Abstract 摘要
Genetically programmed circuits allowing bifunctional dynamic regulation of enzyme expression have far-reaching significances for various bio-manufactural purposes. However, building a bio-switch with a post log-phase response and reversibility during scale-up bioprocesses is still a challenge in metabolic engineering due to the lack of robustness. Here, we report a robust thermosensitive bio-switch that enables stringent bidirectional control of gene expression over time and levels in living cells. Based on the bio-switch, we obtain tree ring-like colonies with spatially distributed patterns and transformer cells shifting among spherical-, rod- and fiber-shapes of the engineered Escherichia coli. Moreover, fed-batch fermentations of recombinant E. coli are conducted to obtain ordered assembly of tailor-made biopolymers polyhydroxyalkanoates including diblock- and random-copolymer, composed of 3-hydroxybutyrate and 4-hydroxybutyrate with controllable monomer molar fraction. This study demonstrates the possibility of well-organized, chemosynthesis-like block polymerization on a molecular scale by reprogrammed microbes, exemplifying the versatility of thermo-response control for various practical uses.
遗传编程电路允许双功能动态调控酶表达,这对各种生物制造目的具有深远意义。然而,构建一个具有对数后期响应和可逆性的生物开关(bio-switch)在规模化生物过程期间,仍然是代谢工程(metabolic engineering)中的挑战,因为缺乏稳健性。在这里,我们报道了一个稳健的热敏生物开关,它能够在活细胞中对基因表达的时间和水平实现严格的双向控制。基于这个生物开关,我们获得了树轮状菌落,具有空间分布模式,以及变形细胞,在工程大肠杆菌中切换球形、杆状和纤维状形态。此外,对重组大肠杆菌进行 fed-batch 发酵,以获得有序组装的定制生物聚合物聚羟基烷酸酯,包括二嵌段和随机共聚物,由 3-羟基丁酸和 4-羟基丁酸组成,具有可控的单体摩尔分数。本研究展示了通过重新编程的微生物在分子尺度上进行良好组织、类似化学合成的块状聚合的可能性,体现了热响应控制的多功能性,用于各种实际应用。
Similar content being viewed by others
其他人正在查看类似内容
Introduction 引言
Synthetic biology aiming to perform computational programs in living cells enables revolutionary developments in biotechnology1,2. Notably, scalable and robust gene circuits for uni- and bidirectional control of target genes over various timings and levels triggered by specific signals are desirable for diverse applications3,4,5. Generally, a dynamic control system of customized functions can be achieved by leveraging a variety of signal-sensing modules, including chemicals6,7, intermediate metabolites8,9,10, temperature11,12,13, light14,15,16, and cell population17 responsive biosensors. In previous studies, many successful systems have been constructed for different purposes, such as enhanced production of various metabolic products including fuels18, drugs19, or other valuable chemicals20 by microbes engineered using bidirectional dynamic control strategy.
合成生物学 (synthetic biology) 旨在在活细胞中执行计算程序,这使得生物技术领域实现了革命性的发展 1,2 。值得注意的是,可扩展且稳健的基因电路 (gene circuits) 能够对目标基因进行单向和双向控制,在各种时间和水平上响应特定信号,这对于多样化的应用是可取的 3,4,5 。一般来说,动态控制系统可以通过利用各种信号感应模块来实现定制功能,包括化学物质 6,7 、中间代谢物 8,9,10 、温度 11,12,13 、光 14,15,16 和细胞种群 17 响应的生物传感器。在先前的研究中,已经构建了许多成功的系统,用于不同的目的,例如通过使用双向动态控制策略工程化的微生物来增强各种代谢产物的生产,包括燃料 18 、药物 19 或其他有价值的化学物质 20 。
More importantly, in contrast to the traditional uses for metabolic control of endogenous or heterogenous pathways as a “metabolic valves”14,21, genetic programs of defined function demonstrate inconceivable capability of generating versatile patterns in narrowed down scales from colony size to molecular scales inspired by nature22,23,24, for instance, programmable cell consortia and space-sensing coordinate patterning2,25,26 based on quorum-sensing systems, block-like amyloid nano-fibers assembly using toggle switch27, and so on.
更重要的是,与传统用于内源性或异源性途径代谢控制的“代谢阀门” 14,21 相比,具有明确功能的遗传程序展示了难以想象的能力,能够在从菌落规模到分子规模的缩小范围内生成多样的模式,这些模式受自然启发 22,23,24 ,例如基于群体感应系统的可编程细胞联合体和空间感知坐标模式 2,25,26 、使用切换开关组装的块状淀粉样纳米纤维 27 等。
However, to achieve dynamic optimization of cell factory engineering or nature-inspired patterning, there are still many challenges ahead of us to construct a well-designed circuit conferring exquisite, reversable and dynamic control for practical uses, which requires stringent and scalable control activity, instantaneous removal of sensing signal, fast-response availability in the post-log-phase, etc. Compared with the chemical- and cell density-response biosensing machinery used for dynamic metabolic control28, temperature and light are attractive strategies with reversibility to address these challenges. In particular, thermosensitive genetic tools, which have been engineered for different uses, such as dynamic regulation for defining cell growth and production synthesis phases11,12, gene therapy29, etc., are more ideal for practical utilizations because of the convenient collocation, low cost, easy operability, and good dispersity of heat-transfer required in varied bioprocesses30,31. Therefore, engineering thermal-switchable bioswitch of bifunction can make possible the simultaneous activation and depression of distinct sets of genes in a temperature-dependent manner.
然而,要实现细胞工厂工程的动态优化或受自然启发的模式构建,仍面临许多挑战,我们需要构建设计精良的电路,以提供精细、可逆和动态控制,用于实际应用,这要求严格且可扩展的控制活动、即时移除感应信号、在对数后期快速响应能力等。与用于动态代谢控制的化学和细胞密度响应生物传感器机制 28 相比,温度和光是具有可逆性的吸引人策略,可解决这些挑战。特别是,热敏遗传工具已被工程用于不同用途,例如动态调控以定义细胞生长和生产合成阶段 11,12 、基因疗法 29 等,由于热传递在各种生物过程的便利搭配、低成本、易操作和良好分散性 30,31 ,这些工具更适合实际应用。因此,工程热敏可切换的双功能生物开关可以实现根据温度依赖方式同时激活和抑制不同基因集。
To address the interests, we demonstrate a versatile thermosensitive system, termed T-switch, for bifunctional dynamic control of gene expression in recombinant Escherichia coli, based on a temperature-associated transcriptional regulator CI857 32. Using the T-switch and its derivates, we first generated tunable and hierarchical tree ring-like patterned colonies under periodically temperature-changing circumstance, inspired by the natural tree ring formation responded to the seasonal variation of environmental temperature and humidity. Besides, we also obtained transformer cells among spherical, rod, and fiber shapes by controlling the expression of morphology-associated genes at different temperatures. Furthermore, T-switch was employed to modulate the biosynthesis of two building blocks of polyhydroxyalkanoates (PHA)33,34, enabling the chemosynthesis-like polymerization of diblock copolymer, poly(3-hydroxybutyrate)-block-poly(4-hydroxybutyrate), short as PHB-b-P4HB. Our results thus revealed the sophisticated gene expression control for narrowed down patterning, from tree ring-like colony on macroscopic scale, morphology-changable bacteria on microscopic scale, to ordered-assembled block biopolymers on molecular scale, which opens the possibility for ingenious tailor-made molecular assembly in vivo.
