Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks
低氮胁迫促进草莓根系氮素吸收和同化:激素网络的贡献
江苏省徐州市徐州农业科学研究所, 徐州 221131, 中国
中国江苏省徐淮221121区徐州农业科学研究所通山检测站
北京市昌平区农业技术推广站, 北京 102200
通信应收件人的作者。
这些作者对这项工作做出了同样的贡献。
园艺2023, 9(2), 249;https://doi.org/10.3390/horticulturae9020249
收到意见书:2022 年 12 月 20 日 / 修订日期:2023 年 2 月 7 日 / 录用日期: 2023-02-10 / 发布日期:2023 年 2 月 12 日
(本文属于特刊 生物和非生物胁迫下的园艺作物生理反应)
Abstract 抽象
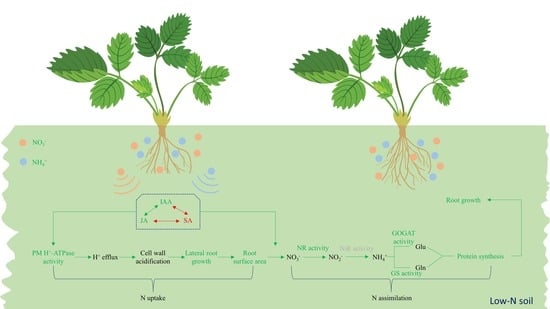
低氮胁迫严重阻碍了作物的生长和生产力。关于许多植物物种的根系对低氮条件的适应已经进行了大量研究。然而,草莓 (Fragaria × ananassa Duch.) 根对低 NO3- 或低 NH4+ 胁迫的形态反应机制仍然知之甚少。草莓植株在 1 mM NO3−、1 mM NH4+ 和对照 (15 mM NO3−) 条件下水培培养,以评估其根系对低氮胁迫的生理反应。因此,低氮胁迫增加了根的鲜重、侧根密度和根表面积,增强了吲哚-3-乙酸和茉莉酸的积累,同时显著降低了根系中的水杨酸。相应地,低氮胁迫增加了 PM H+-ATP 酶活性。低 NO3− 胁迫增强了硝酸盐还原酶和谷氨酰胺合成酶的活性,而低 NH4+ 处理导致更高的谷氨酰胺合成酶和谷氨酸合酶活性。总的来说,目前的结果表明,低氮胁迫通过调节激素(吲哚-3-乙酸、茉莉酸和水杨酸)来增强草莓根的氮吸收,从而介导 PM H+-ATP 酶活性,同时通过上调硝酸还原酶、谷氨酰胺合成酶和谷氨酸合酶的活性来促进氮代谢。总之,低氮条件可能有助于草莓根系更有效地从土壤中获取有效氮。
1. Introduction 1. 引言
氮 (N) 是参与作物生长中许多关键生理和代谢反应的最重要的营养元素之一 [1]。氮供应不足限制了作物的生长,从而降低了作物的产量 [2]。为了追求更高的作物产量,种植者倾向于施用过量的氮,这远远超过了作物的实际需求。在全球范围内,每年约有 1 亿吨氮用于农业生产 [3]。然而,由于氮肥利用效率低(25-50%)[4],氮素损失显著,进一步导致了经济和环境效益的显著损失[5]。因此,当务之急是发展低氮需求、高氮利用效率的作物,以实现环境友好型和可持续农业。
硝酸盐 (NO3−) 和铵态氮 (NH4+) 是土壤中氮的两种主要来源,是调节植物各种生理反应的重要信号分子,包括基因表达和根结构修饰 [6,7]。根是植物吸收养分的主要器官。有充分的证据表明,根系结构重塑是植物适应不断变化的环境(如土壤养分波动)的主要策略[8]。例如,玉米根系通过增强根系的水平和垂直伸展来响应低氮胁迫[9,10],而水稻缺磷可以刺激侧根的形成[11]。因此,了解作物通过根系可塑性响应养分胁迫的机制有助于推进通过根系表型提高作物养分利用效率的育种目标。
许多研究表明,激素在缺氮情况下调节根系生长中起着关键作用。例如,生长素已被广泛认为是其他激素影响根系发育的关键基础 [12]。茉莉酸 (JA) 是一种调节作物生长和各种防御反应的关键植物激素 [13,14]。既往研究表明,JA 可以抑制初级根发育 [15],刺激侧根启动 [16],并诱导根再生 [17]。除了吲哚-3-乙酸 (IAA) 和 JA,水杨酸 (SA) 也是植物抗性反应期间激素调节复杂网络的重要组成部分。据报道,SA 可以显着抑制主根的发育,但促进侧根生长 [18] 和根原基的形成 [19]。几项研究已经确定,在多种激素的协同作用下,根对氮波动做出反应[20],这种反应在很大程度上取决于基因型、环境条件以及低氮胁迫的强度或持续时间。
在被植物利用之前,NO3- 和 NH4+ 必须经过由一系列酶调节的同化过程。NH4+ 被根吸收后,被谷氨酰胺合成酶 (GS) 和谷氨酸合酶 (GOGAT) 转化为氨基酸。对于 NO3−,在通过根部吸收后,它会被硝酸盐还原酶 (NR) 还原成 NO2−,然后被亚硝酸盐还原酶 (NiR) 还原成 NH4+,最后被有机氮同化。此外,广泛的研究表明,NO3− 和 NH4+ 作为信号分子在诱导许多与 N 同化相关的基因表达中起关键作用 [21]。例如,NH4+ 可以在根尖诱导 GOGAT 基因表达 [22]。
质膜 (PM) H+-ATP 酶作为细胞膜中的重要离子泵,可以建立 H+ 的跨膜化学梯度 [23],从而驱动细胞壁的酸化,从而促进根系生长 [24,25]。一些报告指出,PM H+-ATP 酶活性受各种环境信号的调节,包括一些经典的植物激素和 N(在 [23,26] 中已论述)。
草莓是一种流行的新鲜水果,在世界大部分地区广泛种植。氮是草莓生长和果实品质的主要贡献者 [27]。低氮严重抑制草莓生长,导致叶片萎黄[28],果实中叶面积、干物质[29]和氨基酸含量减少[30]。尽管有一些关于草莓缺氮响应根系形态变化的报道,但激素对草莓缺氮下根系重塑的调控机制仍知之甚少。我们的假设是,低 N 刺激根重塑并丰富根结构以更好地获取 N,这是受荷尔蒙相互作用调节的。因此,本研究旨在评价草莓根系对低氮胁迫的生理和形态响应,并初步阐明可能的调控机制。这些发现有望为草莓生产中的遗传改良和更有效的氮利用提供理论基础。
2. Materials and Methods 2. 材料和方法
2.1. Cultivation Conditions
2.1. 栽培条件
从温室中挑选草莓匍匐茎(苗香 3 号),用杀菌剂浸泡,然后转移到蒸馏水中。当新叶出现时,将均匀的幼苗在含有营养液的塑料盆中培养(每盆 5 棵幼苗)。幼苗在 25/20 °C(昼/夜)的人工气候室中生长,光照周期为 16/8 小时(亮/暗),相对湿度为 70%,平均光照强度为 350 μmol m-2 s-1 PPFD。
营养液中含有 2.05 mM KCl、2.001 mM K2HPO4·3H2O、1.0 mM MgSO4·7H2O、0.03 mM EDTA-Fe、0.025 mM H3BO4、2.0 μM MnSO4·H2O、0.78 μM CuSO4、3.21 μM ZnSO4·H2O 和 0.5 μM Na2MoO4。草莓幼苗用含有15.0 mM NO3−的半强度营养液保存2 d [31],然后在含有1 mM NO3−(LN处理)、1 mM NH4+(LA处理)或15 mM NO3−(对照)的溶液中培养。NO3− 被添加为 Ca (NO3)2;NH4+ 添加为 NH4Cl;CaCl2 用于在所有处理中维持相同的 Ca2+ 浓度。营养液的 pH 值保持在 6.5,KOH 和 HCl 。营养液每两天更新一次,花盆随机放置。
在每个采样时间,先用蒸馏水洗涤幼苗,用吸水纸干燥,然后分成两部分。新鲜样品用于生物量、根形态和侧根密度测定。另一部分用液氮处理并储存在 -80 °C 下,用于测定可溶性蛋白质、酶活性和激素。
2.2. Biomass Determination
2.2. 生物量测定
草莓幼苗用蒸馏水洗涤,并在处理后 24 、 48 和 96 h 用吸水纸干燥。茎和根分别称重。
2.3. Root Morphology Examination
2.3. 牙根形态检查
在处理后 0、48 和 96 小时,用蒸馏水洗涤根部并漂浮在装有 2-3 毫米水的 PVC 托盘中,然后用 EPSON V850 PRO 扫描仪(EPSON,中国北京)扫描。使用 WinRHIZO 2017a(Regent Instruments Inc.,魁北克,QC,加拿大)分析图像中总根长、根表面积、平均直径和每株植物的根尖数。
2.4. Lateral Root Density Determination
2.4. 侧根密度测定
处理后 48 h 用蒸馏水清洗不定根后,在显微镜下计数出出的侧根数量和不定根的长度。侧根密度 (LRD) 记录为出现的侧根数量与距离的比值,以 cm-1 根长表示 [32]。
2.5. Enzyme Activity Assay
2.5. 酶活性测定
草莓根用蒸馏水洗涤,并在处理后 12 和 48 h 用吸水纸干燥。研磨成粉末后,这些根用于测定酶活性。
NR 活性是通过参考 Majláth 等人 [33] 的方法确定的。将草莓根 (1 g) 在 1 mL 0.2 M 磷酸盐缓冲液 (pH 7.5) 中充分研磨,并在 4 °C 下以 12,000× g 离心 5 分钟。 将约 0.5 mL、0.1 M KNO3 和 0.3 mL、2.0 mg mL-1 NADH 加入 0.2 mL 上清液中,并在 25 °C 的水浴中反应 30 分钟。然后,通过加入 1 mL 30% 三氯乙酸终止反应。将反应混合物加入 2 mL 1% 磺酰胺和 2 mL 0.2% α-萘胺中,并充分混合。静置 15 分钟后,使用 Multiskan GO 1510 分光光度计(Thermo Fisher Scientific,Vantaa,Finland)在 520 nm 波长下进行比色法测定。一个单位的 NR 活性 (U g-1) 定义为每小时每克样品消耗的 1 μmol NADH 量。
在适应 Caroline 等人 [34] 研究的条件下测定 NiR 活性。简而言之,将 0.1 g 草莓根和 1 mL 缓冲液 (pH 8.0) 在冷冻研钵中研磨,并在 4 °C 下以 12,000 × g 离心 2 分钟。 缓冲液是 50 mM Tris-HCl 和 3 mM EDTA 的混合物。向 1.2 mL 反应溶液中加入约 0.1 mL 上清液(包括 100 mM 磷酸钾缓冲液 (pH 7.5)、10 mM KNO2、15 mg mL−1 甲基紫精、H2O 和 5% 连二亚硫酸钠)。混合物反应在 25 °C 水浴中反应 30 min。然后,加入 1 mL 1% 磺酰胺和 1 mL 0.2% α-萘胺并充分摇匀,静置 30 min 后在 540 nm 处测定吸光度。一个单位的 NiR 活性 (U g-1) 定义为 1 μmol NO 2-g-1 h-1 的还原量。
