenvironment associated with fibrosis may be a more effective antitumor strategy. Indeed, the increased experimental pancreatic adenocarcinoma aggression induced by ablating α-smooth muscle actin-positive fibroblasts and depleting fibrillar collagen was accompanied by a massive infiltration of protumorigenic myeloid cells, which our analyses suggest may have driven tumor aggression and compromised antitumor immunity through their aberrant metabolism66. Consistent with this, therapies that reduce myeloid infiltrates in the TME have beneficial effects with and without ICB67,68, whereas treatments intended to target fibroblasts in the TME have thus far shown limited clinical benefit11. As we and others have illustrated through metabolic supplementation or the use of Arg-ECN, metabolic remediation of the TME may be an effective approach to improve antitumor immunity. Additionally, as an SLC25A15-expressing subset of TAMs is likely to be the major contributor to arginine depletion and ornithine enrichment in the TME23, identifying ways to ablate this TAM population selectively may be sufficient to prevent myeloid protumor programming while maintaining antitumor myeloid populations in the TME. Overall, these types of approaches might have a higher potential to overcome recent failures encountered with antistromal therapies due to factors including non-specificity, poor penetration or stromal fibroblast heterogeneity11.
Arginine supplementation can potentiate antitumor immunity and the efficacy of checkpoint blockade therapies15. However, ingested arginine can be metabolized in the gut or liver, generating ornithine, which, based on our analyses, could exert suppressive effects on CTL proliferation and activation69. Furthermore, our results indicate that although arginine supplementation can exert beneficial effects on CTL function, it also simultaneously increases the circulating concentration of ornithine, which would potentially compromise its beneficial impact on antitumor immunity. Consistent with this, chronic viral infections of the liver increase the circulating ornithine levels, which impede virus-specific T cell expansion, indicating that ornithine is a systemic immunomodulatory metabolite for CTLs70. Accordingly, therapies that locally increase arginine through ornithine consumption, as opposed to systemic arginine supplementation, may offer a greater ability to enhance checkpoint blockade therapies and promote antitumor immunity. Toward this objective, local synthesis of arginine by Arg-ECN, which was designed to consume environmental ammonia and excrete arginine in the TME, was recently shown to improve checkpoint blockade15. Such findings indicate that this Arg-ECN strain may improve the arginine–ornithine concentration ratio in the TME, thereby enhancing ICB efficacy and potentiating antitumor immunity.
One of the unresolved questions from our work is why the physical properties of the TME would promote a collagen ECM-synthetic TAM phenotype at the expense of antitumor inflammation. We hypothesize that the physical properties of the TME subvert antitumor responses in favor of protumor ECM-synthetic TAM programming due to an evolutionarily beneficial, but maladaptive in the tumor context, wound-healing response triggered by the fibrillar ECM content of the TME or site of injury. Open and closed wounds initially display inflammatory programs regardless of sterility. These inflammatory programs are initiated by endogenous damage-associated molecular patterns (DAMPs) in sterile wounds that collaborate with exogenous pattern-associated molecular patterns (PAMPs) in open wounds to elicit inflammatory programs that remodel the site of injury for efficient surveillance and clearance of the sources of PAMPs/DAMPs. However, inflammatory programming is not static; as PAMPs/DAMPs are cleared, temporary reconstructive scaffolding (for example, fibrin) is used to physically support the site of inflammation that is subsequently replaced by a more permanent fibrillar collagen matrix. This fibrillar collagen matrix is synthesized and mechanically entrained by neighboring cells (for example, fibroblasts or keratinocytes), and it can dictate the recruitment of immune cells57 or a programmatic homeostasis between stromal cell types71. Similar to wound-healing responses, the inflammatory programming of myeloid cells is not static. Dynamic phenotypic transitions occur in phases at sites of injury, where myeloid-derived cells that initially express DAMP-driven proinflammatory signatures transition into anti-inflammatory states that produce TGFβ1 and synthesize specific types of ECM that facilitate and typify the resolution of wound healing72,73.
Physically, the similarities between the phased responses observed in wound healing or tumor progression are intuitively obvious. The early inflamed (that is, PAMP/DAMP-enriched) microenvironment is soft due to loss of tissue structure and cell death or clearance. As inflammatory signals are cleared or tolerated74, ECM synthesis and cell contractility mediate tissue stiffening in specific regions of the TME (for example, invasive borders). Microenvironmental physical cues synergize with PAMP/DAMP signaling to inform infiltrating cells to express transcriptional phenotypes intended to match the stage of healing (for example, fibronectin is a Toll-like receptor 4 ligand that becomes inaccessible once incorporated into the fibrillar matrix). Physical properties of healing wounds reflect the stage of repair, where a stiff wound has reached the phase in which the source of DAMP/PAMP has been cleared and the wound is structurally entrained to its surrounding tissue. Our data suggest that one mechanism by which the physical properties of the TME drive TAMs to a protumor/repair phenotype is by dictating the metabolic fate of arginine, which is a hallmark difference between pro- and anti-inflammatory phenotypes of myeloid cells75. Myeloid arginine metabolism has an immunomodulatory role by restricting the proliferation and activity of T cells76, which may serve to lessen inflammatory responses in healing wounds or progressing tumors77. Overall, our results suggest that mechano-metabolic programming induced by the physical properties of a tissue may instruct and support infiltrating myeloid cell programming to match a presumed stage of wound healing, which is speciously subverted in tumor progression.
Methods
Oversight and ethics
All mice were maintained under pathogen-free conditions, and all mouse studies were performed with strict adherence to the use authorizations (AN194983 and AN184232) provided by the Institutional Animal Care and Use Committee of the University of California San Francisco.
Cell culture
Bone marrow was isolated from the femurs of 8- to 12-week-old female C57BL/6J mice (through dissection and syringe-mediated PBS hydraulic pressure) and cultured in 25 mM glucose DMEM (GenClone, 25-500) supplemented with 10% FBS (Hyclone, SH30071.03) and penicillin/streptomycin (Gibco, 151140-122) with 25 ng ml−1 recombinant murine macrophage colony-stimulating factor 1 (CSF1) (Peprotech, 315-02) for 7 days (medium changes every 3 days, first 20 ml and then 25 ml) in 15-cm2 tissue culture polystyrene culture plates (GenClone, 25-203) to generate BMDMs. BMDMs were treated with IL-4 (5 ng ml−1) (Peprotech, 214-14) with and without TGFβ1 (R&D Systems, 7666-MB).