为了应对这些兴趣点,我们展示了一个多功能的热敏系统,称为 T-switch,用于在重组大肠杆菌中实现基因表达的双功能动态控制,该系统基于温度相关的转录调控因子 CI 857 32 。使用 T-switch 及其衍生物,我们首先在周期性温度变化的环境下生成了可调和分层的树轮状菌落模式,这受到了自然树轮形成对环境温度和湿度季节变化的响应启发。此外,我们还通过在不同温度下控制形态相关基因的表达,获得了球形、杆状和纤维状之间的变形细胞。而且,T-switch 被用于调节聚羟基脂肪酸酯 (PHA) 两个构建模块的生物合成 33,34 ,从而实现了类似化学合成的二嵌段共聚物聚合,即聚(3-羟基丁酸)-嵌段-聚(4-羟基丁酸),简称 PHB-b-P4HB。我们的结果揭示了精细的基因表达控制,用于缩小范围的模式构建,从宏观尺度的树轮状菌落到微观尺度的可变形态细菌,再到分子尺度的有序组装嵌段生物聚合物,这为体内巧妙定制的分子组装提供了可能性。
Results 结果
Prototyping bifunctional dynamic control of T-switch
T-switch 的双功能(bifunctional)动态控制原型设计
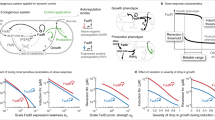
In order to develop a temperature-dependent bifunctional bioswitch, namely, T-switch, of robust gene expression control in engineered E. coli, two cascaded modules were constructed based on a thermosensitive transcriptional regulator CI857 and a TetR-family repressor PhlF encoded, respectively, by gene cI857 and phlF35 (Fig. 1a). The recombinant E. coli JM109SGL came from E. coli JM109SG with deficiency of sad and gabD gene reported in previous study, by an additional deletion of lacI (Supplementary Fig. 1)36. For the temperature sensory module (construct 165), the constitutive expression of cI857, a widely used mutant of cI from bacteriophage λ as a thermo-genetic tool37, exhibits strong repression on PR promoter at 30 °C by forming dimmer CI857 complex to achieve active and inactive transcriptional control of repressor PhlF and reporter mRFP under 37 °C and 30 °C, respectively. To engineer bidirectional control function, construct 155 containing reporter sfGFP expression module controlled by PPhlF, the corresponding promoter tightly inhibited by PhlF, was cascaded to receive the propagated signal of PhlF from temperature-sensing panel. Thus, the switchable bidirectional control mediated by temperature modulation between 30 and 37 °C can be characterized through the ON/OFF expression performance of sfGFP and mRFP (Fig. 1a).
为了开发一种温度依赖的双功能生物开关(bifunctional bioswitch),即 T-switch,用于在工程化的大肠杆菌 E. coli 中实现稳健的基因表达控制,构建了两个级联模块,分别基于热敏(thermosensitive)转录调控因子 CI 857 和 TetR 家族抑制因子 PhlF,由基因 cI 857 和 phlF 35 分别编码(图 1a)。重组大肠杆菌 E. coli JM109SGL 源自 E. coli JM109SG,该菌株缺乏 sad 和 gabD 基因,如先前的研究报道,通过额外删除 lacI 基因(补充图 1) 36 。对于温度感应模块(构建 165),cI 857 的组成型表达是一种广泛使用的 cI 突变体,源自噬菌体 λ 作为热遗传工具 37 ,在 30 °C 时通过形成二聚体 CI 857 复合物对 P R 启动子施加强抑制,从而在 37 °C 和 30 °C 下分别实现对抑制因子 PhlF 和报告基因 mRFP 的活性抑制和非抑制转录控制。为了工程双向控制功能,构建 155 包含由 P PhlF 控制的报告基因 sfGFP 表达模块,该启动子被 PhlF 紧密抑制,并级联连接以从温度感应模块接收 PhlF 的传播信号。因此,通过 30 和 37 °C 之间的温度调控,可以通过 sfGFP 和 mRFP 的 ON/OFF 表达性能表征可切换的双向控制(图 1a)。
图 1:热敏(thermosensitive)双功能(bifunctional)生物开关的设计和表征 (T-switch)。
a Circuits of T-switch using a NOT gate design enables bifunctional gene expression, of which the input is the repressor PhlF related to a temperature-controlled system based on cI857(cI), and the output is a reporter sfgfp encoding sfGFP under the promoter PphlF. Another output is a reporter mrfp encoding mRFP placed downstream the phlF gene (phlF-mrfp) for achieving bifunctional control of gene expression. b Promoter activity and orthogonality of the CI857-regulated panel are characterized based on two expression cassettes: sfgfp as a reporter alone in a and phlF-mrfp cluster. c Data generated using the constructs from part a via cytometry analysis are used to characterize the bidirectional temperature-response functions of the T-switch. d Data gathered onto a single transfer function between two reporters, namely, sfGFP and mRFP, are identical to those shown in c from the same experiments. Each point represents a condition of cultural temperature from 30 ℃ to 37 ℃. e The fluorescence intensity was measured from each temperature from 30 °C to 37 °C for controlling the expression levels of sfgfp and phlF-mrfp. The fluorescence ratios between sfGFP and mRFP expression from each temperature point collapse onto a single function for linear regression analysis with R2 = 0.9868 and slope close to 1 (1.065), which was plotted in log-log coordinates (log10). Data are presented as mean ± s.d. of three replicates. FI, Fluorescence Intensity in arbitrary unit (a.u.).
a 使用 NOT gate 设计的 T-switch 电路实现了双功能基因表达 (bifunctional gene expression),其输入是与基于 cI 857 (cI) 的温度控制系统相关的阻遏物 PhlF,输出是在启动子 P phlF 下编码 sfGFP 的报告基因 sfgfp。另一个输出是置于 phlF 基因下游的编码 mRFP 的报告基因 mrfp (phlF-mrfp),用于实现基因表达的双功能控制。b CI 857 调控面板的启动子活性和正交性 (orthogonality) 通过两个表达盒 (expression cassettes) 进行表征:即 a 中的 sfgfp 单独作为报告基因,以及 phlF-mrfp 簇。c 通过细胞计量学 (cytometry) 分析对 a 部分构建体产生的数据,用于表征 T-switch 的双向温度响应函数 (bidirectional temperature-response functions)。d 将两个报告基因 sfGFP 和 mRFP 之间的数据整合到一个传递函数 (transfer function) 中,这些数据与 c 中相同实验所示的数据一致。每个点代表培养温度 (cultural temperature) 从 30 ℃ 到 37 ℃ 的条件。e 从 30 °C 到 37 °C 的每个温度点测量荧光强度 (fluorescence intensity),以控制 sfgfp 和 phlF-mrfp 的表达水平。从每个温度点的 sfGFP 和 mRFP 表达之间的荧光比率收敛到一个单一函数,用于线性回归分析 (linear regression),R 2 = 0.9868,斜率接近 1 (1.065),并在对数-对数坐标 (log 10 ) 中绘制。数据以三重复的均值 ± 标准差 (s.d.) 表示。FI, Fluorescence Intensity in arbitrary unit (a.u.)。
To study the temperature-response function of T-switch from 30 to 37 °C, FACS analysis was carried out using recombinant cells of E. coli JM109SGL harboring constructs 155+165 grown in a 96-deep well plate for 12 h at different temperatures in Luria-Bertani (LB) medium. The control performance of construct group 155+165 is summarized in Fig. 1c, showing 35- and 1819-fold dynamic range of red fluorescence (mRFP) and green fluorescence (sfGFP), respectively, between 30 and 37 °C. Meanwhile, the performance of construct 155+165 cultured in the chemical defined minimal medium (M9) was tested, obtaining similar dynamic range in contrast to a LB medium (Supplementary Fig. 2). Interestingly, the low-temperature responsive ON-performance characterized by sfGFP panel exhibited a higher sensitivity and lower leakage in the OFF-stage (sfGFP under 37 °C vs. mRFP under 30 °C) compared to the high-temperature responsive panel characterized by mRFP (Fig. 1c). Significant decrease of leakage was observed with the addition of degradation tag AVV to the C-terminal of sfGFP and mRFP, respectively, resulting in a higher dynamic range except the performances at 30 °C (Supplementary Fig. 3). The topology-like performance of T-switch was studied by plotting the fluorescent intensity (FI) of sfGFP against mRFP (Fig. 1d), forming two separated regions A and B with dominant green and red fluorescence, respectively. Notably, these performances also conferred the simultaneous up- and downregulation, namely bifunctional control, of target genes by replacing sfgfp and mrfp at altering temperatures from 30 to 37 °C. Furthermore, the expression levels of two independent modules, including phlF-mrfp cluster from construct 165 and sfgfp (Fig. 1b), were characterized for linear regression analysis with R2 = 0.9868 and slope k = 1.065 (Fig. 1e), indicating negligible expression variance among different target gene clusters controlled by temperature sensing panel derived from construct 165. In addition, considering the compatibility of T-switch circuit functioning in the start host at 30 and 37 °C, transcriptome profiling was performed to study the effects of genome-wide mRNA levels. Results showed highly consistent total mRNA abundance and distribution under different conditions except 250 genes that were significantly downregulated at 37 °C, probably due to the minor crosstalk of PhlF expression in cellular regulatory network (Supplementary Figs. 4 and 5). Generally, a stringent and robust T-Switch had been successfully constructed to achieve bifunctional regulation of gene expression with a significant tunable dynamic fold-change.