根据Singh等[35]测量GOGAT活性,并稍作修改。将草莓根 (0.1 g) 在 1 mL 25 mM Tris-HCl 缓冲液中充分研磨,并在 4 °C 下以 8000 × g 离心 10 分钟。 将上清液与 20 mM 谷氨酰胺、100 mM α-酮戊二酸和 3 mM NADH 充分混合。然后,在 340 nm 处测量 GOGAT 的活性,并表示为消耗的 1 nmol NADH 的量 g-1 min-1。
根据Wang等[36]描述的方法测定GS活性。将草莓根 (1 g) 和 4 mL 0.05 mM 磷酸盐缓冲液(含有 0.4 M 蔗糖和 4 mM L-半胱氨酸)在冷冻研钵中彻底研磨。在 12,00×0 g 和 4 °C 下离心 15 分钟后,将 1 mL 上清液加入 3 mL 酶反应溶液中,该溶液由 50 mM L-谷氨酸钠、4 mM ATP-2Na、40 mM 羟胺、20 mM 硫酸镁、10 mM L-半胱氨酸和 40 mM 磷酸盐缓冲液组成。反应在 30 °C 下进行 15 分钟。然后,向混合物中加入 30% 三氯乙酸、5.5 M HCl 和 8% FeCl3 以终止反应。静置 10 分钟后,在 540 nm 处测量吸光度。GS 活性表示为 1 nmol γ-谷氨酰异羟肟酸盐产生的量 g-1 min-1。
2.6. Soluble Protein Content Determination
2.6. 可溶性蛋白含量测定
根据Bradford等[37]描述的方法测定可溶性蛋白含量,并稍作修改。处理后 12 h 和 48 h,将草莓根 (0.2 g) 充分研磨,并在 4 °C 下以 5000× g 离心 10 分钟。 将约 0.1 mL 上清液与 5 mL Komas Brilliant Blue G-250 溶液(由 100 mg L -1 Komas Brilliant Blue G-250、4.7% 乙醇 (v/v) 和 8.5% (w/v) 磷酸组成)混合,静置 2 分钟后在 595 nm 处测量吸光度。使用基于牛血清蛋白的标准曲线计算可溶性蛋白含量。
2.7. Total N Content Measurement
2.7. 总氮含量测量
使用凯氏定氮法测定草莓根中的总氮含量 [38]。处理后 48 小时,将干草莓根 (0.1 g) 放入装有硫酸 (5 mL) 和过氧化氢的凯氏定氮瓶中消化。混合物澄清后,使用凯氏定氮仪(FOSS Kjeltec™ 8400,Hilleroed,丹麦)获得蒸馏液,并用 0.01 mol L-1 1/2 H2SO4 滴定。
2.8. PM H+-ATPase Activity Assay
2.8. PM H+-ATPase 活性测定
根据Zhang等[39]测定PM H + -ATP酶活性。在处理后 12 小时和 48 小时,将草莓根在缓冲液(含有 250 mM 蔗糖、4 mM DTT、7.2 μg mL-1 PMSF、50 mM Tris、8 mM EDTA 和 1.5% PVP)中匀浆。在 4 °C 下以 10,00×0 g 离心 15 分钟后,将上清液在 4 °C 下再次以 10,000 × g 离心 30 分钟。 将沉淀物重新溶解在含有 250 mM 蔗糖、2 mM DTT 和 5 mM 管道的缓冲液中。用蔗糖梯度溶液和 KCl 处理后,将获得的沉淀加入 0.5 mL 反应溶液中,该溶液由 250 mM HEPES-Tris、25 mM ATP-Na2、3 mM Na2MoO4、1 mM NaN3、1 mM EDTA 和 0.02% Triton X-100 组成。反应终止后,加入 50 μL 10% 抗坏血酸,静置 40 分钟后在 660 nm 处测定吸光度。
2.9. Determination of Plant Hormones
2.9. 植物激素的测定
根据Yang等[40]测定草莓根中的激素,并稍作修改。处理后 12 h 和 48 h,将草莓根 (0.1 g) 用液氮研磨并加入 1 mL 提取溶液(由 0.4 mL 甲醇、0.4 mL 乙腈和 0.2 mL 水组成)中,然后在 4 °C 下避光提取 12 h,然后以 14,00×0 g 离心 10 g。使用氮气蒸发器干燥约 0.8 mL 上清液, 然后重新溶解在 0.2 mL 50% 甲醇中,并以 14,000× g 离心 10 分钟,得到上清液。
通过 ExionLC™ AD 系列高效液相色谱系统 (AB SCIEX, Framingham, MA, USA) 和 Kinetex® C18 色谱柱 (1.7 μm, 150 × 2.1 mm;Phenomenex, Torrance, CA, USA)。流动相A采用含0.04% (v/v)甲酸的蒸馏水,流动相B采用甲醇。洗脱梯度如下:0–5.5 min,流动相B在10%至95%范围内线性变化;5.6–7 min,流动相B保持在95%;7.1–7.5 min,流动相B在95%至10%范围内线性变化;7.6–10 min,流动相 B 保持在 10%。使用 AB Sciex Triple Quad 3500(AB SCIEX,Framingham,MA,USA)完成正/负离子模式下的质谱分析。使用 Multi Quant 软件提取色谱图的峰面积和保留时间。根据标准曲线计算草莓根中的激素含量。
2.10. Statistical Analysis
2.10. 统计分析
使用 SPSS V.26 (SPSS Statistics, Armonk, NY, USA) 进行数据分析。Duncan 检验用于检测 95% 概率水平的显著差异。如果处理之间的差异很大,则使用不同的字母。
3. Results 3. 结果
3.1. Biomass of Strawberry Roots
3.1. 草莓根的生物量
与对照相比,低氮胁迫在 24 、 48 和 96 h 对地上部鲜重没有显著影响 (图 1a),但在 48 h 时显著增加了草莓根的鲜重 (图 1b)。
图 1. 低氮胁迫对 24 h、48 h 和 96 h 草莓芽和根鲜重的影响。(a) 地上部鲜重。(b) 根鲜重。值表示 5 次生物学重复的 SEM ±平均值。不同字母表示在 p < 0.05 处差异显著。
3.2. Morphology of Strawberry Roots
3.2. 草莓根的形态
低 N 胁迫改变了草莓的根系结构。与对照相比,LN 处理下 48 h 和 96 h 的总根长分别增加了 30.34% 和 27.71%,但在 LA 处理下 48 h 和 96 h 分别增加了 23.67% 和 8.5%(图 2a)。
图 2. 不同氮处理下草莓 0 h、48 h 和 96 h 的根系形态。(a) 总根长。(b) 总表面积。(c) 根尖总数。(d) 平均根直径。值表示三个生物学重复的平均 ± SEM。不同字母表示在 p < 0.05 处差异显著。
48 h时,LN和LA处理下根系表面积较对照分别增加21.23%和22.93%。然而,LN 和 LA 处理在 96 小时时相对于对照的根表面积没有显着差异(图 2b)。
在低氮胁迫下,根尖的数量显著增加(图 2c)。与对照相比,LN 和 LA 处理在 48 h 时根尖数量分别增加了 15.63% 和 8.26%,在 96 h 时也观察到类似的趋势。
然而,与 48 h 对照相比,LN 和 LA 处理下的平均根直径分别减少了 9.02% 和 7.46%(图 2d)。96 h时,与对照相比,平均根径分别减少了11.34%和6.40%。
3.3. Lateral Root Density
3.3. 侧根密度
LN 和 LA 处理显着促进了 48 h 侧根的形成(图 3)。与对照 (4.28 ± 0.14 cm−1 根长) 相比,LN 处理下 LRD 增加了 36.69% (5.86 ± 0.32 cm-1 根长),LA 处理下增加了 24.82% (5.35 ± 0.38 cm-1 根长)。
3.4. Hormone Contents in Strawberry Roots
3.4. 草莓根中的激素含量
与对照相比,LN 和 LA 处理在 12 h 时草莓根中的 IAA 浓度分别提高了 42.37% 和 49.51%(图 4a)。然而,在 3 种处理中,48 h 根系 IAA 水平没有显著差异。
图 4. 不同氮处理下草莓根系 IAA 浓度 (a) 、 JA 浓度 (b) 和 SA 浓度 (c) 在 12 h 和 48 h 时。值表示三个生物学重复的平均 ± SEM。不同字母表示在 p < 0.05 处差异显著。
在低 N 应力下,JA 浓度显著增加(图 4b)。与对照相比,LN 和 LA 处理下 JA 浓度在 12 h 分别提高了 64.49% 和 36.68%,在 48 h 时也观察到类似的趋势。LN 处理下根系 JA 浓度比 LA 处理下高 49.13%。
然而,与对照相比,低氮胁迫下的根系 SA 浓度显著降低(图 4c)。12 h时,LN和LA处理下根系SA浓度较对照分别下降了35.55%和47.69%,48 h时也呈类似趋势。然而,LN 和 LA 处理在 12 h 和 48 h 时没有显著差异。
3.5. PM H+-ATPase Activity
3.5. PM H+-ATPase 活性
在 12 小时时,与对照相比,LN 处理下 PM H+-ATP 酶活性显着升高,但在 LA 处理下仅略有增加,无显著差异(图 5)。与对照相比,LN 和 LA 处理下 48 h PM H+-ATP 酶活性显著增加。
图 5. 不同氮处理对草莓根系 12 h 和 48 h H+-ATPase 酶活性的影响值表示三个生物学重复的平均 ± SEM。不同字母表示在 p < 0.05 处差异显著。
3.6. Enzyme Activity Related to Nitrogen Assimilation
3.6. 与氮同化相关的酶活性
在 12 h 时,低氮处理与对照之间草莓的根 NR 活性没有显著差异(图 6a)。48 h 时,LN 和 LA 处理使 NR 活性分别提高了 19.83% 和 10.48%。然而,LN 和 LA 处理在 12 小时和 48 小时时相对于对照的 NiR 活性没有显着差异(图 6b)。
图 6. 低氮胁迫对草莓根系 12 h 和 48 h NR (a) 、 NiR (b) 、 GS (c) 和 GOGAT (d) 活性的影响。值表示三个生物学重复的平均 ± SEM。不同字母表示在 p < 0.05 处差异显著。
与对照相比,LN 处理在 12 h 和 48 h 时显着增加 GS 活性,而 LA 处理仅在 48 h 时显着增加 GS 活性(图 6c)。