CD8+ CTLs were isolated from the spleen and inguinal and axillary lymph nodes of 8- to 12-week-old female C57BL/6J (Jax: 000664) or OT-I (C57BL/6-Tg(TcraTcrb)1100Mjb/J, Jax: 003831) mice. Tissues were pulverized with a 1-ml syringe plunger through a PBS-wetted 70-µm cell strainer (Corning, 352350). The resulting cell suspension was treated with ACK lysis buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.3) to remove red blood cells, and CD8+ T cells were isolated using EasySep Mouse CD8+ T Cell Isolation Kit (STEMCELL Technologies, 19853) per the manufacturer’s instructions. CD8+ T cells were activated with Dynabeads Mouse T-Activator CD3/CD28 for T Cell Expansion and Activation (Gibco, 11456D) without exogenous recombinant IL-2 supplementation. OT-I CD8+ T cells were isolated and cocultured with PyMT-OVA tumor cells61 that display the model antigen chicken OVA (SIINFEKL) in 25 mM glucose DMEM (GenClone, 25-500) supplemented with or without l-ornithine (RPI, 020040).
ECM-coated polyacrylamide hydrogel cell culture surfaces
Cleaned (10% ClO, 1 M HCl and then 100% ethanol) round #1 German glass coverslips (Electron Microscopy Services) were coated with 0.5% v/v (3-aminopropyl)triethoxysilane (APTES) (Sigma, 440140), 99.2% v/v ethanol and 0.3% v/v glacial acetic acid for 2 h and then cleaned in 100% ethanol on an orbital shaker at 22 °C. APTES-activated coverslips were coated with PBS-buffered acrylamide/bis-acrylamide (Bio-Rad, 1610140 and 1610142) solutions (3%/0.05% for 400 Pa, 7.5%/0.07% and 10%/0.5% for 60,000 Pa) polymerized with TEMED (0.1% v/v) (Bio-Rad, 1610801) and potassium persulfate (0.1% w/v) (Thermo Fisher, BP180) to yield a final thickness of ~85 µm. Polyacrylamide hydrogel cell culture surfaces (PA-gels) were washed with 70% ethanol and sterile PBS before coating with sterile filtered 3,4-dihydroxy-l-phenylalanine (DOPA) in pH 10 (10 mM) Tris buffer for 5 min at 22 °C protected from light78. DOPA-coated PA-gels were washed twice with sterile PBS, and ECM was functionalized with 10 µg ml−1 type I collagen (Corning, 354236) in sterile PBS for 1 h at 37 °C.
Fibroblast-derived ECM culture surfaces
Fibroblast-derived ECM was generated based on previously described methods79. Multipotent subcutaneous adipose-derived fibroblasts80 were seeded in gelatin-coated tissue culture dishes, cultured for 8 days in a complete medium (DMEM, 10% FBS, 1% antibiotics/antimycotics) with 1 ng ml−1 TGFβ1 and 50 µg ml−1 ascorbic acid added every 48 h to support collagen synthesis. Cell cultures were washed with PBS and decellularized using a prewarmed (37 °C) extraction buffer for 2–5 min (0.5% Triton X-100, 20 mM NH4OH in PBS). Residual ECM was then washed three times with PBS before seeding BMDMs on top of the fibroblast-derived ECM.
qPCR
Total RNA was isolated from biological samples with TRIzol (Invitrogen, 15596-018) according to the manufacturer’s instructions. cDNA was synthesized with 250 ng of total RNA in a 10-µl reaction volume using Moloney murine leukemia virus (M-MLV) reverse transcriptase (BioChain, Z5040002-100K) and 5× reaction buffer (BioChain, Z5040002-100K), random hexamers (Roche, 11034731001), dNTPs and 1 U of RiboLock (Thermo Fisher Scientific, EO0384). According to the reverse transcription thermocycler program, random hexamers and RNA were incubated at 70 °C for 10 min and then held at 4 °C until the addition of the M-MLV reverse transcriptase, dNTPs, RiboLock and M-MLV reverse transcriptase; then, they were incubated at 50 °C for 1 h, followed by incubation at 95 °C for 5 min; finally, they were stored at −20 °C until qPCR was performed. The reverse transcription reaction was then diluted to a 50-µl total volume with double-distilled H2O, rendering a concentration of 20 ng RNA per 1 µl used in subsequent qPCR reactions. qPCR was performed in triplicate using PerfeCTa SYBR Green FastMix (Quantabio, cat. no. 95072-05 K) with Eppendorf Mastercycler RealPlex2 and qPCR primer sequences (Supplementary Table 1). The qPCR thermocycler program consisted of 10 min at 95 °C and then 40 cycles of 95 °C for 15 s, 60 °C for 20 s, followed by a melt curve at 60–95 °C for 10 min. Melt curves and gel electrophoresis were used to validate the quality of amplified products. The ΔCt values from independent experiments were used to calculate the fold change of expression using the 2−ΔΔCt method. For each gene measured, the s.e.m. of the ΔCt values was calculated and used to generate positive and negative error values in the 2−ΔΔCt fold change space. Plots of qPCR data display bars representing the mean fold change ± s.e.m. and individual points representing the fold change value for each experiment relative to the mean.
In vitro respirometry
Mitochondrial stress tests were performed with a Seahorse XF24e cellular respirometer on non-permeabilized cells (50,000 cells per well) in V7 microplates, with XF assay medium supplemented with 1 mM pyruvate (Gibco), 2 mM glutamine (Gibco) and 25 mM glucose (Sigma) at pH 7.4 and sequential additions, through injection ports, of oligomycin (1 µM final), FCCP (1 µM final) and antimycin A/rotenone (1 µM final) during respirometry (concentrated stock solutions solubilized in 100% ethanol (2.5 mM) for mitochondrial stress test compounds). Oxygen consumption rate values are presented with the non-mitochondrial oxygen consumption deducted.
RNAseq
RNAseq data from TAMs were generated using spontaneous PyMT mammary tumors collected in DMEM on ice and then minced with a prison-shank-like razor blade. Tissues were digested in two 30-min rounds with collagenase and DNase with agitation at 37 °C. At the end of each round, digested tissue was washed through a 100-µm cell strainer on ice. Red blood cells were lysed with ACK buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.3), and the remaining cells were washed and counted. Cells were stained with anti-mouse CD45, anti-mouse CD11b, anti-mouse Ly6C, anti-mouse CD14, anti-mouse I-A/I-E, anti-mouse CD11c, anti-mouse CD24, anti-mouse CD29, anti-mouse CD31 and Zombie NIR live/dead. Sorted macrophages were CD45+, MHCII+ and CD14+. Sorted cells were pelleted and snap frozen on dry ice. RNA was harvested using TRIzol, measured on a spectrophotometer and run on a bioanalyzer to confirm quality. Libraries were prepared using Nugen Ovation plus Nextera according to the manufacturer’s instructions. Multiplexed libraries were sequenced on an Illumina HiSeq 4000, and reads were aligned to the human genome (hg19) using RNA STAR81. Aligned reads were counted using HOMER82, and hierarchical clustering was performed using Cluster83 and visualized with Java TreeView. GO and motif analyses were performed using Metascape84. Referenced accession number: GSE157290. Strains used: FVB/NJ (Jax: 001800) and FVB/N-Tg(MMTV-PyVT)634Mul/J (Jax: 002374).