为研究 T-switch 从 30 到 37 °C 的温度响应功能,使用携带构建体 155+165 的重组大肠杆菌 (E. coli) JM109SGL 细胞,在 Luria-Bertani (LB) 培养基中于 96 深孔板中在不同温度下培养 12 小时后,进行流式细胞术分析 (FACS)。构建体组 155+165 的控制性能总结在图 1c 中,显示在 30 到 37 °C 之间,红色荧光 (mRFP) 和绿色荧光 (sfGFP) 的动态范围分别为 35 倍和 1819 倍。与此同时,在化学定义的最小培养基 (M9) 中培养的构建体 155+165 的性能被测试,获得了与 LB 培养基类似的动态范围 (补充图 2)。有趣的是,由 sfGFP 表征的低温响应 ON-性能 (sfGFP 在 37 °C 下 vs. mRFP 在 30 °C 下) 相对于由 mRFP 表征的高温响应性能,显示出更高的敏感性和更低的泄漏 (图 1c)。通过分别在 sfGFP 和 mRFP 的 C 端添加降解标签 (degradation tag) AVV,观察到泄漏显著减少,从而导致更高的动态范围,但 30 °C 下的性能除外 (补充图 3)。T-switch 的拓扑-like 性能通过绘制 sfGFP 的荧光强度 (FI) 与 mRFP 的关系来研究,形成两个分离区域 A 和 B,分别主导绿色和红色荧光 (图 1d)。值得注意的是,这些性能还赋予了在 30 到 37 °C 的温度变化下,同时上调和下调目标基因,即双功能控制,通过替换 sfgfp 和 mrfp 来实现。此外,两个独立模块的表达水平,包括来自构建体 165 的 phlF-mrfp 簇和 sfgfp (图 1b),被用于线性回归分析,得到 R² = 0.9868 和斜率 k = 1.065 (图 1e),表明由构建体 165 派生的温度感应模块控制的不同目标基因簇之间,表达变异性可以忽略不计。另外,考虑到 T-switch 电路在起始宿主中在 30 和 37 °C 下功能的兼容性,转录组分析 (transcriptome profiling) 被进行,以研究不同条件下全基因组 mRNA 水平的影响。结果显示,除了 250 个在 37 °C 下显著下调的基因外,总 mRNA 丰度和分布在不同条件下高度一致,这可能是由于 PhlF 表达在细胞调控网络中的轻微串扰 (补充图 4 和 5)。总体上,一个严格且稳健的 T-Switch 已被成功构建,以实现基因表达的双功能调控,并具有显著的可调动态倍数变化。
Optimization and characteristics of T-switch
T-switch 的优化与特性
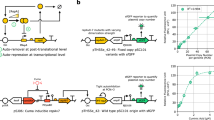
On the basis of the original T-switch, constructs 155+165, further bioswitch engineering was developed by introducing negative feedback loop control of the temperature sensing panel (Fig. 2a). Specifically, repressor LacI, encoded by lacI under PPhlF promoter, associated operator LacO, was introduced to achieve a negative feedback control of the transcriptional activity of PR promoter under low-temperature (30 °C) responded sfGFP-ON stage. First, the seed cultures of recombinant cells harboring constructs 155+165 and 147+167, respectively, were prepared at 30 °C, then inoculated and grown at 37 °C for 12 h, followed by the time course monitoring of fluorescence intensity of both reporters. The static temperature-response performances of two T-switch combinations were thus characterized. Constructs 147+167 exhibited a tighter sfGFP-OFF control with a lower leakage and lag-activation of mRFP-ON control compared to the original constructs 155+165 (black triangle shown in Fig. 2b).
基于原始的 T-switch,在构建体 155+165 的基础上,通过引入温度感应面板的负反馈回路控制(negative feedback loop)(Fig. 2a),开发了进一步的生物开关工程。具体而言,编码于 P PhlF 启动子下的 lacI 基因表达的抑制蛋白 LacI,与相关操作子 LacO 被引入,以实现对 P R 启动子转录活性的负反馈控制(negative feedback control),在低温(30 °C)响应下的 sfGFP-ON 阶段。首先,分别携带构建体 155+165 和 147+167 的重组细胞的种子培养物在 30 °C 下准备,然后接种并在 37 °C 下生长 12 小时,随后对两个报告基因的荧光强度进行时程监测。从而表征了两种 T-switch 组合的静态温度响应性能(temperature-response performances)。与原始构建体 155+165 相比,构建体 147+167 展示了更严格的 sfGFP-OFF 控制,具有更低的泄漏和 mRFP-ON 控制的滞后激活(lag-activation)(Fig. 2b 中的黑三角表示)。
Fig. 2: 双功能(bifunctional)开和关响应(on- and off-responses)的开关测试(switch-testing)。
a Constructs based on T-switch, the 155+165 group, by introducing negative feedback loop control of input signals, namely 147+167 group. lacI gene was co-expressed with sfgfp to stringently repress the promoter activity of PR with the downstream insertion of a LacI-associated operator, LacO. b Fluorescence intensity (FI) monitoring of time course of T-switch circuits by altering temperature from 30 °C to 37 °C right after inoculation to characterize the response-curve under thermal inductions. Cells were grown at 30 °C throughout the pre-cultivation from a single colony. c On- and off-response performance of T-switch constructs shown in a in different growth phases. Recombinant cells were grown for 12 h after changing temperature from 30 °C to 37 °C. Left: distribution and variance of fluorescence, including sfGFP and mRFP, monitored using a flow cytometer; right: quantitative comparisons of on/off performances. Data in b and c are presented as mean ± s.d. of three replicates. d 12 h Time-lapse photography of the temperature-responsive performances of engineered E. coli harboring T-switch, and constructs 155+165, during a shift from 37 °C to 30 °C. Samples comprising 1 µL of the cell suspension were injected and incubated underneath a layer of solid LB with 1.0% agarose (~1.5 mm) containing the relevant antibiotics at 30 °C from overnight cultures at 37 °C. Since the tightness and ultra-sensitivity of tetR family repressor PhlF, the activation of sfGFP lagged behind the quenching of mRFP by at least 4 h.
a 基于 T-switch 的构建,包括 155+165 组,通过引入输入信号的负反馈回路(negative feedback loop)控制,即 147+167 组。lacI gene 与 sfgfp 共表达,以严格抑制 P R 的启动子活性(promoter activity),并在下游插入 LacI 相关操作子 LacO。
b 通过在接种后立即将温度从 30 °C 改为 37 °C,对 T-switch 电路的时间进程进行荧光强度(FI)监测,以表征热诱导(thermal inductions)下的响应曲线。细胞从单个菌落开始,在整个预培养过程中都在 30 °C 下生长。
c a 中所示 T-switch 构建在不同生长阶段的开和关响应性能。重组细胞在将温度从 30 °C 改为 37 °C 后培养 12 h。左边:使用流式细胞仪(flow cytometer)监测 sfGFP 和 mRFP 等荧光的分布和变异性;右边:开/关性能的定量比较。b 和 c 中的数据以三重复的平均值 ± s.d. 呈现。
d 在温度从 37 °C 降至 30 °C 期间,对携带 T-switch 和 155+165 构建的工程化 E. coli 的温度响应性能进行 12 h 时间序列摄影(time-lapse photography)。取 1 µL 细胞悬液样本,从 37 °C 过夜培养物中注入,并在 30 °C 下置于含有相关抗生素的 1.0% 琼脂糖固体 LB 层(~1.5 mm)下方进行培养。由于 tetR 家族阻遏蛋白 PhlF 的紧密性和超高敏感性,sfGFP 的激活至少落后 mRFP 的淬灭 4 h。
More combinatory designs were constructed with the addition of degradation tags, AAV and LVA (sequences are shown in Supplementary Table 2), to the C-terminal of sfGFP and mRFP, respectively, to study the dynamic temperature-response performances during different growth phases at altering temperatures from 30 to 37 °C in 0–12 h (at 0, 2, 4, 6, 8, 10, and 12 h) after inoculation (Fig. 2c and Supplementary Fig. 6). In contrast, cell cultures maintained at 30 °C for 24-h growth after inoculation were used as control group labeled with “–.” Results showed that the mRFP-ON response controlled by the temperature sensing panel was still active before 10 h with less than 50% decrease of fluorescence intensity. However, the sfGFP-OFF control could be activated in the first 6 h, approximately in the mid-log phase (Fig. 2c and Supplementary Fig. 13a). Constructs 147+168 exhibited a poor mRFP-ON performance with discrete distribution of fluorescence due to the joint weakening of LacO and AAV tag (Supplementary Fig. 7). In addition, correlations of four phlF-mrfp modules with combinatory supplementation of LacO operator and AAV tag controlled by the temperature-sensing panel were characterized. They had highly pairwise linear correlations with R2 (square of Pearson correlation coefficient) higher than 0.93 (Supplementary Fig. 8). Interestingly, the insertion of LacO resulted in a nearly five-fold increase of output level, including the leakage under OFF-stage at 30 °C. On the contrary, the addition of AAV tag displayed about 100-fold decrease of basal leakage.