LN 处理在 12 小时和 48 小时时产生与对照相似的 GOGAT 活性(图 6d)。然而,与对照相比,LA 处理在 12 h 和 48 h 时分别使 GOGAT 活性提高了 15% 和 14.85%。
3.7. Soluble Protein Content in Strawberry Roots
3.7. 草莓根中的可溶性蛋白含量
低氮胁迫对 12 h 时草莓根中的可溶性蛋白含量没有显着影响(图 7)。相比之下,在 48 h 时,LN 和 LA 处理的可溶性蛋白含量分别比对照提高了 96.97% 和 84.25%。在 12 h 和 48 h 时,LN 处理导致可溶性蛋白含量略高于 LA 处理,无显著差异。
3.8. Total Nitrogen Content in Strawberry Roots
3.8. 草莓根中的总氮含量
48 h时,LN 和 LA 处理均显著降低草莓根系的总 N 含量(图 8)。与对照相比,LN 和 LA 处理分别降低了 17.86% 和 24.4% 的总 N 含量。
4. Discussion 4. 讨论
4.1. Root Architecture Changes for Better N Uptake
4.1. 根结构变化以获得更好的 N 吸收
根系结构可塑性对于植物对复杂多变的生活环境的适应性反应至关重要。迄今为止,大量研究表明,根系结构的变化可以显著提高作物的养分效率[41]。相应地,根系结构会受到营养条件的影响,如氮供应[42]。本试验结果表明,在低氮条件下,48 h时根系鲜重显著增加。因此,我们更侧重于根的分析。进一步检查根结构表明,与对照相比,低氮处理显著增加了总根长、根表面积和总根尖数,但明显降低了 48 h 时的平均根直径。这些结果与以前的研究一致,这些研究报告称,作物会发育出更深的根系和更大的根表面积,从而更有效地获取氮,从而更好地适应氮缺乏[43,44,45]。
植物激素在控制根系发育方面起重要作用,包括侧根生长和根毛形成[46],并与N有显著的相互作用[47]。Jia等[48]揭示了低氮胁迫可以上调YUC8及其同源物和TAA1基因的转录,从而增强拟南芥根中局部IAA的生物合成。最近的一些研究表明,NO3− 和 NH4+ 信号转导可以介导生长素从芽到根的转运 [49],并调节其在侧根原基上皮细胞中的积累,进而刺激侧根的出现和生长 [50]。Sun等[51]报道,低氮胁迫可以增加根系中生长素的积累,通过几种生长素介导的途径促进根系发育。在本研究中,我们观察到在低 N 处理下 12 h IAA 浓度显着增加。基于上述结果,可以推测低 NO3- 和低 NH4+ 信号可以诱导草莓植株局部 IAA 生物合成并增强其细胞间转运,从而提高根 IAA 浓度并促进根系生长。
如上所述,JA 和 SA 也与调节根结构的信号通路密切相关。Wang等[52]和Sun等[53]认为,茉莉酸酯的应用可以增加水稻和拟南芥的侧根数。Gutierrez等[54]报道,JA对不定根形成产生负面影响。此外,广泛的研究揭示了 JA 和生长素之间的密切相关性。几项研究表明,JA信号通路通过调节生长素相关基因的表达与生长素稳态呈正相关[16,55]。Xu等[12]揭示了JA通过调节PIN基因促进生长素转运,而生长素通过调节GH3.3/5/6基因来调节JA稳态。我们的研究揭示了在低氮胁迫下草莓 LRD 、 IAA 和 JA 浓度的显著增加。因此,可以推断 JA 和 IAA 之间存在协同关联,以促进缺氮下侧根的形成。
以前的研究强调了 SA 在减少拟南芥幼苗侧根数量方面的作用 [18,19,56]。此外,几项研究表明,SA 促进或抑制侧根生长取决于其自身的浓度 [57] 和 IAA 浓度 [58]。该领域的研究记录了 SA 和 IAA 之间的相互作用。Kitakura等[59]观察到,高水平的SA会干扰生长素的分布。Llorente等[60]发现,SA可以通过与生长素抑制蛋白结合来提高生长素抑制蛋白在生长素信号转导过程中的稳定性,从而使生长素信号网络短路。同样,SA 和 JA 在调节根形态中的拮抗关系也得到了广泛的研究 [61,62]。SA 诱导的 ANAC032 和 GRX480 表达可以抑制植物免疫中的 JA 信号传导 [63,64]。有趣的是,JA 信号转导还通过调节多种 NAC 转录因子(NAM、ATAF 和 CUC 转录因子)的活性来阻断 SA 生物合成 [65]。本研究观察到低氮胁迫下草莓根系 SA 浓度显著降低,这与 IAA 和 JA 的趋势相反。因此,可以合理地推测 SA 可能通过干扰 IAA 和 JA 的信号转导来负向调节侧根。
PM H+-ATP酶提供的H+外排促进了细胞壁松动[66],这是细胞扩增和植物生长的直接原因[67]。Sperandio 等 [68] 评估了 PM H+-ATP 酶活性在水稻适应缺氮中的重要性。进一步的研究表明,氮的饥饿和再供应可以促进 PM H+-ATP 酶活性和根系生长 [69]。根据酸生长理论,IAA 可以诱导 PM H+-ATP 酶活性 [24]。此外,最近的研究证实,PM-ATP酶活性与生长素结合蛋白1(ABP1)有关,生长素积累可以激活[70]。在这项研究中,我们观察到在低氮胁迫下草莓中 LRD 、 IAA 浓度和 H+-ATP 酶活性也有相同的增加趋势。一般来说,IAA 的积累似乎增加了 PM H+-ATP 酶活性,进而促进了侧根起始和原基发育。这一推测与Sun等[51]和Lv等[71]对玉米和小麦的研究结果一致,他们报道,在低氮胁迫下,玉米和小麦根系IAA的增加导致H+外排增加和质外体空间酸化,并最终促进侧根生长。此外,添加原钒酸钠 (Na3VO4,PM H+-ATP 酶活性抑制剂) 或 2,3,5-三碘苯并酸 (TIBA,生长素极性转运抑制剂) 消除了缺氮对根伸长的增强作用。
用茉莉酸甲酯浸泡可以显著提高姜根茎中 H+-ATP 酶活性和 H+ 跨膜转运 [72]。Chen等[73]报道了在多种胁迫(食草动物胁迫和盐胁迫)下JA信号传导介导的H+-ATP酶活性增强。此外,SA 还参与 PM H+-ATP 酶的调节。最近的研究表明,H+-ATP酶活性在SA预处理的幼苗中可以上调,从而增强对盐胁迫的耐受性[74]。有趣的是,先前对温度胁迫的研究表明,SA 预处理还可以刺激葡萄 [75] 和豌豆 [76] 中的 PM H+-ATP 酶活性。在本研究中,在低氮胁迫下,草莓的 JA 浓度和 H+-ATP 酶活性显著增加,而 SA 浓度显著降低。考虑到 IAA、JA 和 SA 之间的协同和/或拮抗作用,可以推测低 N 应激刺激激素之间串扰网络的不对称激活,并有助于相互作用的最终平衡,这可能导致 H+-ATP 酶活性和 H+ 外排的增加,从而促进侧根的形成和生长。
Lv等[71]观察到,在低氮条件下,根系NO3−和NH4+流入量显著减少,这与根系总氮含量的降低一致。这一发现与我们的研究基本一致。此外,在低氮条件下,根重显著高于对照,这可能与光合产物的再分配有关[77,78]。
4.2. Improving N Utilization via Enzyme Activities Changes
4.2. 通过改变酶活性提高氮利用率
NO3− 被根吸收后,NR 和 NiR 开始第一阶段的氮同化,然后是负责将无机氮转化为有机氮的 GS/GOGAT 循环 [79]。NR 是 N 同化中的第一种,也是一种限速酶 [80]。GS是一种多功能酶,其水平可以反映氮同化的强度[81]。Li等[82]观察到,随着氮的消耗,假绿球菌生长培养基中的NR活性逐渐增加。与 NR 相比,NiR 似乎不受 N 水平和形态的影响 [83]。Xiong等[83]证明,低氮水平刺激柑橘,但高氮水平抑制柑橘的GS活性。周等[84]表明,低氮处理诱导生菜中较高的GOGAT活性。在这里,我们发现低 NO3− 导致较高的 NR 和 GS 活性,而低 NH4+ 导致较高的 GS 和 GOGAT 活性。考虑到低氮胁迫下草莓根系可溶性蛋白含量在 48 h 时的显著增加,我们推测低氮主要通过诱导 NR、GS 和 GOGAT 活性来促进氮同化,从而促进蛋白质生物合成。
有充分的证据表明,与 N 同化相关的酶的活性也受植物激素的调节。在以前的研究中,在铜 (Cu) 胁迫下施用外源 IAA,菠菜幼苗表现出更高的 NR、GS、GOGAT 活性和可溶性蛋白含量 [85]。同样,Parihar等[86]观察到,茉莉酸甲酯的应用可以增加丝瓜中的NR、GS和GOGAT活性。几项研究表明,低浓度的 SA 可以增加小麦 [87] 和玉米 [88] 中的 NR 活性;另一方面,在高 SA 浓度下观察到抑制作用 [89]。在本研究中,与对照相比,低氮胁迫显著改变了 NR 、 GS 和 GOGAT 活性以及 IAA 、 JA 和 SA 浓度。因此,激素之间的串扰很可能会增加 N 同化相关酶的活性,这有助于草莓根在低 N 条件下保持更高水平的 N 同化和蛋白质生物合成。
5. Conclusions 5. 结论
本研究阐明了激素在草莓缺氮下复杂调控网络中的核心作用。草莓植株根结构的变化有两种可能的机制,可以更好地获取氮。首先,形成更大的根表面积以增强根在土壤中探索更多氮的能力。这个过程可能由激素相互作用(包括 IAA、JA 和 SA)的最终平衡介导,这可以增加 PM H+-ATP 酶活性并最终加速细胞壁酸化,从而增强侧根的形成和生长。其次,NR、GS 和 GOGAT 活性增强,以改善根 N 同化和蛋白质生物合成,从而促进侧根生长。这个过程也可能与荷尔蒙网络的调节有关。综上所述,草莓根系可以通过增加氮吸收面积和氮同化来更好地适应缺氮环境。
Author Contributions 作者贡献
M.W.、W.Z.、T.Z. 和 LZ 设计了这项研究。S.W. 和 M.S. 准备了植物。W.Z.、T.Z.、S.W.、J.Z. 和 MS 进行了实验。W.Z.、T.Z. 和 W.L. 分析了这些数据。M.W.、W.Z.、T.Z. 和 W.L. 撰写了手稿。所有作者均已阅读并同意手稿的已发表版本。
Funding 资金
本研究由徐州市科技计划项目 (KC22085) 资助。
Data Availability Statement
数据可用性声明
Conflicts of Interest 利益冲突
作者声明没有利益冲突。
References 引用
- Urban, A.; Rogowski, P.; Wasilewska-Debowska, W.; Romanowska, E. Understanding Maize Response to Nitrogen Limitation in Different Light Conditions for the Improvement of Photosynthesis. Plants 2021, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