RNAseq data from human tumors were reanalyzed for myeloid expression levels of transcripts associated with the collagen-containing ECM GO category (data published previously30) (referenced accession number: GSE184398). Additionally, transcriptomic data for the breast invasive carcinoma, glioblastoma multiforme, kidney renal clear cell carcinoma, lung adenocarcinoma and pancreatic adenocarcinoma projects were acquired from TCGA repository. To control for the disparity in total RNA collected for each sample, observed library sizes were scaled into effective library sizes through the trimmed mean of M values (TMM) method85 in calcNormFactors (edgeR v3.34.1). Briefly, the TMM method assumes that most genes are not differentially expressed between samples and scales gene-level counts to alleviate artifactual expression differences that arise due to differing initial RNA abundances and sequencing depths. RNAseq counts were filtered to remove lowly expressed genes and then normalized using calcNormFactors (edgeR v3.34.1); GSVA (v1.40.1) was applied to estimate the enrichment of collagen genes and macrophage M1- and M2-like signatures driven by IL-4 or lipopolysaccharide29.
Picrosirius red staining, polarized light microscopy and second-harmonic generation
Formalin-fixed paraffin-embedded tissue sections were stained using 0.1% picrosirius red (PS-red) (Direct Red 80, Sigma, 365548) in picric acid solution (Sigma, P6744) and counterstained with Weigert’s hematoxylin (Cancer Diagnostics, cat. no. CM3951). Polarized light images were acquired using an Olympus IX81 microscope fitted with an analyzer (U-ANT) and a polarizer (U-POT, Olympus) oriented parallel and orthogonal to each other.
Second-harmonic generation imaging was performed using a custom-built two-photon microscope setup equipped with resonant-scanning instruments based on published designs containing a five-photomultiplier tube array (Hamamatsu, C7950). The setup consisted of two-channel simultaneous video rate acquisition through two photomultiplier tube detectors and an excitation laser (2-W MaiTai titanium–sapphire laser, 710- to 920-nm excitation range). Second-harmonic generation imaging was performed on a Prairie Technology Ultima System attached to an Olympus BX-51 fixed-stage microscope equipped with a ×25 (numerical aperture 1.05) water immersion objective. Paraformaldehyde-fixed or formalin-fixed paraffin-embedded tissue sections were exposed to polarized laser light at a wavelength of 830 nm, and emitted light was separated using a filter set (short-pass filter, 720 nm; dichroic mirror, 495 nm; band-pass filter, 475/40 nm). Images of x–y planes at a resolution of 0.656 mm per pixel were captured using an open-source Micro-Magellan software suite. Macrophages were depleted in MMTV-PyMT mice by intraperitoneal injections of 1 mg anti-CSF1 (Bio X Cell, cat. no. BE0204-A025, clone 5A1) or an immunoglobulin G1 (IgG1) control (Bio X Cell, cat. no. BP0088-A025) every 7 days starting at 4 weeks of age. Mice were killed at 11 weeks of age for PS-red and polarized light microscopy.
The presence of fibrosis was scored by a pathologist who assessed the occupied percentage of the stromal area as follows: 0%, no stromal fibrosis (0); <10%, minimal (1); 10–30%, mild (2); 30–60%, moderate (3); >60%, high (4). Strains used: FVB/NJ (Jax: 001800) and FVB/N-Tg(MMTV-PyVT)634Mul/J (Jax: 002374).
Liquid chromatography–MS metabolomics and arginine- or glutamine-derived 13C flux
BMDMs (500,000 cells) were seeded on 50-mm2 varied stiffness ECM-coated PA-gels and cultured for 24 h in 25 mM glucose DMEM or 20–22 h in 25 mM glucose DMEM. Then, the medium was changed to DMEM lacking glutamine (Gibco, 11960044), replaced with [13C5]glutamine (Cambridge Isotope Laboratories, CLM-1822-H-PK), for 2 h, or SILAC DMEM (Gibco, 88364) lacking arginine and lysine, replaced with [13C6]arginine (Cambridge Isotope Laboratories, CLM-2265-H-PK) and l-lysine (Sigma, L5501), for 4 h. Activated CD8+ T cells (500,000 cells) (CD3/CD28 Dynabeads) were cultured in DMEM supplemented with l-ornithine for prescribed time points, with the medium changed after 48 h for the 3-day activation. Cells were washed twice with PBS and extracted with MS-grade 80% methanol (Fisher, A456-1) and 20% water (Fisher, W6500) supplemented with 1 nmol dl-norvaline (Sigma, N7502). Flash-frozen tumors/tissues were pulverized with liquid nitrogen in a mortar and pestle on dry ice, and 400-µg protein equivalents of tissue were extracted with MS-grade 80% methanol (Fisher, A456-1) and 20% water (Fisher, W6500) supplemented with 1 nmol dl-norvaline (Sigma, N7502). Tissue or insoluble material from cell extracts was used to calculate protein equivalents by resuspension in 0.2 M NaOH, heated to 95 °C for 25 min and determined by BCA (Pierce, 23225). Dried metabolites were resuspended in 50% acetonitrile/water, and one-tenth of the volume was loaded onto a Luna 3 μm NH2 100 A (150 × 2.0 mm) column (Phenomenex). Chromatographic separation was performed on a Vanquish Flex system (Thermo Fisher Scientific) with mobile phases A (5 mM NH4AcO, pH 9.9) and B (acetonitrile) and a flow rate of 200 µl min−1. A linear gradient from 15% A to 95% A over 18 min was followed by 9-min isocratic flow at 95% A and re-equilibration to 15% A. Metabolites were detected with a Thermo Fisher Scientific Q Exactive mass spectrometer run with polarity switching (+3.5 kV/−3.5 kV) in the full-scan mode with an m/z range of 65–975. TraceFinder 4.1 (Thermo Fisher Scientific) was used to quantify the targeted metabolites by the area under the curve using the expected retention time and accurate mass measurements (<5 ppm). Values were normalized to cell numbers and sample protein concentrations. Relative amounts of metabolites were calculated by summing the values for all isotopologues of a given metabolite. The fractional contribution of 13C carbons to the total carbon for each metabolite was calculated as previously described86. Data analysis was accomplished with in-house developed open-source R scripts (available at https://rdrr.io/github/graeberlab-ucla/MetabR/).