构建了更多组合设计,通过在 sfGFP 和 mRFP 的 C 末端(C-terminal)分别添加降解标签(degradation tags)、AAV 和 LVA(序列见补充表 2),以研究在接种后 0-12 小时内温度从 30 到 37 °C 变化期间不同生长阶段的动态温度响应性能(在 0、2、4、6、8、10 和 12 小时,Fig. 2c 和补充图 6)。相比之下,接种后在 30 °C 下培养 24 小时的细胞培养物作为对照组,标记为“–”。结果显示,由温度感应模块控制的 mRFP-ON 响应在 10 小时前仍保持活性,荧光强度(fluorescence intensity)下降不足 50%。然而,sfGFP-OFF 控制可在前 6 小时激活,大约在对数生长期(mid-log phase)中期(Fig. 2c 和补充图 13a)。构型 147+168 显示 mRFP-ON 性能较差,荧光分布离散,这是由于 LacO 和 AAV 标签的联合削弱作用(补充图 7)。此外,对四个 phlF-mrfp 模块的关联进行了表征,这些模块通过温度感应模块结合补充 LacO 操纵子和 AAV 标签控制;它们具有高度成对线性相关性,R²(Pearson correlation coefficient 的平方)高于 0.93(补充图 8)。有趣的是,插入 LacO 导致输出水平几乎增加五倍,包括在 30 °C 关闭阶段(OFF-stage)的漏泄。相反,添加 AAV 标签显示基础漏泄约减少 100 倍。
However, the reversible control of bifunction from 37 to 30 °C cannot be achieved possibly due to the durable tight control of PhlF. Thus, time-lapse photography was applied to study the dynamic response from 37 to 30 °C right after inoculation in a single-cell level leveraging confocal online monitoring with recombinant cells incubated underneath a layer of solid 1.0% LB agarose (~1.5 mm) (Fig. 2d). Under this situation, the dynamic regulation of sfGFP-ON and mRFP-OFF could be obtained. However, at least 4-h time-lag of sfGFP-ON activation was observed after the entire quenching of mRFP (Supplementary File 1), demonstrating that long hours of degradation and multiple generations of cell growth dilution of PhlF are necessary to generate effective dynamic control of bifunction from 37 to 30 °C.
然而,bifunction(双功能)从 37 到 30 °C 的可逆控制可能无法实现,这可能是由于 PhlF 的持久紧密控制所致。因此,time-lapse photography(延时摄影)被用于研究在单细胞水平上从 37 到 30 °C 的动态响应,利用 confocal online monitoring(共焦在线监测),对接种后的重组细胞进行监测,这些细胞被置于固体 1.0% LB agarose(约 1.5 mm)层下方(Fig. 2d)。在这种情况下,能够获得 sfGFP-ON 和 mRFP-OFF 的动态调控。然而,在 mRFP 的整个 quenching(猝灭)过程完成后,观察到 sfGFP-ON 激活至少有 4 小时的时滞(Supplementary File 1),这表明需要 PhlF 的长时间降解以及多个细胞生长世代的 dilution(细胞生长稀释),以产生从 37 到 30 °C 的 bifunction 有效动态控制。
Tree ring-like patterning by temperature-response grown colonies
通过温度响应生长的菌落形成的树轮状图案(patterning)(temperature-response)
Artificial genetic circuits are powerful tools to explore the fundamental insights of hierarchical patterns of nature. To illustrate the feasibility of T-switch designs, we tested their periodic thermo-response performances of bifunctional control during colony formation by mimicking the formation of natural tree ring under the seasonal variation of temperature and humidity. Colonies of recombinant cells showed spatially distributed double-color ring patterns of mRFP (red) and sfGFP (green). First, recombinant cells harboring the start constructs 155+165 were spread on 10LB agar plate for 72-h growth by changing the temperature periodically, with 10-h growth first at 37 °C then 14-h at 30 °C per day as a cycle (Fig. 3a). The colonies with fluorescence rings were photographed under bright- and dark-field microcopy at the end of each cycle (Fig. 3b). Interestingly, the inner green ring (G1) turned yellow in the next two cycles of incubation due to the integration of green (sfGFP) and red (mRFP) colors. Notably, the sfGFP rings were distinguishable, while the mRFP FI was stepwise enhanced from outer- to inner-region, because the in situ memorial expression of mRFP was periodically activated and stacked once the colony was exposed at 37 °C (see cartoon video in Supplementary File 2). To form the distinct red rings, the OFF-stage of mRFP should be steadily retained whenever the temperature changed from 30 to 37 °C.
人工遗传回路是探索自然分层模式基本洞察的强大工具(artificial genetic circuits)。为了说明 T-switch 设计(T-switch designs)的可行性,我们测试了其在重组细胞菌落形成过程中双功能控制的周期性热响应性能(thermo-response performances),通过模拟季节性温度和湿度变化下的自然年轮形成。重组细胞菌落显示出空间分布的双色环模式,包括 mRFP(红色)和 sfGFP(绿色)。首先,将携带起始构建体 155+165 的重组细胞在 LB 琼脂平板上均匀涂布,进行 72 小时生长,温度周期性变化,每日一个周期,先在 37 °C 下生长 10 小时,然后在 30 °C 下生长 14 小时(图 3a)。每个周期结束时,使用明场和暗场显微镜拍摄具有荧光环的菌落(图 3b)。有趣的是,内层绿色环(G1)在接下来的两个培养周期中因绿色(sfGFP)和红色(mRFP)颜色的整合而变为黄色。值得注意的是,sfGFP 环可被区分,而 mRFP 的荧光强度(FI)从外层向内层逐步增强,因为一旦菌落暴露在 37 °C 下,mRFP 的原位记忆表达(in situ memorial expression)会周期性激活并累积(见补充文件 2 中的卡通视频)。为了形成明显的红色环,mRFP 的关闭阶段(OFF-stage)应在温度从 30 °C 变化到 37 °C 时稳定保持。
图 3: 可调 (Tunable) 形成树轮状菌落。
a Three-day tree ring-like colony formation by thermally controlled expression of sfGFP and mRFP. Specifically, plates spread with recombinant cells from cell cultures at 37 °C were incubated in periodical changing circumstances at 37 °C and 30 °C for 10 h and 14 h, respectively. b The colony growth image was recorded every 24 h to study the forming process of rings in red or green. The band width of outer rings became more and more narrow due to the gradually saturated growth of colony, especially for green rings compared to that of the red ones. c Growth of a single colony harboring various T-switch circuits, including 155+165 from part b, 147+167 and 147+168 groups, by periodically changing temperature between 30 °C and 37 °C. Construct of 168 was derived from 167 by adding AAV degradation tag to reporter mrfp to reduce the memory effects of thermal controlled system. Fluorescence intensity (FI) was measured by imageJ from images and normalized by the maximum value of sfGFP and mRFP measured from every single image, ranging from 0 to 100. d Diameter of tree ring-like colonies increased with longer temperature cycles at 30 °C. e Ring-width of sfGFP- and mRFP-ring were obviously affected by different T-switch designs and temperature cycles from 30 °C to 37 °C. Hollow and solid squares represent the visible and missing formation of the first red ring (R1) generated by constructs 147+167, respectively, namely 147+167-D (2 colonies) and 147+167-S (4 colonies), respectively. Data in d and e are presented as mean ± s.d. of at least 2 collected colonies. p values of d and e are displayed in Supplementary Fig. 12.