厄本,A.;罗戈夫斯基,P.;瓦西莱夫斯卡-德博斯卡,W.;Romanowska, E. 了解玉米在不同光照条件下对氮限制的响应以改善光合作用。植物2021, 10, 1932。[谷歌学术搜索][交叉引用][公共医学] - Quan, X.Y.; Qian, Q.F.; Ye, Z.L.; Zeng, J.B.; Han, Z.G.; Zhang, G.P. Metabolic Analysis of Two Contrasting Wild Barley Genotypes Grown Hydroponically Reveals Adaptive Strategies in Response to Low Nitrogen Stress. J. Plant Physiol. 2016, 206, 59–67. [Google Scholar] [CrossRef] [PubMed]
全,XY;钱,Q.F.;叶,Z.L.;曾, JB;韩,Z.G.;Zhang, GP 水培种植的两种截然不同的野生大麦基因型的代谢分析揭示了响应低氮胁迫的适应性策略。J. 植物生理学。2016, 206, 59-67.[谷歌学术搜索][交叉引用][公共医学] - Glass, A.D.M. Nitrogen Use Efficiency of Crop Plants: Physiological Constraints Upon Nitrogen Absorption. Crit. Rev. Plant Sci. 2003, 22, 453–470. [Google Scholar] [CrossRef]
Glass, ADM 农作物的氮利用效率:氮吸收的生理限制。Crit. Rev. Plant Sci.2003, 22, 453–470。[谷歌学术搜索][交叉引用] - Undurraga, S.F.; Ibarra-Henriquez, C.; Fredes, I.; Alvarez, J.M.; Gutierrez, R.A. Nitrate Signaling and Early Responses in Arabidopsis Roots. J. Exp. Bot. 2017, 68, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
Undurraga, SF;伊瓦拉-恩里克斯,C.;弗雷德斯,I.;阿尔瓦雷斯,JM;Gutierrez, RA 拟南芥根中的硝酸盐信号传导和早期反应。J. Exp. Bot.2017, 68, 2541–2551.[谷歌学术搜索][交叉引用][公共医学] - Zhang, Y.T.; Wang, H.Y.; Lei, Q.L.; Luo, J.F.; Lindsey, S.; Zhang, J.Z.; Zhai, L.M.; Wu, S.X.; Zhang, J.S.; Liu, X.X.; et al. Optimizing the Nitrogen Application Rate for Maize and Wheat Based on Yield and Environment on the Northern China Plain. Sci. Total Environ. 2018, 618, 1173–1183. [Google Scholar] [CrossRef]
张 Y.T.;王 H.Y.;雷,Q.L.;罗,J.F.;林赛,S.;张 J.Z.;翟,LM;吴,S.X.;张,JS;刘 X.X.;等。基于产量和环境的华北平原玉米和小麦氮肥施用量优化.Sci. Total Environ.2018, 618, 1173–1183.[谷歌学术搜索][交叉引用] - Forde, B.G. Local and Long-Range Signaling Pathways Regulating Plant Responses to Nitrate. Annu. Rev. Plant Biol. 2002, 53, 203–224. [Google Scholar] [CrossRef]
Forde, BG 调节植物对硝酸盐反应的局部和远程信号通路。植物生物学年鉴。2002, 53, 203–224.[谷歌学术搜索][交叉引用] - Krouk, G.; Ruffel, S.; Gutierrez, R.A.; Gojon, A.; Crawford, N.M.; Coruzzil, G.M.; Lacombe, B. A Framework Integrating Plant Growth with Hormones and Nutrients. Trends Plant Sci. 2011, 16, 178–182. [Google Scholar] [CrossRef]
克鲁克,G.;鲁菲尔,S.;古铁雷斯,RA;Gojon, A.;新墨西哥州克劳福德;科鲁齐尔,通用汽车;拉科姆,B.将植物生长与激素和营养物质相结合的框架。趋势植物科学。2011, 16, 178–182.[谷歌学术搜索][交叉引用] - Wang, X.; Shen, J.; Liao, H. Acquisition or Utilization, Which Is More Critical for Enhancing Phosphorus Efficiency in Modern Crops? Plant Sci. 2010, 179, 302–306. [Google Scholar] [CrossRef]
王 X.;沈 J.;Liao, H. 获取或利用,哪个对提高现代作物的磷效率更重要?植物科学。2010, 179, 302–306.[谷歌学术搜索][交叉引用] - Gaudin, A.C.M.; McClymont, S.A.; Holmes, B.M.; Lyons, E.; Raizada, M.N. Novel Temporal, Fine-Scale and Growth Variation Phenotypes in Roots of Adult-Stage Maize (Zea Mays L.) in Response to Low Nitrogen Stress. Plant Cell Environ. 2011, 34, 2122–2137. [Google Scholar] [CrossRef]
高丁,A.C.M.;麦克莱蒙特,SA;福尔摩斯,BM;里昂,E.;Raizada, M.N. 成年期玉米 (Zea mays L.) 根系响应低氮胁迫的新型时间、精细尺度和生长变异表型。植物细胞环境。2011, 34, 2122–2137.[谷歌学术搜索][交叉引用] - Liu, J.; Li, J.; Chen, F.; Zhang, F.; Ren, T.; Zhuang, Z.; Mi, G. Mapping Qtls for Root Traits under Different Nitrate Levels at the Seedling Stage in Maize (Zea Mays L.). Plant Soil 2008, 305, 253–265. [Google Scholar] [CrossRef]
刘 J.;李 J.;陈, F.;张 F.;任, T.;庄 Z.;Mi, G. 绘制玉米幼苗期不同硝酸盐水平下根性状的图谱 (Zea Mays L.)。植物土壤2008, 305, 253–265。[谷歌学术搜索][交叉引用] - Kirk, J.G.D.; Du, L.V. Changes in Rice Root Architecture, Porosity, and Oxygen and Proton Release under Phosphorus Deficiency. New Phytol. 1997, 135, 191–200. [Google Scholar] [CrossRef]
柯克,J.G.D.;Du, L.V. 缺磷下水稻根系结构、孔隙率、氧和质子释放的变化。新植物醇。1997, 135, 191–200.[谷歌学术搜索][交叉引用] - Xu, P.; Zhao, P.X.; Cai, X.T.; Mao, J.L.; Miao, Z.Q.; Xiang, C.B. Integration of Jasmonic Acid and Ethylene into Auxin Signaling in Root Development. Front. Plant Sci. 2020, 11, 271. [Google Scholar] [CrossRef] [PubMed]
徐 P.;赵 P.X.;蔡,X.T.;毛, J.L.;苗,Z.Q.;Xiang, C.B. 茉莉酸和乙烯在根发育中的生长素信号传导中的整合。前面。植物科学。2020, 11, 271.[谷歌学术搜索][交叉引用][公共医学] - Glazebrook, J. Genes Controlling Expression of Defense Responses in Arabidopsis—2001 Status. Curr. Opin. Plant Biol. 2001, 4, 301–308. [Google Scholar] [CrossRef]