In vitro TGFβ1 ELISA
IL-4-stimulated (5 ng ml−1) BMDMs (200,000 cells) were cultured on varied stiffness ECM-coated PA-gels for 48 h in 25 mM glucose DMEM. The culture medium was collected and assayed for the presence of TGFβ1 through sandwich ELISA per the manufacturer’s instructions (R&D Systems, DB100C) without activation of latent TGFβ1.
Immunofluorescence microscopy
Cells or tissues were fixed in 4% paraformaldehyde (Electron Microscopy Services, 15710) in PBS for 30 min at room temperature (for cell cultures) or overnight at 4 °C (for tissues), washed and blocked with blocking buffer (HBSS fortified with 10% FBS (Hyclone), 0.1% BSA (Fisher, BP1600), 0.05% saponin (EMD, L3771) and 0.1% Tween-20 (Fisher, BP337500)). The samples were incubated with primary antibodies (1:100–1:200) for 2 h at room temperature (22–23 °C) or 24 h at 4 °C. Then, the samples were incubated with secondary antibodies (1:1,000) for 2 h at room temperature. Samples were imaged with a Nikon Eclipse Ti spinning-disc microscope, Yokogawa CSU-X, Andor Zyla sCMOS, Andor Multi-Port Laser unit and Molecular Devices MetaMorph imaging suite. The antibodies and concentrations used were as follows: cleaved caspase-3 (1:200) (Cell Signaling, 9661, AB_2341188), collagen XII (1:200) (Sigma, SAB4500395, AB_10743871), collagen VI (1:200) (Abcam, ab6588, AB_305585), CD8 (1:150) (eBioscience, 14-0808-82, AB_2572861), PyMT (1:100) (SCBT, sc-53481, AB_630138) and F4/80 (1:200) (Cell Signaling, 70076, AB_2799771).
Fibrillar collagen tumor organoids and orthotopic tumors
Collagen type I (4.8 mg ml−1 final) (rat tail, Corning, 354249) was suspended in 0.1% glacial acetic acid with (stiff) or without (soft) non-metabolizable l-ribose (0.5 mM final) (Chem-Impex International, 28127) for at least 28 days at 4 °C to facilitate complete collagen glycation/cross-linking32. To generate orthotopic tumors or 3D tumor cell organoids, we suspended 1 M ml−1 PyMT tumor cells in collagen with or without l-ribose supplemented with basement membrane extract (20% v/v final) (R&D Systems, Cultrex BME, 3532-005-02) and DMEM (1× final) and adjusted the pH to ~7.3. Tumor cell–collagen suspensions were kept on ice until implantation into the fourth/fifth inguinal mammary fat pad of 8-week-old female mice (varied genotypes) through a 25-gauge syringe (100 µl per animal) or pipetted (20 µl) into culture dishes containing growth medium for in vitro tumor organoid cultures.
Primary tumor growth
Tumor growth was monitored by caliper measurement and volume estimation based on V = (W2 × L)/2, where V = volume, W = width and L = length, or absolute measurement of mass. Mice were monitored to ensure that the observable tumors did not exceed the ethics committee-permitted diameter of 2 cm. Mice were maintained in a 12-h light/12-h dark cycle, ambient temperature of 20–22 °C, relative humidity of between 30% and 70%, and fed 5058 PicoLab Mouse Diet 20 ad libitum. Strains used: C57BL/6J (Jax: 000664), C57BL/6-Arg1tm1Pmu/J (Jax: 008817), B6.129P2-Lyz2tm1(cre)Ifo/J (Jax:004781) and B6;129-Tgfbr2tm1Karl/J (Jax: 012603) backcrossed to pure C57BL/6J mice.
Amino acid supplementation and PD-1 checkpoint blockade
Fibrillar collagen orthotopic tumors were generated with 100,000 PyMT cells in 100 µl collagen suspension per mouse, implanted through 25-gauge syringe injection into the fourth/fifth inguinal mammary fat pad of 8-week-old female C57BL/6J mice. Every day until the humane endpoint, the mice were gavaged with 100 µl water or 2 g kg−1 glycine (Fisher, BP3815), ornithine (RPI, 020040) or arginine (TCI, A0528) suspended in water (~100 µl). For PD-1 checkpoint blockade, mice were gavaged with glycine, ornithine or arginine daily for the 2 weeks after orthotopic tumor implantation. On days 5, 9 and 13 after orthotopic tumor implantation, mice were intraperitoneally injected with 200 μg of an anti-PD-L1 monoclonal antibody (RMP1-14, Bio X Cell, BE0146, lot no. 810421D1) or isotype control (IgG2a, Bio X Cell, BE0089, lot no. 815021S1). For PD-1 checkpoint blockade, mice were humanely killed when the tumors passed 1,000 mm3 (no tumors exceeded 2 cm in diameter).
Quantitative analysis of TIF amino acid levels
Tumors were harvested, weighed and rinsed with room-temperature saline, 150 mM NaCl, and blotted dry on filter paper. Tumors were then placed on 20-µm nylon filters (Spectrum Labs, 148134) atop 50-ml conical tubes and centrifuged at 1,000,000g for 10 min at 4 °C. The extracted TIF was removed from the conical tube, frozen on dry ice and stored at −80 °C until further analysis. Approximately 100 µl of TIF was collected from each tumor. A 5-µl aliquot of a sample was mixed with 45 µl of acetonitrile/methanol/formic acid (75:25:0.1) extraction mixture spiked with 13C-labeled internal standards for each amino acid measured (Cambridge Isotope Laboratory). Samples were then vortexed for 10 min at 4 °C, and the insoluble material was sedimented by centrifugation at 15,000g for 10 min at 4 °C. A 20-µl sample of the soluble polar metabolite extract was taken for liquid chromatography (LC)–MS analysis. All solvents used in the extraction mixture were high-performance LC grade.