a 热控制表达 sfGFP 和 mRFP 导致的三天树轮状菌落形成。具体而言,将从 37 °C 细胞培养物中获得的重组细胞涂布的平板在周期性变化的环境中孵育,温度分别为 37 °C 和 30 °C,时间分别为 10 小时和 14 小时。
b 菌落生长图像每 24 小时记录一次,以研究红色或绿色环的形成过程。由于菌落生长的逐渐饱和,外部环的带宽变得越来越窄,尤其是绿色环相比红色环更为明显。
c 携带各种 T-switch 电路的单个菌落通过在 30 °C 和 37 °C 之间周期性改变温度来生长,包括 b 部分中的 155+165、147+167 和 147+168 组。168 构建体是从 167 构建体衍生而来的,通过向报告基因 mrfp 添加 AAV 降解标签来减少热控系统的记忆效应。荧光强度 (FI) 通过 imageJ 从图像中测量,并使用每个单张图像中 sfGFP 和 mRFP 的最大值进行归一化,范围从 0 到 100。
d 树轮状菌落的直径随着 30 °C 温度周期的延长而增加。
e sfGFP-环和 mRFP-环的环宽明显受不同 T-switch 设计和从 30 °C 到 37 °C 的温度周期影响。空心和实心方块分别表示由 147+167 构建体生成的第一个红色环 (R1) 的可见和缺失形成,分别为 147+167-D (2 个菌落) 和 147+167-S (4 个菌落)。d 和 e 中的数据以至少 2 个收集菌落的平均值 ± 标准差呈现。d 和 e 的 p 值显示在 Supplementary Fig. 12 中。
Therefore, two more T-switch designs, constructs 147+167 and 147+168, were joined to generate rigorous colony ring pattern with stringent control of mRFP and sfGFP by introducing negative feedback control from LacI and degradation tag AAV (Fig. 3c). Normalized FI by ImageJ was used to characterize the expression-level variation of sfGFP and mRFP involved in different color rings. More specifically, the color formation of inner green ring (G1) was significantly improved with reduced integration effect of red color, resulting from fluctuant radical distribution of normalized mRFP FI compared to the stepwise tendency of constructs 155+165. Accordingly, typical peaks of normalized green fluorescence appeared in the valley of the red ones, indicating an optimized staggered formation of red and green rings with distinctive boundaries. Furthermore, characteristics of incubation time at 37 °C (X = 6, 8, and 10 h) of recombinant cells in each cycle were performed to achieve tunable pattern formation (Supplementary Figs. 9–11). Statistically, longer incubation time (X) at 37 °C generally leads to formation of a larger size colony with increased red ring width (R1, R2, and R3) and decreased green ring width (G1, G2, and G3) (Fig. 3d, e and Supplementary Fig. 12), which is possibly relevant to the growth phase of colony formation. The protein expression activity decreased while the colony growth reached the stationary phase in the third cycle incubation, which also led to decreased fluorescent peak values from the inner to outer color rings. Notably, the inner red ring (R1) was drowned in the inner green ring (G1) because of the short-term (6 and 8 h) growth at 37 °C except two of the six collected colonies from constructs 147+168 exhibited recognizable double inner rings of R1 and G1 (hollow square in green in Fig. 3d, termed 147+168-D, 8 h). In conclusion, the tunable formation of tree ring-like colony pattern by engineered E. coli carrying various T-switch designs demonstrated promising thermal control capability of bifunction for further application trial in functional gene expression control.
因此,额外设计了两种 T-switch 设计,即构建体 147+167 和 147+168,通过引入 LacI 的负反馈控制和降解标签 AAV(Fig. 3c),以实现对 mRFP 和 sfGFP 的严格控制,从而生成精确的菌落环状模式。利用 ImageJ 对归一化 FI 进行分析,以表征不同颜色环中 sfGFP 和 mRFP 的表达水平变化。更具体地说,内层绿色环(G1)的颜色形成得到显著改善,减少了红色影响的整合效应,这源于归一化 mRFP FI 的波动性径向分布,与构建体 155+165 的阶梯式趋势相比有所不同。因此,归一化绿色荧光的典型峰值出现在红色荧光的谷值中,表明红绿环以优化交错方式形成,具有清晰边界。此外,还考察了重组细胞在每个循环中 37 °C 下培养时间(X = 6、8 和 10 h)的特性,以实现可调图案形成(Supplementary Figs. 9–11)。统计学上,更长的 37 °C 培养时间(X)通常导致菌落尺寸增大,红色环宽度(R1、R2 和 R3)增加,而绿色环宽度(G1、G2 和 G3)减少(Fig. 3d、e 和 Supplementary Fig. 12),这可能与菌落形成的生长阶段相关。在第三个循环培养中,当菌落生长进入平稳期时,蛋白表达活性降低,这也导致从内层到外层颜色环的荧光峰值递减。值得注意的是,由于 37 °C 下短期生长(6 和 8 h),内层红色环(R1)通常被内层绿色环(G1)掩盖,但从构建体 147+168 的六个收集菌落中,有两个显示出可识别的双层内环(R1 和 G1),如 Fig. 3d 中的绿色空心方块所示(标记为 147+168-D,8 h)。总之,工程化大肠杆菌(E. coli)携带各种 T-switch 设计可调形成树轮状菌落图案,展示了双功能热调控能力,具有进一步应用于功能性基因表达控制的潜力。
Thermal regulation of cell morphology
细胞形态(cell morphology)的热调控(thermal regulation)
Dynamic morphology control over time and levels promote the process of understanding fundamental morphological insights as well as shaping engineered cells in industry38,39,40. Here, we engineered a prototype T-switch (constructs 145+221, termed TM-switch) to modulate the expression of morphology-associated genes instead of reporters, including cell skeleton gene mreB and cell division gene ftsZ, respectively, related to the formation of spherical and fiber shapes (Fig. 4a). First, static test was performed to culture the recombinant cells at 30 °C, 33 °C, and 35 °C to form spherical, rod, and fiber shapes, respectively (Fig. 4b). Particularly, because of the low expression level of T-switch at 33 °C with a green FI value close to 200 in bifunctional control panels (Fig. 1d), cells harboring TM-switch were maintained in normal rod shape compared to the heteromorphic phenotype at 30 and 35 °C. Quantitative cell length measurement of at least 150 cells from confocal images from each group revealed remarkable increasing trend along with the activation of ftsZ and inactivation of mreB gene from 30 to 35 °C (Fig. 4c and Supplementary Files 3–5).
动态形态控制在时间和水平上的应用促进了理解基本形态学洞察(morphological insights)以及塑造工业中工程细胞的过程 38,39,40 。在这里,我们设计了一个原型 T-switch(constructs 145+221,termed TM-switch),用于调节形态相关基因的表达,而不是报告基因,包括细胞骨架基因 mreB 和细胞分裂基因 ftsZ,分别与球状和纤维状形成的相关(Fig. 4a)。首先,进行静态测试,在 30 °C、33 °C 和 35 °C 培养重组细胞,分别形成球状、杆状和纤维状(Fig. 4b)。特别地,由于 T-switch 在 33 °C 时的低表达水平,绿色 FI 值接近 200,在双功能控制面板中(Fig. 1d),携带 TM-switch 的细胞保持正常杆状,与 30 °C 和 35 °C 时的异形表型(heteromorphic phenotype)相比。每个组从共聚焦图像中对至少 150 个细胞进行定量细胞长度测量,显示出从 30 °C 到 35 °C 伴随 ftsZ 激活和 mreB 基因失活而显着的增加趋势(Fig. 4c and Supplementary Files 3–5)。
Fig. 4:热响应细胞形态从杆状到球状或到纤维状的变化。
a Construct for controlling cell morphology. The expression of two morphology related genes, mreB and ftsZ, enabling formations of sphere- and fiber-shape cells, respectively, were controlled by the T-switch system. b Confocal imaging of cells harboring 145+221 constructs cultured at constant temperature, 30 °C, 33 °C and 35 °C, scale bar = 10 μm. c Quantitative measurements of cell lengths by imageJ from at least 9 images from part b containing over 150 captured cells manually. d Bifunctional dynamic control of cell morphology among shapes of spheres (mreB overexpressing), rods (normal cell type) and fibers (ftsZ overexpressing) were on-line recorded in a microfluidics with a scale bar of 2 μm. Sample sizes of collected cells of each time point varied significantly depending on the growth phase, 3–10 cells at 1 h, 10–40 cells at 3 h, and 100–240 cells at 5 h. All data in c and d are displayed in Box-plot: a value of median, quantiles, mini- and maxi-mum. One-way ANOVA with Tukey-Kramer test was used in c and d. p value: N.S. not significant; *p < 0.0332; **p < 0.0021; ***p < 0.0002; and ****p < 0.0001.
a 用于控制细胞形态的构建体。两个与形态相关的基因 mreB 和 ftsZ 的表达,分别使细胞形成球形和纤维状,被 T-switch system 控制。
b 搭载 145+221 构建体的细胞在恒温条件下(30 °C、33 °C 和 35 °C)的共聚焦成像(confocal imaging),比例尺 = 10 μm。
c 使用 imageJ 对来自 b 部分的至少 9 张图像中手动捕获的超过 150 个细胞的细胞长度进行定量测量。
d 细胞形态的双功能动态控制,包括球形(mreB 过表达)、杆状(正常细胞类型)和纤维状(ftsZ 过表达)的形状,在微流控芯片中实时记录,比例尺 = 2 μm。每个时间点的收集细胞样本量根据生长阶段有显著变化,1 小时时 3–10 个细胞,3 小时时 10–40 个细胞,5 小时时 100–240 个细胞。c 和 d 部分的所有数据以箱线图(box-plot)显示:包括中位数、分位数、最小值和最大值。使用了一元 ANOVA 和 Tukey-Kramer test。p 值:N.S. 表示不显著;*p < 0.0332;**p < 0.0021;***p < 0.0002;****p < 0.0001。
To further explore dynamic morphology controls, recombinant cells carrying TM-switch were cultured in a chamber within a microfluidic device for online recording of cell growth with thermo-response that shapes variation under different control strategies (Fig. 4d), including single-step control from 30 to 35 °C (left panel), from 33 to 35 °C (middle panel), and dual-step control from 30 to 33 to 35 °C (right panel, Supplementary File 8). Specifically, for single-step control groups, overnight seed cultures at 30 and 33 °C were injected in chip chambers for 8 h growth at 35 °C in flowing fresh LB medium, while dual-step control group was able to maintain the cell growth at 30 °C for 1.5 h, the same for the seed cultures, before changing the temperature to 33 and 35 °C orderly after 1.5- and 4-h growth, respectively. Video captured at 1, 3, and 5 h of single-step control groups exhibited remarkable cell elongations from spherical and rod stage (Supplementary Files 6 and 7). However, minor elongation of cells was observed at 4 and 6 h captured images with temperature changing from 33 to 35 °C from dual-step control group, probably due to the saturated cell population since longer cells were easier to be observed in neighbor chambers with less cell counts (Supplementary File 8). This study not only illuminates the thermo-response bifunctional control of target genes of functions, but also hints at the practical uses of efficient output in timing control of temperature alteration.