- Turner, J.G.; Ellis, C.; Devoto, A. The Jasmonate Signal Pathway. Plant Cell 2002, 14, S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, J.Q.; Zhai, Q.Z.; Zhou, W.K.; Qi, L.L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.L.; Qi, J.; et al. The Basic Helix-Loop-Helix Transcription Factor MYC2 Directly Represses PLETHORA Expression During Jasmonate-Mediated Modulation of the Root Stem Cell Niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.T.; Xu, P.; Zhao, P.X.; Liu, R.; Yu, L.H.; Xiang, C.B. Arabidopsis Erf109 Mediates Cross-Talk between Jasmonic Acid and Auxin Biosynthesis During Lateral Root Formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Zhao, F.; Chen, Y.Q.; Pan, Y.; Sun, L.J.; Bao, N.; Zhang, T.; Cui, C.X.; Qiu, Z.Z.; Zhang, Y.J.; et al. Jasmonate-Mediated Wound Signalling Promotes Plant Regeneration. Nat. Plants 2019, 5, 491–497. [Google Scholar] [CrossRef]
- Conesa, C.M.; Saez, A.; Navarro-Neila, S.; de Lorenzo, L.; Hunt, A.G.; Sepulveda, E.B.; Baigorri, R.; Garcia-Mina, J.M.; Zamarreno, A.M.; Sacristan, S.; et al. Alternative Polyadenylation and Salicylic Acid Modulate Root Responses to Low Nitrogen Availability. Plants 2020, 9, 251. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic Acid Affects Root Meristem Patterning Via Auxin Distribution in a Concentration-Dependent Manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis Root Development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [Green Version]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct Signalling Pathways and Transcriptome Response Signatures Differentiate Ammonium- and Nitrate-Supplied Plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef]
- Ishiyama, K.; Kojima, S.; Takahashi, H.; Hayakawa, T.; Yamaya, T. Cell Type Distinct Accumulations of Mrna and Protein for Nadh-Dependent Glutamate Synthase in Rice Roots in Response to the Supply of Nh4+. Plant Physiol. Biochem. 2003, 41, 643–647. [Google Scholar] [CrossRef]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-Atpase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef]
- Rayle, D.L.; Cleland, R.E. The Acid Growth Theory of Auxin-Induced Cell Elongation Is Alive and Well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef]
- Rober-Kleber, N.; Albrechtova, J.T.P.; Fleig, S.; Huck, N.; Michalke, W.; Wagner, E.; Speth, V.; Neuhaus, G.; Fischer-Iglesias, C. Plasma Membrane H+-Atpase Is Involved in Auxin-Mediated Cell Elongation During Wheat Embryo Development. Plant Physiol. 2003, 131, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Duby, G.; Boutry, M. The Plant Plasma Membrane Proton Pump Atpase: A Highly Regulated P-Type Atpase with Multiple Physiological Roles. Pflug. Arch. Eur. J. Physiol. 2009, 457, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C.M. Changes in the Concentration of Leaf Nitrogen over the Season Affect the Diagnosis of Deficiency or Sufficiency in Strawberries in the Subtropics. Agriculture 2018, 8, 126. [Google Scholar] [CrossRef]
- Nestby, R.; Lieten, F.; Pivot, D.; Lacroix, C.R.; Tagliavini, M. Influence of Mineral Nutrients on Strawberry Fruit Quality and Their Accumulation in Plant Organs: A Review. Int. J. Fruit Sci. 2005, 5, 139–156. [Google Scholar] [CrossRef]
- Yoshida, Y.; Goto, T.; Hirai, M.; Masuda, M. Anthocyanin Accumulation in Strawberry Fruits as Affected by Nitrogen Nutrition; International Society for Horticultural Science: Leuven, Belgium, 2002; pp. 357–360. [Google Scholar]
- Ojeda-Real, L.A.; Lobit, P.; Cárdenas-Navarro, R.; Grageda-Cabrera, O.; Farías-Rodríguez, R.; Valencia-Cantero, E.; Macías-Rodríguez, L. Effect of Nitrogen Fertilization on Quality Markers of Strawberry (Fragaria× Ananassa Duch. Cv. Aromas). J. Sci. Food Agric. 2009, 89, 935–939. [Google Scholar] [CrossRef]
- Kun, C.; Yimin, C.; Hong, Z.; Tongyong, L.; Qing, X.; Lei, W. Effects of Different No3− Concentrations on Growth and Photosynthetic Characteristics in Strawberry Seedling. Chin. Agric. Sci. Bull. 2012, 28, 221–224. [Google Scholar]
- Placido, D.F.; Sandhu, J.; Sato, S.J.; Nersesian, N.; Quach, T.; Clemente, T.E.; Staswick, P.E.; Walia, H. The Lateral Root Density Gene Regulates Root Growth During Water Stress in Wheat. Plant Biotechnol. J. 2020, 18, 1955–1968. [Google Scholar] [CrossRef] [Green Version]
- Majláth, I.; Darko, E.; Palla, B.; Nagy, Z.; Janda, T.; Szalai, G. Reduced Light and Moderate Water Deficiency Sustain Nitrogen Assimilation and Sucrose Degradation at Low Temperature in Durum Wheat. J. Plant Physiol. 2016, 191, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Bowsher, C.G.; Emes, M.J.; Cammack, R.; Hucklesby, D.P. Purification and Properties of Nitrite Reductase from Roots of Pea (Pisum Sativum Cv. Meteor). Planta 1988, 175, 334–340. [Google Scholar] [CrossRef]