LC–MS analysis was performed on the sample extracts using a Q Exactive Orbitrap mass spectrometer equipped with an Ion Max source and a heated electrospray ionization (HESI) probe coupled to a Dionex Ultimate 3000 ultra-performance LC system (Thermo Fisher Scientific). External mass calibration was performed every 7 days, and internal mass calibration (lock masses) was not used. A 2-μl aliquot of each sample was injected onto a ZIC-pHILIC 2.1 × 150 mm analytical column equipped with a 2.1 × 20 mm guard column (both 5-μm particle size, EMD Millipore). The autosampler and column oven were held at 4 °C and 25 °C, respectively. Buffer A consisted of 20 mM ammonium carbonate and 0.1% ammonium hydroxide, whereas buffer B was acetonitrile. The chromatographic gradient was run at a flow rate of 0.150 ml min−1, as follows: 0–20 min, linear gradient from 80% to 20% B; 20–20.5 min, linear gradient from 20% to 80% B; 20.5–28 min, held at 80% B. The mass spectrometer was operated in the full-scan, polarity-switching mode with the spray voltage set to 3.0 kV, the heated capillary held at 275 °C and the HESI probe held at 350 °C. The sheath gas flow rate was set to 40 units, the auxiliary gas flow was set to 15 units and the sweep gas flow was set to 1 unit. MS data acquisition was performed in an m/z range of 70–1,000, with the resolution set to 70,000, the automatic gain control target at 1 × 106 and the maximum injection time at 20 ms. Metabolite identification was performed with XCalibur 2.2 software (Thermo Fisher Scientific) using a 5-ppm mass accuracy and a 0.5-min retention time window. For metabolite identification, external standard pools were used to assign the metabolites to peaks at given m/z and retention time values and to determine the limit of detection for each metabolite, which ranged from 100 nM to 3 µM (see ref. 41 for the m/z, retention time and limit of detection for each metabolite analyzed).
Fluorescence-activated cell sorting
Fluorescence-activated cell sorting (FACS) was performed as described previously87. Tumors were processed into single-cell suspensions through razor blade-mediated pulverization and enzymatic digestion with DNase (200 mg ml−1) (Sigma-Aldrich, 10104159001), collagenase I (100 U ml−1) and collagenase type IV (500 U ml−1) (Worthington Biochemical, LS004197 and LS004189) on a shaking incubator (37 °C) for 30 min. Enzymatic activity was quenched by doubling the volume of the tissue digest with FACS buffer (2% FCS in PBS). The resulting cell suspensions were filtered through a 70-µm cell strainer (Corning, 352350) to obtain single-cell suspensions. Lymph nodes and tumor-draining lymph nodes were pulverized with a 1-ml syringe plunger through a PBS-wetted 70-µm cell strainer to generate single-cell suspensions. For each sample, 5–10 × 106 cells were washed with PBS and stained with Zombie NIR Fixable live/dead dye (BioLegend, 423106) at 4 °C for 20 min. Cells were washed with PBS, followed by surface staining at 4 °C with directly conjugated antibodies (see the list below) diluted in FACS buffer containing anti-CD16/32 (Bio X Cell, BE0307, RRID:AB_2736987 or BioLegend, 101320, RRID:AB_1574975) for 30 min. Cells were washed again with FACS buffer. For intracellular staining, cells were restimulated in RPMI (Gibco, 11875085) supplemented with 10% FCS (Benchmark, NC1643060), penicillin–streptomycin–glutamine (Gibco, 10378016) and β-mercaptoethanol (50 µM) (Gibco, 21985023) containing phorbol 12-myristate 12-acetate (PMA) (50 ng ml−1) (Sigma, P8139), ionomycin (500 ng ml−1) (Invitrogen, I24222) and brefeldin A (3 mg ml−1) (Sigma, B7651) in 5% CO2 at 37 °C for 3–5 h. After surface staining, cells were fixed at 4 °C for 20 min and washed in permeabilization buffer from the fixation/permeabilization kit (BD Biosciences, 554714). Antibodies against intracellular targets were diluted in permeabilization buffer, and cells were incubated at 4 °C for 30 min, followed by another wash before assessment with a BD LSR Fortessa SORP cytometer. All flow cytometry data were generated and analyzed in a blinded fashion.
Statistics and reproducibility
Mouse and in vitro data were derived from experiments that were repeated at least two times and analyzed with GraphPad Prism. No statistical methods were used to predetermine sample sizes unless specified in the reporting summary. Data distribution was assumed to be normal; if found to be otherwise, a post-test to determine outliers was used. Flow cytometry experiments were conducted in a blinded fashion, whereas other experiments were not, as the experiments were conducted and analyzed by the same researchers. Mouse tumor experiments were randomized so that the groups had no differences in mean body weight at the start of the experiment. Individual data points that significantly deviated from the group mean were identified using Grubb’s test; the removal of the data points in question did not affect whether a P value was <0.05 for a given comparison (specifically, one data point was removed from the ornithine group in Fig. 4e).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNAseq data described here have been deposited in Gene Expression Omnibus and is publicly available (referenced accession numbers: GSE157290 and GSE184398), and data for the breast invasive carcinoma, glioblastoma multiforme, kidney renal clear cell carcinoma, lung adenocarcinoma and pancreatic adenocarcinoma projects were acquired from TCGA repository (https://portal.gdc.cancer.gov/). Source data for Figs. 1b–d, 3f,h,i,j, 4a–d,h,j, 5e,g,h, 6b–f,h and Fig. 7b–f,i and Extended Data Figs. 1a,b,d, 2, 3a,d,e, 4a,c–i, 5a,e,f,g and 6b have been provided as source data files. Minimally processed metabolomics datasets are provided in Supplementary Table 2. All other data supporting the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Open-source R scripts for metabolomics analysis can be found at https://rdrr.io/github/graeberlab-ucla/MetabR/.
References
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Bagaev, A. et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell 39, 845–865 (2021).
Sun, X. et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 599, 673–678 (2021).
Nicolas-Boluda, A. et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. eLife 10, e58688 (2021).
Acerbi, I. et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. (Camb.) 7, 1120–1134 (2015).
Maller, O. et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. 20, 548–559 (2021).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Chakravarthy, A., Khan, L., Bensler, N. P., Bose, P. & De Carvalho, D. D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9, 4692 (2018).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. Cell 169, 570–586 (2017).
Geiger, R. et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842 (2016).
Canale, F. P. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598, 662–666 (2021).
Ma, E. H. et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells. Immunity 51, 856–870 (2019).
Rodriguez, P. C. et al. l-Arginine consumption by macrophages modulates the expression of CD3ζ chain in T lymphocytes. J. Immunol. 171, 1232–1239 (2003).
Reinfeld, B. I. et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Grzywa, T. M. et al. Myeloid cell-derived arginase in cancer immune response. Front. Immunol. 11, 938 (2020).
Wei, Z., Oh, J., Flavell, R. A. & Crawford, J. M. LACC1 bridges NOS2 and polyamine metabolism in inflammatory macrophages. Nature https://doi.org/10.1038/s41586-022-05111-3IF: 50.5 Q1 (2022).
Menjivar, R. E. et al. Arginase 1 is a key driver of immune suppression in pancreatic cancer. eLife https://doi.org/10.7554/eLife.80721IF: 6.4 Q1 (2023).