为了进一步探索动态形态控制,重组细胞携带 TM-switch 被培养在微流控装置内的腔室中,用于在线记录具有热响应性细胞生长,该生长在不同控制策略下塑造变异(图 4d),包括从 30 到 35 °C 的单步控制(左面板)、从 33 到 35 °C 的单步控制(中面板),以及从 30 到 33 到 35 °C 的双步控制(右面板,Supplementary File 8)。具体而言,对于单步控制组,过夜种子培养物在 30 °C 和 33 °C 下被注入芯片腔室中,在流动的新鲜 LB 培养基(LB medium)中于 35 °C 下生长 8 小时,而双步控制组能够在 30 °C 下维持细胞生长 1.5 小时,与种子培养物相同,随后在生长 1.5 小时和 4 小时后分别有序地将温度改变至 33 °C 和 35 °C。视频在单步控制组的 1、3 和 5 小时时捕获,展示了从球形和杆状阶段的显著细胞延长(Supplementary Files 6 和 7)。然而,在双步控制组的温度从 33 °C 改变至 35 °C 时,捕获的 4 和 6 小时图像中观察到细胞延长较轻微,这可能是由于细胞种群饱和所致,因为在邻近腔室中细胞计数较少时,更易观察到较长细胞(Supplementary File 8)。本研究不仅阐明了热响应双功能控制目标基因功能(bifunctional control),还暗示了在温度改变定时控制中高效输出的实际应用(thermo-response)。
Ordered assembly of block-copolymer poly(3-hydroxybutyrate)-block-poly(4-hydroxybutyrate)
block-copolymer poly(3-hydroxybutyrate)-block-poly(4-hydroxybutyrate) 的有序组装 (ordered assembly)
Engineering dynamic control circuit of mono- or bifunction to modulate target pathways for enhanced bioproduct accumulation is a commonly used strategy to make effective production by decoupling the cell growth phase and production phase41,42,43. However, the de novo ordered assembly of biopolymers on a molecular scale, which requires exquisite expression control over time and levels with well-organized collaborative response, is still challenging to achieve comparable performance of chemosynthesis polymerization. To our knowledge, PHA is a family of microbially synthesized biodegradable44 biopolymers with tunable thermo-mechanical properties depending on the components, molecular weights, and polymerization manners including homopolymer, random-, and block-copolymer (Supplementary Fig. 14)33. Therefore, in addition to the successes of tree ring-like colony and transformer cell, herein, further efforts were carried out to expand the T-switch into more difficult uses of in vivo ordered polymerization of diblock PHA composed of poly (3-hydroxybutyrate) (PHB) and poly (4-hydroxybutyrate) (P4HB) blocks, namely PHB-b-P4HB (Fig. 5a).
工程动态控制电路的单功能或双功能设计,用于调控目标通路以增强生物产物积累,是一种常用的策略,通过将细胞生长阶段与生产阶段解耦,来实现高效生产 41,42,43 。然而,从头(de novo)有序组装生物聚合物在分子尺度上的过程,需要对表达进行精细的时间和水平控制,并具有良好组织的协同响应,这在实现与化学合成聚合(chemosynthesis polymerization)相当的性能方面仍面临挑战。到我们所知,PHA 是一类由微生物合成的可生物降解(biodegradable)生物聚合物,具有可调的热机械性能(thermo-mechanical properties),这取决于其组分、分子量以及聚合方式,包括均聚物、随机共聚物和嵌段共聚物(Supplementary Fig. 14) 33 。因此,除了树轮状菌落和变形细胞的成功之外,本文在此进一步开展了努力,将 T-switch 扩展到更具挑战性的体内(in vivo)有序聚合应用中,即由聚(3-羟基丁酸酯)(PHB)和聚(4-羟基丁酸酯)(P4HB)嵌段组成的二嵌段 PHA,即 PHB-b-P4HB(Fig. 5a)。
Fig. 5: 通过重组 E. coli 合成嵌段共聚物 (block copolymer) PHB-b-P4HB。
a PHB-b-P4HB synthesis pathway from glucose as a sole carbon source, which is grouped into three modules including syntheses of 3-hydroxybutyrate-CoA synthesis (highlighted in light green, activated at 37 ℃), 4-hydroxubutyrate-CoA (highlighted in light orange, activated at 30 °C) and constitutive expression of PHA synthase PhaC encoded by phaC controlled by porin promoter, respectively. b Debugging the expression sensitivity of 3-hydroxybutyrate-CoA and 3-hydroxybutyrate-CoA synthesis pathways under the control of tac promoter induced by various concentrations of IPTG, respectively. 4HB synthesis pathway has more active performance in a low expression level with saturated P4HB accumulation in the presence of over 2 mg L-1 IPTG. Error bars, mean ± s.d. of three replicates. c Fed-batch fermentative production of PHB-b-P4HB was carried out for 3 times utilizing the same bioprocess under changing temperatures from 30 °C to 37 °C after 18 h growth in a 7 L bioreactor. Upper panel displays the accumulations of block PHB and P4HB in PHB-b-P4HB during the bioprocessing; middle panel shows 4HB molar fractions during the formation of PHB-b-P4HB; bottom panel displays the accumulative tendency of cell dry weights and PHA content of time course. d D-value of PHB-b-P4HB production from Batch-I determined by NMR C13. x-axis: chemical shift (ppm); y-axis: intensity of chemical shift. e Thermodynamics assays of PHB-b-P4HB from three repeated batches including melting temperature (Tm) and glass transfer temperature (Tg), using differential scanning calorimetry (DSC). PHB from Sigma and P4HB from Tepha were used as control for comparative analysis. x-axis: temperature range of cooling-heating cycles; y-axis: relative heat flow rate generated from DSC scanning.
一种从葡萄糖作为唯一碳源合成 PHB-b-P4HB 的途径,该途径分为三个模块,包括 3-羟基丁酸辅酶 A 合成(以浅绿色突出,37℃ 时激活)、4-羟基丁酸辅酶 A 合成(以浅橙色突出,30℃ 时激活)以及由 porin 启动子调控的 PHA 合成酶 PhaC 的组成型表达(由 phaC 编码)。
b 调试 3-羟基丁酸辅酶 A 和 3-羟基丁酸辅酶 A 合成途径的表达敏感性,这些途径分别受 tac 启动子调控,并通过不同浓度的 IPTG 诱导。4HB 合成途径在低表达水平下表现出更高活性,并在 IPTG 浓度超过 2 mg L⁻¹ 时实现 P4HB 饱和积累。误差条表示三重复的平均值 ± 标准差。
c 在 7 L 生物反应器中,进行分批发酵生产 PHB-b-P4HB,过程重复 3 次,在生长 18 小时后将温度从 30℃ 变化至 37℃。上图显示 PHB-b-P4HB 过程中块状 PHB 和 P4HB 的积累;中图显示 PHB-b-P4HB 形成期间的 4HB 摩尔分数;下图显示细胞干重和 PHA 含量的累积趋势随时间变化。
d 通过 NMR 测定 Batch-I 的 PHB-b-P4HB 生产 D-值。x 轴:化学位移 (ppm);y 轴:化学位移强度。
e 对三个重复批次的 PHB-b-P4HB 进行热力学分析,包括熔点 (T_m) 和玻璃化转变温度 (T_g),使用差示扫描量热法 (DSC)。Sigma 公司的 PHB 和 Tepha 公司的 P4HB 用作对照进行比较分析。x 轴:冷却-加热循环的温度范围;y 轴:DSC 扫描产生的相对热流率。
Before the circuit construction, two major tests including cell growth characterization in various scales and transcription-response sensitivity of target pathways were implemented to determine the operational time range for temperature alteration and pathway control strategy, respectively, with the “Begin with the end in mind” rational design (Supplementary Figs. 14 and 15). Notably, two heterogenous pathways, including phaA-phaB encoding β-ketothiolase (PhaA) and NADH-dependent acetoacetyl-CoA reductase (PhaB)36, and orfZ-sucD-4hbd encoding CoA transferase (OrfZ), succinate semi-aldehyde dehydrogenase (SucD), and 4-hydroxybutyrate dehydrogenase (4HBD)45, involved in the synthesis of 3HB-CoA and 4HB-CoA, the polymerization precursors for PHB-b-P4HB mediated by PHA synthase encoded by phaC, were constructed in the start host E. coli JM109SGL independently under the control of tac promoter. These constructs allow us to study their transcriptional levels for both PHB and P4HB synthesis induced by the same dosage of IPTG (Fig. 5b). Remarkably, the constitutive co-expression of PHB synthesis cluster from Cupriavidus necator (namely Ralstonia eutropha H16) was employed to reduce the toxicity of 4HB-CoA synthesis pathway for normal cell growth46. Results showed that the 4HB-CoA synthesis pathway exhibited lower expression-level limit with saturated activity in 4HB-CoA synthesis in the presence of 2 mg L−1 IPTG compared to the one of 3HB-CoA synthesis (Fig. 5b and Supplementary Fig. 15c, d).