- Singh, R.P.; Srivastava, H.S. Increase in Glutamate Synthase Nadh Activity in Maize Zea-Mays Cultivar Ganga-Safed-2 Seedlings in Response to Nitrate and Ammonium Nitrogen. Physiol. Plant. 1986, 66, 413–416. [Google Scholar] [CrossRef]
- Yuefu, W.; Zhenwen, Y.; Shangxia, L.; Songlie, Y. Effect of Nitrogen Nutrition on the Change of Key Enzyme Activity During the Nitrogen Metabolism and Kernel Protein Content in Winter Wheat. Zuo Wu Xue Bao 2002, 28, 743–748. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Saez-Plaza, P.; Jose Navas, M.; Wybraniec, S.; Michalowski, T.; Garcia Asuero, A. An Overview of the Kjeldahl Method of Nitrogen Determination. Part Ii. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, L.; Ji, Y.; Fang, Y. Effects of Nacl Stress on Activity and Expression of Plasma Membrane H+-Atpase in Broussonetia Papyrifera. J. Beijing For. Univ. 2011, 33, 21–26. [Google Scholar]
- Yang, L.; Jon, C.-S.; Wang, L.; Zou, Y.; Liu, L.; Ri, H.-C.; Zhao, J.; Cui, M.; Shang, H.-B.; Li, D. Analysis of Multiple-Phytohormones During Fruit Development in Strawberry by Using Miniaturized Dispersive Solid-Phase Extraction Based on Ionic Liquid-Functionalized Carbon Fibers. J. Food Compos. Anal. 2022, 106, 104262. [Google Scholar] [CrossRef]
- van der Bom, F.J.; Williams, A.; Bell, M.J. Root Architecture for Improved Resource Capture: Trade-Offs in Complex Environments. J. Exp. Bot. 2020, 71, 5752–5763. [Google Scholar] [CrossRef]
- Kenobi, K.; Atkinson, J.A.; Wells, D.M.; Gaju, O.; De Silva, J.G.; Foulkes, M.J.; Dryden, I.L.; Wood, A.T.A.; Bennett, M.J. Linear Discriminant Analysis Reveals Differences in Root Architecture in Wheat Seedlings Related to Nitrogen Uptake Efficiency. J. Exp. Bot. 2017, 68, 4969–4981. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chen, F.; Yuan, L.; Zhang, F.; Mi, G. A Comprehensive Analysis of Root Morphological Changes and Nitrogen Allocation in Maize in Response to Low Nitrogen Stress. Plant Cell Environ. 2015, 38, 740–750. [Google Scholar] [CrossRef]
- Ju, C.; Buresh, R.J.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Root and Shoot Traits for Rice Varieties with Higher Grain Yield and Higher Nitrogen Use Efficiency at Lower Nitrogen Rates Application. Field Crops Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Mi, G.; Chen, F.; Wu, Q.; Lai, N.; Yuan, L.; Zhang, F. Ideotype Root Architecture for Efficient Nitrogen Acquisition by Maize in Intensive Cropping Systems. Sci. China-Life Sci. 2010, 53, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bucio, J.; Cruz-Ramirez, A.; Herrera-Estrella, L. The Role of Nutrient Availability in Regulating Root Architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal Control of Nitrogen Acquisition: Roles of Auxin, Abscisic Acid, and Cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; von Wiren, N. Local Auxin Biosynthesis Acts Downstream of Brassinosteroids to Trigger Root Foraging for Nitrogen. Nat. Commun. 2021, 12, 5437. [Google Scholar] [CrossRef]
- Meier, M.; Liu, Y.; Lay-Pruitt, K.S.; Takahashi, H.; von Wiren, N. Auxin-Mediated Root Branching Is Determined by the Form of Available Nitrogen. Nat. Plants 2020, 6, 1136–1145. [Google Scholar] [CrossRef]
- Liu, Y.; von Wiren, N. Integration of Nutrient and Water Availabilities Via Auxin into the Root Developmental Program. Curr. Opin. Plant Biol. 2022, 65, 102117. [Google Scholar] [CrossRef]
- Sun, X.; Chen, H.; Wang, P.; Chen, F.; Yuan, L.; Mi, G. Low Nitrogen Induces Root Elongation Via Auxin-Induced Acid Growth and Auxin-Regulated Target of Rapamycin (Tor) Pathway in Maize. J. Plant Physiol. 2020, 254, 153281. [Google Scholar] [CrossRef]
- Wang, S.C.; Ichii, M.; Taketa, S.; Xu, L.L.; Xia, K.; Zhou, X. Lateral Root Formation in Rice (Oryza Sativa): Promotion Effect of Jasmonic Acid. J. Plant Physiol. 2002, 159, 827–832. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Ye, S.; Jiang, H.; Chen, Q.; Liu, F.; Zhou, W.; Chen, R.; Li, X.; Tietz, O.; et al. Arabidopsis Asa1 Is Important for Jasmonate-Mediated Regulation of Auxin Biosynthesis and Transport During Lateral Root Formation. Plant Cell 2009, 21, 1495–1511. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin Biosynthesis by the Yucca Flavin Monooxygenases Controls the Formation of Floral Organs and Vascular Tissues in Arabidopsis. Genes Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentrich, M.; Boettcher, C.; Duechting, P.; Cheng, Y.; Zhao, Y.; Berkowitz, O.; Masle, J.; Medina, J.; Pollmann, S. The Jasmonic Acid Signaling Pathway Is Linked to Auxin Homeostasis through the Modulation of Yucca8 and Yucca9 Gene Expression. Plant J. 2013, 74, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.P.; Zhang, C.L.; Zheng, H.H.; Sun, M.; Zhang, F.; Zhang, M.Y.; Cui, F.H.; Lv, D.P.; Liu, L.J.; Guo, S.Y.; et al. Antagonistic Interaction between Auxin and Sa Signaling Pathways Regulates Bacterial Infection through Lateral Root in Arabidopsis. Cell Rep. 2020, 32, 108060. [Google Scholar] [CrossRef]