Li, S. et al. Metabolism drives macrophage heterogeneity in the tumor microenvironment. Cell Rep. 39, 110609 (2022).
Mouw, J. K. et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med. 20, 360–367 (2014).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014).
Afik, R. et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J. Exp. Med. 213, 2315–2331 (2016).
Schnoor, M. et al. Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J. Immunol. 180, 5707–5719 (2008).
Karsdal, M. A. (ed.) Biochemistry of Collagens, Laminins and Elastin: Structure Function and Biomarkers https://doi.org/10.1016/C2015-0-05547-2 (Elsevier, 2016).
Combes, A. J. et al. Discovering dominant tumor immune archetypes in a pan-cancer census. Cell 185, 184–203 (2022).
Yu, X. et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity 47, 903–912 (2017).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Peranzoni, E. et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc. Natl Acad. Sci. USA 115, E4041–E4050 (2018).
Sinha, P., Clements, V. K. & Ostrand-Rosenberg, S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J. Immunol. 174, 636–645 (2005).
Leone, R. D. & Powell, J. D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 20, 516–531 (2020).
Lim, A. R., Rathmell, W. K. & Rathmell, J. C. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. eLife 9, e55185 (2020).
Bantug, G. R., Galluzzi, L., Kroemer, G. & Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 18, 19–34 (2018).
Rossiter, N. J. et al. CRISPR screens in physiologic medium reveal conditionally essential genes in human cells. Cell Metab. 33, 1248–1263 (2021).
Leney-Greene, M. A., Boddapati, A. K., Su, H. C., Cantor, J. R. & Lenardo, M. J. Human plasma-like medium improves T lymphocyte activation. iScience 23, 100759 (2020).
Cantor, J. R. et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell 169, 258–272 (2017).
Tharp, K. M. et al. Adhesion-mediated mechanosignaling forces mitohormesis. Cell Metab. 33, 1322–1341 (2021).
Sullivan, M. R. et al. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. eLife 8, e44235 (2019).
Benedetti, E. et al. A multimodal atlas of tumour metabolism reveals the architecture of gene–metabolite covariation. Nat. Metab. 5, 1029–1044 (2023).
Kumar, V., Patel, S., Tcyganov, E. & Gabrilovich, D. I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 37, 208–220 (2016).
Raber, P., Ochoa, A. C. & Rodríguez, P. C. Metabolism of l-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol. Invest. 41, 614–634 (2012).
Schwörer, S. et al. Proline biosynthesis is a vent for TGFβ-induced mitochondrial redox stress. EMBO J. 39, e103334 (2020).
Durante, W., Liao, L., Reyna, S. V., Peyton, K. J. & Schafer, A. I. Transforming growth factor-β1 stimulates l-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation 103, 1121–1127 (2001).
Yadav, P. et al. Reciprocal inflammatory signals establish profibrotic cross-feeding metabolism. Preprint at bioRxiv https://doi.org/10.1101/2023.09.06.556606 (2023).
Tran, D. H. et al. Mitochondrial NADP+ is essential for proline biosynthesis during cell growth. Nat. Metab. 3, 571–585 (2021).
Torrino, S. et al. Mechano-induced cell metabolism promotes microtubule glutamylation to force metastasis. Cell Metab. 33, 1342–1357 (2021).
Steggerda, S. M. et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J. Immunother. Cancer 5, 101 (2017).
Stekovic, S. et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 30, 462–476 (2019).
Argüello, R. J. et al. SCENITH: a flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 32, 1063–1075 (2020).
Mak, T. W. et al. Glutathione primes T cell metabolism for inflammation. Immunity 46, 675–689 (2017).
Yarosz, E. L. & Chang, C.-H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw. 18, e14 (2018).
Engelhardt, J. J. et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell 21, 402–417 (2012).
Zaitsev, A. et al. Precise reconstruction of the TME using bulk RNA-seq and a machine learning algorithm trained on artificial transcriptomes. Cancer Cell 40, 879–894 (2022).
Pakshir, P. et al. Dynamic fibroblast contractions attract remote macrophages in fibrillar collagen matrix. Nat. Commun. 10, 1850 (2019).
Pickup, M. W., Mouw, J. K. & Weaver, V. M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253 (2014).
Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 3, a004978 (2011).
Papanicolaou, M. et al. Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nat. Commun. 13, 4587 (2022).
Simões, F. C. et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 11, 600 (2020).
Wishart, A. L. et al. Decellularized extracellular matrix scaffolds identify full-length collagen VI as a driver of breast cancer cell invasion in obesity and metastasis. Sci. Adv. 6, eabc3175 (2020).
Thompson, S. B. et al. Formin-like 1 mediates effector T cell trafficking to inflammatory sites to enable T cell-mediated autoimmunity. eLife 9, e58046 (2020).
Davidson, M. D., Burdick, J. A. & Wells, R. G. Engineered biomaterial platforms to study fibrosis. Adv. Healthc. Mater. 9, e1901682 (2020).
Carey, S. P., Martin, K. E. & Reinhart-King, C. A. Three-dimensional collagen matrix induces a mechanosensitive invasive epithelial phenotype. Sci. Rep. 7, 42088 (2017).
Özdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Germano, G. et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 23, 249–262 (2013).
Mantovani, A., Allavena, P., Marchesi, F. & Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. https://doi.org/10.1038/s41573-022-00520-5IF: 122.7 Q1 (2022).
Dröge, W. et al. Suppression of cytotoxic T lymphocyte activation by l-ornithine. J. Immunol. 134, 3379–3383 (1985).
Lercher, A. et al. Type I interferon signaling disrupts the hepatic urea cycle and alters systemic metabolism to suppress T cell function. Immunity 51, 1074–1087 (2019).
Adler, M. et al. Principles of cell circuits for tissue repair and fibrosis. iScience 23, 100841 (2020).
Nguyen-Chi, M. et al. Identification of polarized macrophage subsets in zebrafish. eLife 4, e07288 (2015).
Foster, D. S., Jones, R. E., Ransom, R. C., Longaker, M. T. & Norton, J. A. The evolving relationship of wound healing and tumor stroma. JCI Insight 3, e99911 (2018).
Timblin, G. A. et al. Mitohormesis reprogrammes macrophage metabolism to enforce tolerance. Nat. Metab. 3, 618–635 (2021).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Van De Velde, L.-A. et al. T cells encountering myeloid cells programmed for amino acid-dependent immunosuppression use Rictor/mTORC2 protein for proliferative checkpoint decisions. J. Biol. Chem. 292, 15–30 (2017).
Eming, S. A., Murray, P. J. & Pearce, E. J. Metabolic orchestration of the wound healing response. Cell Metab. 33, 1726–1743 (2021).