在电路构建之前,进行了两项主要测试,包括在不同规模下的细胞生长表征(cell growth characterization)和目标途径的转录响应敏感性(transcription-response sensitivity),以分别确定温度变化的操作时间范围和途径控制策略,这些测试基于“Begin with the end in mind”的理性设计(rational design)(补充图 14 和 15)。值得注意的是,构建了两个异源途径,包括编码β-酮硫解酶(PhaA)和 NADH 依赖的乙酰乙酰辅酶 A 还原酶(PhaB)的 phaA-phaB 途径 36 ,以及编码 CoA 转移酶(OrfZ)、琥珀酸半醛脱氢酶(SucD)和 4-羟基丁酸脱氢酶(4HBD)的 orfZ-sucD-4hbd 途径 45 ,这些途径参与 3HB-CoA 和 4HB-CoA 的合成,后者是 PHB-b-P4HB 的聚合前体,由 phaC 编码的 PHA 合成酶介导。这些构建体在起始宿主大肠杆菌 E. coli JM109SGL 中独立构建,并在 tac 启动子的控制下。通过这些构建体,我们可以研究在相同剂量的 IPTG 诱导下,PHB 和 P4HB 合成的转录水平(Fig. 5b)。值得一提的是,采用了从耐盐铜菌 Cupriavidus necator(即 Ralstonia eutropha H16)来源的 PHB 合成簇的组成型共表达(constitutive co-expression),以降低 4HB-CoA 合成途径的毒性,从而维持正常细胞生长 46 。结果显示,在 2 mg/L IPTG −1 的存在下,4HB-CoA 合成途径表现出较低的表达水平阈值,并具有 4HB-CoA 合成的饱和活性,与 3HB-CoA 合成途径相比(Fig. 5b 和补充图 15c、d)。
Based on these pre-debugging knowledge, the control strategy for PHB-b-P4HB synthesis was developed using constructs 169+170 carrying 4HB-CoA synthesis cluster controlled by PPhlF with tight-control leakage (activated at 30 °C) and 3HB-CoA synthesis cluster controlled by temperature-sensing panel with acceptable leakage (activated at 37 °C), while the phaC gene was constitutively expressed using porin promoter for continuous polymerization activity (Fig. 5a and Supplementary Fig. 16a). In addition, two degradation tags, LVA and AAV, were added to the C-terminal of PhaA and PhaB, respectively, to reduce the leakage under PR promoter. Subsequently, a pre-culture test was conducted in 1-L quadruple fermentors (Infors, Switzerland) by alternating temperature from 30 to 37 °C at 9, 12, 15, and 18 h, respectively, during 32 h of cultivation to identify the optimal time moment for PHB-b-P4HB synthesis (Supplementary Fig. 16b, c).
基于这些预调试知识(pre-debugging knowledge),开发了 PHB-b-P4HB 合成的控制策略(control strategy),采用构建体 169+170,该构建体携带由 P PhlF 控制的 4HB-CoA 合成簇,具有紧密控制泄漏(activated at 30 °C),以及由温度感应面板控制的 3HB-CoA 合成簇,具有可接受泄漏(activated at 37 °C),同时 phaC 基因使用孔蛋白启动子进行本构表达(constitutively expressed),以实现连续聚合活性(Fig. 5a 和 Supplementary Fig. 16a)。此外,在 PhaA 和 PhaB 的 C 端分别添加了两个降解标签 LVA 和 AAV(degradation tags),以减少 P R 启动子下的泄漏。随后,在 1-L 四联发酵罐(Infors, Switzerland)中进行预培养测试(pre-culture test),通过在 32 小时培养过程中分别在 9、12、15 和 18 小时时将温度从 30 °C 切换到 37 °C,以确定 PHB-b-P4HB 合成的最佳时间点(Supplementary Fig. 16b, c)。
Generally, a diblock copolymer should have a D-value greater than 10 calculated from C13 NMR spectrum. Meanwhile, two characteristic peaks of melting points (Tm) resulted from the diblock regions can be detected by differential scanning calorimetry (DSC) profiling47. Interestingly, prolonging the cultural time at 30 °C could significantly increase the 4HB molar fraction and D-value of PHB-b-P4HB block copolymer but with noticeable decline of cell mass and product titer because of the long-term activation of toxic 4HB-CoA synthesis pathway (Supplementary Figs. 16 and 17). Among these four fed-batch fermentations, the temperature alteration time at 18 h from 30 to 37 °C displayed dominant diblock features, having a D-value over 23 (Supplementary Fig. 17d), and two distinguished Tm peaks in DSC profiling, which represented the typical melting points (Tm) of homopolymer of PHB and P4HB in the diblock copolymer (Supplementary Fig. 18).
一般来说,一个双嵌段共聚物(diblock copolymer)应该具有大于 10 的 D 值(D-value),该 D 值是从 C 13 NMR 谱计算得到的。同时,来自双嵌段区域的两个熔点特征峰(T m )可以通过差示扫描量热法(differential scanning calorimetry)分析(DSC profiling)检测到。有趣的是,延长 30°C 下的培养时间可以显著增加 PHB-b-P4HB 嵌段共聚物的 4HB 摩尔分数(molar fraction)和 D 值,但由于有毒的 4HB-CoA 合成途径的长期激活,细胞质量和产物滴度会明显下降(补充图 16 和 17)。在这些四次分批发酵(fed-batch fermentations)中,将温度从 30°C 改为 37°C 的时间点设定在 18 小时时,显示出明显的双嵌段特征,具有大于 23 的 D 值(补充图 17d),并且在 DSC 分析中出现两个明显的 T m 峰,这些峰代表了双嵌段共聚物中 PHB 和 P4HB 均聚物的典型熔点(T m )(补充图 18)。
Subsequently, three independent trials of fed-batch cultivations were conducted in a 7-L bioreactor (Thermal, USA) to obtain significant amount of PHB-b-P4HB containing 69.7% (Batch-I), 64.5% (Batch-II), and 50.9% (Batch-III) molar fraction of P4HB with D-values larger or close to 10 (Fig. 5c, d and Supplementary Fig. 19). Based on the time course recording of PHB and P4HB contents, the switching performance of ON- and OFF-control of PHB and P4HB synthesis were tightly and smoothly manipulated by changing temperatures from 30 to 37 °C at 18 h of growth (dash line) with undetected leakage of PHB accumulation (green dots) before 18 h, and effective termination of P4HB synthesis (orange dots) after 18 h compared to the gradually climbing of cell dry weight and PHB-b-P4HB titer (Fig. 5c).