- Echevarria-Machado, I.; Escobedo-Gm, R.M.; Larque-Saavedra, A. Responses of Transformed Catharanthus Roseus Roots to Ferntomolar Concentrations of Salicylic Acid. Plant Physiol. Biochem. 2007, 45, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic Acid in Root Growth and Development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef]
- Kitakura, S.; Vanneste, S.; Robert, S.; Lofke, C.; Teichmann, T.; Tanaka, H.; Friml, J. Clathrin Mediates Endocytosis and Polar Distribution of Pin Auxin Transporters in Arabidopsis. Plant Cell 2011, 23, 1920–1931. [Google Scholar] [CrossRef]
- Llorente, F.; Muskett, P.; Sanchez-Vallet, A.; Lopez, G.; Ramos, B.; Sanchez-Rodriguez, C.; Jorda, L.; Parker, J.; Molina, A. Repression of the Auxin Response Pathway Increases Arabidopsis Susceptibility to Necrotrophic Fungi. Mol. Plant 2008, 1, 496–509. [Google Scholar] [CrossRef]
- Hou, S.; Tsuda, K. Salicylic Acid and Jasmonic Acid Crosstalk in Plant Immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.B.A.; Liu, S.D.; Zhang, S.P.; Ge, C.W.; Shen, Q.; Ma, H.J.; Zhang, X.M.; Dong, H.L.; Zhao, X.H.; et al. Nitrogen Stress Inhibits Root Growth by Regulating Cell Wall and Hormone Changes in Cotton (Gossypium Hirsutum L.). J. Agron. Crop Sci. 2021, 207, 1006–1023. [Google Scholar] [CrossRef]
- Allu, A.D.; Brotman, Y.; Xue, G.P.; Balazadeh, S. Transcription Factor Anac032 Modulates Ja/Sa Signalling in Response to Pseudomonas Syringae Infection. Embo Rep. 2016, 17, 1578–1589. [Google Scholar] [CrossRef]
- Ndamukong, I.; Al Abdallat, A.; Thurow, C.; Fode, B.; Zander, M.; Weigel, R.; Gatz, C. Sa-Inducible Arabidopsis Glutaredoxin Interacts with Tga Factors and Suppresses Ja-Responsive Pdf1.2 Transcription. Plant J. 2007, 50, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Spivey, N.W.; Zeng, W.Q.; Liu, P.P.; Fu, Z.Q.; Klessig, D.F.; He, S.Y.; Dong, X.N. Coronatine Promotes Pseudomonas Syringae Virulence in Plants by Activating a Signaling Cascade That Inhibits Salicylic Acid Accumulation. Cell Host Microbe 2012, 11, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Obroucheva, N.V.; Lityagina, S.V.; Sinkevich, I.A. Activation and Activity of Plasma Membrane H+-Atpase: Key Events in Germinating Vicia Faba Seeds. Seed Sci. Res. 2021, 31, 76–82. [Google Scholar] [CrossRef]
- Anderson, C.T.; Kieber, J.J. Dynamic Construction, Perception, and Remodeling of Plant Cell Walls. Annu. Rev. Plant Biol. 2020, 71, 39–69. [Google Scholar] [CrossRef]
- Sperandio, M.V.L.; Santos, L.A.; Tavares, O.C.H.; Fernandes, M.S.; de Freitas Lima, M.; de Souza, S.R. Silencing the Oryza Sativa Plasma Membrane H+-Atpase Isoform OsA2 Affects Grain Yield and Shoot Growth and Decreases Nitrogen Concentration. J. Plant Physiol. 2020, 251, 153220. [Google Scholar] [CrossRef]
- Sperandio, M.V.L.; Santos, L.A.; Bucher, C.A.; Fernandes, M.S.; de Souza, S.R. Isoforms of Plasma Membrane H+-Atpase in Rice Root and Shoot Are Differentially Induced by Starvation and Resupply of No3− or Nh4+. Plant Sci. 2011, 180, 251–258. [Google Scholar] [CrossRef]
- Vanneste, S.; Friml, J. Auxin: A Trigger for Change in Plant Development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Y.; Hu, L.; Zhang, Y.; Zhang, B.; Xia, H.; Du, W.; Fan, S.; Kong, L. Low-Nitrogen Stress Stimulates Lateral Root Initiation and Nitrogen Assimilation in Wheat: Roles of Phytohormone Signaling. J. Plant Growth Regul. 2021, 40, 436–450. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Wei, M.; Ge, Y.; Hou, J.; Cheng, Y.; Chen, J. Methyl Jasmonate Maintained Antioxidative Ability of Ginger Rhizomes by Regulating Antioxidant Enzymes and Energy Metabolism. Sci. Hortic. 2019, 256, 108578. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, C.; Guo, Z.; Zhang, Q.; Li, S.; Zhang, X.; Gong, J.; Shen, Y. Herbivore Exposure Alters Ion Fluxes and Improves Salt Tolerance in a Desert Shrub. Plant Cell Environ. 2020, 43, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi-Golezani, K.; Abdoli, S. Improving Atpase and Ppase Activities, Nutrient Uptake and Growth of Salt Stressed Ajowan Plants by Salicylic Acid and Iron-Oxide Nanoparticles. Plant Cell Rep. 2021, 40, 559–573. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Liu, H.; Huang, W. Salicylic Acid or Heat Acclimation Pre-Treatment Enhances the Plasma Membrane-Associated Atpase Activities in Young Grape Plants under Heat Shock. Sci. Hortic. 2008, 119, 21–27. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, H.T.; Pan, Q.H.; Yang, H.R.; Zhan, J.C.; Huang, W.D. The Plasma Membrane H+-Atpase Is Related to the Development of Salicylic Acid-Induced Thermotolerance in Pea Leaves. Planta 2009, 229, 1087–1098. [Google Scholar] [CrossRef]