Wouters, O. Y., Ploeger, D. T. A., van Putten, S. M. & Bank, R. A. 3,4-Dihydroxy-l-phenylalanine as a novel covalent linker of extracellular matrix proteins to polyacrylamide hydrogels with a tunable stiffness. Tissue Eng. Part C Methods 22, 91–101 (2016).
Kaukonen, R., Jacquemet, G., Hamidi, H. & Ivaska, J. Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat. Protoc. 12, 2376–2390 (2017).
Tharp, K. M. et al. Actomyosin-mediated tension orchestrates uncoupled respiration in adipose tissues. Cell Metab. 27, 602–615 (2018).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Lin, Y. C. et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat. Immunol. 11, 635–643 (2010).
Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl Acad. Sci. USA 95, 14863–14868 (1998).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Cameron, A. M. et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 20, 420–432 (2019).
Kersten, K. et al. Spatiotemporal co-dependency between macrophages and exhausted CD8+ T cells in cancer. Cancer Cell 40, 624–638 (2022).
Abe, M. et al. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216, 276–284 (1994).
Acknowledgements
We thank E. Benedetti and E. Reznik for access to the Cancer Atlas of Metabolic Profiles datasets. This work was supported by 1F32CA236156-01A1, 5T32CA108462-15 and the Sandler Program for Breakthrough Biomedical Research (postdoctoral independence award) to K.M.T.; R35 CA242447-01A1, R01CA192914 and R01CA222508-01 to V.M.W.; and the National Institutes of Health Shared Instrumentation Grant S10 OD016387. Quantitative analysis of amino acids in interstitial fluid was performed by H. Shah and R. AminiTabrizi at the University of Chicago Comprehensive Cancer Center Metabolomics Platform, which receives financial support from the University of Chicago Comprehensive Cancer Center Support Grant (P30-CA014599).
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Costas Lyssiotis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Tumor progression is associated with ECM synthetic myeloid programming.
a. H&E-stained histological sections of 8 week and 11 week FVB/N PyMT mammary tumors, quantification of the grade of stromal fibrosis (n = 10 or 9 mice). b. Quantification of pathological assessment for area of hyperplasia, DCIS with early invasion (DCIS) and advanced invasion (invasion) within H&E-stained histological sections of 8 week and 11 week FVB/N PyMT mammary tumors (n = 10 or 9 mice). c. Representative second-harmonic generation (SHG) images of collagen fibers in 11 week old FVB/N PyMT mammary tumors, treated with anti-CSF1 blocking antibody or IgG control weekly from 4 weeks of age until 11 weeks of age (n = 3 mice), (scale bar: 100 µm). d. Relative expression of macrophage polarization-associated gene expression of TAMs derived from 11 week old FVB/N PyMT mammary tumors, relative to TAMs derived from 8 week old PyMT mammary tumors, (n = 5 mice). e. Representative immunofluorescence microscopy of Collagen VI (white) and DNA (blue) of 11 week old FVB/N PyMT mammary tumors treated with or without LOX-inhibition, representative of the effect observed in 2 independent experiments (n = 4 mice) (scale bar: 100 µm). f. Representative immunofluorescence microscopy of F4/80 (red) and DNA (blue) in 11 week old FVB/N PyMT mammary tumors, representative localization of every tumor-stroma border assessed (n = >10 mice from > 10 independent experiments) (scale bar: 40 µm). g. Representative immunofluorescence microscopy of Collagen VI (white) and DNA (blue) of BMDMs treated with or without 5 ng/mL IL4 and 1 ng/mL TGFβ1 on fibroblast synthesized ECM surfaces for 24 h, representative of the effect observed in 3 independent experiments (scale bar: 40 µm). Data shown represent ± SEM via two-tailed unpaired Student t test (b).
Extended Data Fig. 2 ECM-synthetic stage II and IIIA tumors are associated with poor survival.
Kaplan-Meier survival curves of 2506 stage II and IIIA breast tumors, stratified for the top and bottom quartile expression level of the genes comprising the top GO category identified in d (Cox Proportional Hazard model: p = 0.0364, z = 2.092432; LogRank: p = 0.0346).
Extended Data Fig. 3 TGFβ1 signaling and production is mechanosensitive.
a. Relative gene expression of IL4-polarized BMDMs cultured on soft (400 Pa) or stiff (60k Pa) collagen I-coated polyacrylamide hydrogel surfaces treated with 0, 0.1, 1, or 10 ng/mL TGFβ1 for 4 h, qPCR-ΔΔCT normalized to BMDMs treated without IL4 (housekeeping gene: 18 s), (n = 3 independent experiments). b. Representative immunofluorescence microscopy for RETNLA (red) and DNA (blue) of IL4-polarized BMDMs cultured on soft (400 Pa) or stiff (60k Pa) collagen I-coated polyacrylamide hydrogel surfaces, treated with 1 ng/mL TGFβ1 with or without 10 µg/mL TGFβ1-blocking antibody (1D.11) for 24 h, representative of the effect observed in 3 independent experiments (scale bar: 20 µm). c. Representative immunofluorescence microscopy for phosphorylated-SMAD2/3 (red) and DNA (blue) of IL4-polarized BMDMs cultured on soft (400 Pa) or stiff (60k Pa) collagen I-coated polyacrylamide hydrogel surfaces, treated with 1 ng/mL TGFβ1 with or without 10 µg/mL TGFβ1-blocking antibody (1D.11) for 24 h, representative of the effect observed in 3 independent experiments (scale bar: 40 µm). d. Active TGFβ1 in culture medium after 48 h of culture of IL4-polarized BMDMs cultured on soft (400 Pa) or stiff (60k Pa) collagen I-coated polyacrylamide hydrogel surfaces. (n = 4 independent experiments) e. Relative luminescent intensity of TGFβ1-reporter (PAI-1 Luc) expressing mink lung epithelial cells88 when cultured for 24 h in 1:1 conditioned media from IL4-polarized BMDMs cultured on soft (400 Pa) or stiff (60k Pa), (n = 9, 3 independent experiments of 3 technical replicates). f. Representative immunofluorescence microscopy of f4/80 (red), and DNA (blue) of stiff collagen orthotopic C57BL/6J PyMT mammary after 3 weeks of growth in Tgfbr2MyeKO or control animals, (n = 3 mice) (scale bar: 100 µm). Data shown represent ± SEM via two-tailed unpaired Student t test (d-e).