- Remans, T.; Nacry, P.; Pervent, M.; Girin, T.; Tillard, P.; Lepetit, M.; Gojon, A. A Central Role for the Nitrate Transporter Nrt2.1 in the Integrated Morphological and Physiological Responses of the Root System to Nitrogen Limitation in Arabidopsis. Plant Physiol. 2006, 140, 909–921. [Google Scholar] [CrossRef]
- Sun, X.; Jia, X.; Huo, L.Q.; Che, R.M.; Gong, X.Q.; Wang, P.; Ma, F.W. Mdatg18a Overexpression Improves Tolerance to Nitrogen Deficiency and Regulates Anthocyanin Accumulation through Increased Autophagy in Transgenic Apple. Plant Cell Environ. 2018, 41, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Hu, B.; Chu, C.C. Nitrogen Assimilation in Plants: Current Status and Future Prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.T.; Shao, X.Q.; Li, J.X.; Ahammed, G.J.; Yao, Y.L.; Ding, J.; Hu, Z.J.; Yu, J.Q.; Shi, K. Nitrogen Forms and Metabolism Affect Plant Defence to Foliar and Root Pathogens in Tomato. Plant Cell Environ. 2021, 44, 1596–1610. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef]
- Li, Y.T.; Han, D.X.; Sommerfeld, M.; Hu, Q.A. Photosynthetic Carbon Partitioning and Lipid Production in the Oleaginous Microalga Pseudochlorococcum Sp (Chlorophyceae) under Nitrogen-Limited Conditions. Bioresour. Technol. 2011, 102, 123–129. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Ma, H.T.; Hu, B.; Zhao, H.Y.; Wang, J.; Rennenberg, H.; Shi, X.J.; Zhang, Y.Q. Nitrogen Fertilization Stimulates Nitrogen Assimilation and Modifies Nitrogen Partitioning in the Spring Shoot Leaves of Citrus (Citrus Reticulata Blanco) Trees. J. Plant Physiol. 2021, 267, 153556. [Google Scholar] [CrossRef]
- Zhou, W.W.; Liang, X.; Li, K.J.; Dai, P.B.; Li, J.L.; Liang, B.; Sun, C.L.; Lin, X.Y. Metabolomics Analysis Reveals Potential Mechanisms of Phenolic Accumulation in Lettuce (Lactuca Sativa L.) Induced by Low Nitrogen Supply. Plant Physiol. Biochem. 2021, 158, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Li, Z.; Wang, L.; Dai, T.; Kang, Q.; Niu, D. Exogenous of Indole-3-Acetic Acid Application Alleviates Copper Toxicity in Spinach Seedlings by Enhancing Antioxidant Systems and Nitrogen Metabolism. Toxics 2020, 8, 1. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, R.; Singh, A.; Prasad, S.M. Role of Oxylipin on Luffa Seedlings Exposed to Nacl and Uv-B Stresses: An Insight into Mechanism. Plant Physiol. Biochem. 2021, 167, 691–704. [Google Scholar] [CrossRef]
- Hayat, S.; Fariduddin, Q.; Ali, B.; Ahmad, A. Effect of Salicylic Acid on Growth and Enzyme Activities of Wheat Seedlings. Acta Agron. Hung. 2005, 53, 433–437. [Google Scholar] [CrossRef]
- Gautam, S.; Singh, P.K. Salicylic Acid-Induced Salinity Tolerance in Corn Grown under Nacl Stress. Acta Physiol. Plant. 2009, 31, 1185–1190. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of Exogenous Salicylic Acid under Changing Environment: A Review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, T.; Zhang, J.; Lei, W.; Zhao, L.; Wang, S.; Shi, M.; Wei, M. Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks. Horticulturae 2023, 9, 249. https://doi.org/10.3390/horticulturae9020249
Zhang W, Zhang T, Zhang J, Lei W, Zhao L, Wang S, Shi M, Wei M. Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks. Horticulturae. 2023; 9(2):249. https://doi.org/10.3390/horticulturae9020249
Chicago/Turabian StyleZhang, Wenjie, Ting Zhang, Jia Zhang, Weiwei Lei, Lin Zhao, Shuai Wang, Mengyun Shi, and Meng Wei. 2023. "Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks" Horticulturae 9, no. 2: 249. https://doi.org/10.3390/horticulturae9020249
APA StyleZhang, W., Zhang, T., Zhang, J., Lei, W., Zhao, L., Wang, S., Shi, M., & Wei, M. (2023). Low Nitrogen Stress Promotes Root Nitrogen Uptake and Assimilation in Strawberry: Contribution of Hormone Networks. Horticulturae, 9(2), 249. https://doi.org/10.3390/horticulturae9020249