Extended Data Fig. 4 Arginine, TGFβ1, and ECM stiffness affect myeloid ECM synthesis and metabolism.
a. Relative arginine concentrations of stiff collagen orthotopic C57BL/6J PyMT mammary tumors in Tgfbr2MyeKO or control animals for 3 weeks, LC-MS analysis, (n = 7 or 6 mice). b. Representative immunofluorescence microscopy of Collagen VI (orange) and DNA (blue) of IL4-polarized WT or Arg1KO BMDMs cultured with or without 0.4 mM arginine on stiff (60k Pa) collagen I-coated polyacrylamide hydrogel surfaces treated with 1 ng/mL TGFβ1 for 24 h, representative of the effect observed in 3 independent experiments (scale bar:40 µm). c. Relative hydroxyproline (Pro-OH) concentration in BMDMs cultured on soft (400 Pa) or stiff (60k Pa) collagen I-coated polyacrylamide hydrogel surfaces with or without 1 ng/mL TGFβ1 for 24 h, measured via LC-MS, (n = 3 independent experiments). d. Fractional contributions (all isotopologues, m + 5 or m + 3) of 13C derived from 13C5-glutamine in BMDMs cultured on soft (400 Pa) or stiff (60k Pa) collagen I-coated polyacrylamide hydrogel surfaces in medium containing 12C5-glutamine, treated with 1 ng/mL TGFβ1 for 22 h, swapped for fresh medium containing 13C5-glutamine for 2 h, BMDMs were harvested and measured via LC-MS, (n = 3 independent experiments). e. Fractional contributions (all isotopologues, m + 5 or m + 3) of 13C derived from 13C5-glutamine in WT or Tgfbr2MyeKO BMDMs cultured on soft (400 Pa) or stiff (60k Pa) collagen I-coated polyacrylamide hydrogel surfaces in medium containing 12C5-glutamine, treated with or without 1 ng/mL TGFβ1 for 22 h, swapped for fresh medium containing 13C5-glutamine for 2 h, BMDMs were harvested and measured via LC-MS, (n = 3 independent experiments). f. Cellular respirometry of BMDMs cultured with or without 1 ng/mL TGFβ1 for 24 h or 100 ng/mL LPS for 1 h along with sequential additions via injection ports of Oligomycin [1 µM final], FCCP [1 µM final], and Antimycin A/Rotenone [1 µM final] during respirometry measurements, measured with an SeahorseXF24, (n = 5 wells, repeated 3 times) g-i. Relative g. NADH h. NAD i. NADH/NAD ratio in BMDMs cultured on soft (400 Pa) or stiff (60k Pa) collagen I-coated polyacrylamide hydrogel surfaces with or without 1 ng/mL TGFβ1 for 24 h, measured via LC-MS, (n = 3). Data shown represent ± SEM via two-tailed unpaired Student t test (a, d, and f) or one-way ANOVA with Tukey test for multiple comparisons (c, e, g-i) and **P < 0.01 via, ns indicates statistically not significant.
Extended Data Fig. 5 Arginine improves CTL tumor infiltration.
a. LC-MS based metabolomics of medium after 3 h of culture of Arg-ECN or ECN metabolizing a modified M9 medium, lacking ammonia, supplemented with ornithine [3 mM], (n = 3 independent experiments). b. Using the BRCA1 dataset from the Cancer Atlas of Metabolic Profiles42 we compared the levels of arginine and proline in 61 breast tumor to 47 normal adjacent tissues. c. Graphical description of the experimental setup for c-d. d. Tumor mass after 3 weeks of growth in C57BL/6J mice gavaged daily with 2 g/kg glycine, ornithine, arginine, or water, (n = 8 mice) e. Relative serum metabolites derived from retro-orbital isolated blood from mice containing PyMT tumors after 3 weeks of growth in C57BL/6J mice gavaged daily with 2 g/kg glycine, ornithine, arginine, or water (100 µL), 4 h prior to isolation of blood/serum, measured with LC-MS (n = 8 mice pooled and measured as 2 technical replicates). f. Quantitation of cleaved-caspase 3+ %-area per field view of tumor border zone of 3 week old tumors from C57BL/6J mice gavaged daily with 2 g/kg glycine, ornithine, arginine, or water (n = 5 mice). g. Quantitation of CD8+ %-area per field view from the core of 3 week old tumors from C57BL/6J mice gavaged daily with 2 g/kg glycine, ornithine, arginine, or water (n = 4 mice). h. Representative thesholded-area mask for CD8+ from the core of 3 week old tumors from C57BL/6J mice gavaged daily with 2 g/kg glycine, ornithine, arginine, or water (n = 4 mice) (scale bar: 100 µm). i. Representative immunofluorescence microscopy for CD8+ from the core of 3 week old tumors from C57BL/6J mice gavaged daily with 2 g/kg glycine, ornithine, arginine, or water (n = 4 mice) (scale bar: 100 µm). j. Representative immunofluorescence microscopy of CD8+ morphologies observed in orthotopic PyMT mammary tumors in Tgfbr2MyeKO mice gavaged daily with 2 g/kg ornithine (n = 5 mice) (scale bar: 10 µm). Data shown represent ± SEM via two-tailed unpaired Student t test (a) or one-way ANOVA with Tukey test for multiple comparisons (c-e, & f-g).
Extended Data Fig. 6 Ornithine alters CTL metabolism.
a. Heat map of relative metabolite levels of CD3/CD28-activated CD8+ CTLs cultured for 24 h in medium containing a molar ratio of 1:1 [0.5 mM], 3:1 [1.5 mM], and 9:1 [4.5 mM] ornithine:arginine, LC-MS analysis. (n = 3 independent experiments). b. Graphical description of the experimental setup and fractional contribution of 13C5-ornithine to intracellular ornithine in CD3/CD28-activated CD8+ CTLs cultured for 24 h with and without 1 mM Arginine (12C) present in medium, LC-MS analysis, (n = 3 independent experiments). c. Heat map of differentially abundant metabolite levels in b, LC-MS analysis. (n = 3 independent experiments). d. Representative immunofluorescence microscopy of OVA-PyMT tumor cells challenged with GFP+-OTI CTLs (green) for 24 h in medium containing a molar ratio of 1:1 [0.5 mM] or 3:1 [1.5 mM] ornithine:arginine, cleaved-caspase 3 (red) and DNA (blue), representative of the effect observed in 4 independent experiments (scale bar: 40 µm).
Supplementary information
Supplementary Information
Supplementary Fig. 1: flow and RNAseq gating strategy.
Supplementary Data 1
TCGA case list used.
Supplementary Tables
Supplementary Table 1: qPCR primers. Supplementary Table 2: raw/minimally processed LC–MS datasets.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tharp, K.M., Kersten, K., Maller, O. et al. Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment. Nat Cancer 5, 1045–1062 (2024). https://doi.org/10.1038/s43018-024-00775-4IF: 23.5 Q1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-024-00775-4IF: 23.5 Q1