随后,在一个 7 升生物反应器(Thermal, USA)中进行了三项独立的 fed-batch cultivations(fed-batch cultivations)试验,以获得大量含有 P4HB 摩尔分数(molar fraction)为 69.7%(Batch-I)、64.5%(Batch-II)和 50.9%(Batch-III)的 PHB-b-P4HB,其 D-values 大于或接近 10(图 5c、d 和补充图 19)。
基于 PHB 和 P4HB 含量的时程记录,PHB 和 P4HB 合成的 ON- and OFF-control(ON- and OFF-control)切换性能通过在生长 18 小时时(虚线)将温度从 30°C 改为 37°C 被紧密且平滑地操控,在 18 小时前 PHB 积累(绿点)无检测到泄漏,在 18 小时后 P4HB 合成(橙点)有效终止,与细胞干重(cell dry weight)和 PHB-b-P4HB 滴度(titer)的逐渐上升相比(图 5c)。
DSC profiling of three-batch PHB-b-P4HB (dash line) also showed two characteristic Tm peaks compared to PHB (solid line in yellow) and P4HB (solid line in black) (Fig. 5e), of which PHB-b-P4HB from Batch-I exhibited the best performance with dominant Tm peaks and D-value close to 24 (Fig. 5d). Interestingly, by leveraging the leakage nature of temperature-sensing panel and ultra-sensitivity of 4HB-CoA synthesis cluster expression, further attempts of exchanging the control panels of 3HB-CoA and 4HB-CoA synthesis pathways (constructs 149+150) resulted in synthesis of random copolymers P(3HB-co-4HB) with increased 4HB molar fraction from 30 to 89% when maintaining the culture temperature at 30, 31, and 32 °C in a 7-L bioreactor throughout the 32-h fermentation bioprocess (Supplementary Fig. 20). In contrast to the PHB-b-P4HB fermentation process, the synergetic accumulation pattern of PHB and P4HB represents the simultaneous polymerization of 3HB and 4HB monomers involved in random P(3HB-co-4HB) synthesis, leading to D-values close to 1 (Supplementary Fig. 21), and single or undetected Tm peaks in DSC profiling (Supplementary Fig. 22). Otherwise, the mechanical properties of PHB-b-69.7% P4HB (Batch-I in Fig. 5c) displayed remarkable improvements of Young’s modulus (23-fold) and tensile strength (2.3-fold) compared to the copolymer formed with a similar 4HB molar fraction of P(3HB-co-64%4HB) (batch at 31 °C in Supplementary Fig. 20b) (Supplementary Table 3).
对三批 PHB-b-P4HB 的 DSC 分析(虚线)也显示了两个特征性的 T m 峰,与 PHB(黄色实线)和 P4HB(黑色实线)相比(Fig. 5e),其中 Batch-I 的 PHB-b-P4HB 表现最佳,具有主导的 T m 峰和 D 值(D-value)接近 24(Fig. 5d)。有趣的是,通过利用温度感应面板的泄漏特性以及 4HB-CoA 合成簇表达的超高敏感性,进一步尝试交换 3HB-CoA 和 4HB-CoA 合成途径的控制面板(constructs 149+150),结果在 7 L 生物反应器(bioreactor)中保持培养温度在 30、31 和 32 °C,整个 32 小时发酵(fermentation)生物过程,合成了随机共聚物 P(3HB-co-4HB),4HB 摩尔分数从 30% 增加到 89%(Supplementary Fig. 20)。与 PHB-b-P4HB 发酵过程相反,PHB 和 P4HB 的协同积累模式代表了随机 P(3HB-co-4HB) 合成的 3HB 和 4HB 单体的同时聚合,导致 D 值接近 1(Supplementary Fig. 21),以及 DSC 分析中单一或未检测到的 T m 峰(Supplementary Fig. 22)。否则,PHB-b-69.7% P4HB 的机械性能(Fig. 5c 中的 Batch-I)显示了杨氏模量(Young’s modulus)(23 倍)和拉伸强度(tensile strength)(2.3 倍)的显著改善,与具有类似 4HB 摩尔分数的 P(3HB-co-64%4HB) 共聚物相比(Supplementary Fig. 20b 中的 31 °C 批次)(Supplementary Table 3)。
Discussion 讨论 (Discussion)
Generally, a thermal switch has its advantages for scale-up microbial fermentations due to the uniform dispersity and easy operability of temperature control with negligible bias based on the proven industrial heat- and mass-transfer control. Therefore, robust temperature-simulated gene expression could be achieved even in a post-log-phase of cell growth (Supplementary Figs. 6 and 13), which suggests that thermosensitive tools are available in different cultural scales with strong response performances. Meanwhile, temperature-induced bidirectional control for diblock copolymer synthesis were successfully conducted in fed-batch fermentations in a 1 and 7-L bioreactor, respectively, of which the OD600 reached close to 50 for temperature alteration (Supplementary File 10), making the strong case for demonstrating the potential uses of thermal switch in the high cell density growth for larger scale fermentation processes. In addition, the removal of an input signal mediated by the temperature alteration confers the reversable and even periodical control of target genes manually for different purposes, especially for bio-inspired patterning, such as tree ring-like colonies obtained in this study. However, the cells harboring construct 155+165 grown in colony and liquid culture, especially for chemo-state culture with strong and sustainable dilution effects of cell division, exhibit totally different activities, because the recombinant cells grown on plate are spatially immobilized without free division activity but still can sense and response to the signal of temperature changes. For example, cells in the earlier rings formed at 30 °C are able to express mRFP in the later growth at 37 °C (Supplementary File 2), which requires careful design for problem-based complementation, such as construct 147+168 with the introduction of a negative feedback control. As a result, the enlarged transition regions of the green and red rings were observed after the introduction of two repression systems PhlF-PhlO and LacI-LacO (construct 147+168), since the recovered expression of related reporter highly depends on the radial dilution effect of cell division, forming a non-color gap between two neighbor rings. Generally, the compatibility of artificially designed T-switch in recombinant cell systems could be further improved by substituting the TetR-family repressor, PhlF, to avoid the non-objected repression of endogenous gene expression (Supplementary Fig. 5c). Otherwise, engineering thermal-associated regulators to change the operational range of temperature are a promising approach to achieve multiple-functional control29, including various growth rates, morphology, and products. Overall, our T-switch circuits offer an easily established and high-performing solution for bidirectional dynamic regulation of gene expression based on currently used fermentation systems.
一般来说,热开关在扩大规模的微生物发酵中具有优势,因为温度控制具有均匀分散性、易操作性和可忽略偏差的特性,这基于已证实的工业热量和质量传递控制。因此,即使在细胞生长对数后期阶段(Supplementary Figs. 6 and 13),也可以实现稳健的温度模拟基因表达(gene expression),这表明热敏工具可在不同培养规模下使用,并具有强烈的响应性能。同时,在 1 升和 7 升生物反应器(bioreactor)中,分别成功进行了温度诱导的双向控制以合成双嵌段共聚物,该过程采用 fed-batch 发酵方式,其中 OD 600 在温度变化时接近 50(Supplementary File 10),这有力地证明了热开关在高细胞密度生长中的潜在应用,以支持更大规模的发酵过程。此外,通过温度变化介导的输入信号去除,可以实现目标基因的可逆甚至周期性手动控制,用于不同目的,特别是生物启发模式化,例如本研究中获得的树轮状菌落。然而,携带构型 155+165 的细胞在菌落和液体培养中生长,尤其是在具有强烈和持续细胞分裂稀释效应的化学状态培养中,表现出完全不同的活性,因为平板上生长的重组细胞被空间固定,没有自由分裂活性,但仍能感知和响应温度变化的信号。例如,在 30 °C 形成的早期环中的细胞能够在后续 37 °C 生长中表达 mRFP(Supplementary File 2),这需要仔细设计基于问题的互补策略,例如引入负反馈控制的构型 147+168。因此,在引入两个抑制系统 PhlF-PhlO 和 LacI-LacO(构型 147+168)后,观察到绿色和红色环的过渡区域扩大,因为相关报告基因的恢复表达高度依赖于细胞分裂的径向稀释效应,从而在相邻环之间形成无色间隙。一般来说,人工设计的 T-switch 在重组细胞系统中的兼容性可以通过替换 TetR 家族抑制因子 PhlF 来进一步改进,以避免对内源基因表达的非目标抑制(Supplementary Fig. 5c)。否则,工程热相关调节因子以改变温度操作范围是一种有前景的方法,可实现多功能控制 29 ,包括各种生长速率、形态和产物。总体上,我们的 T-switch 电路基于当前使用的发酵系统,提供了一种易于建立且高性能的解决方案,用于双向动态调控基因表达。
The in vivo ordered assembly of biomacromolecules48,49,50,51 or nano complex, such as block-like amyloid fibers27, is an interesting but challenged topic in synthetic biology, which required stringent and collocative control of well-characterized target gene sets. It was reported that the block co-polyesters, including PHA52, PLGA53, and other kinds of block copolymer54, fabricated in chemosynthesis exhibit enhanced performances for material processing and medical uses. Besides, more attempts of various tailor-made biopolymer fabrications have been studied for high value-added applications55,56,57. Here, we used T-switch to dynamically manipulate the expression of two independent pathways for 3HB-CoA and 4HB-CoA synthesis and achieved the de novo synthesis of diblock copolymer PHB-b-P4HB with remarkable improvements on mechanical properties. This study opens a possibility of fabrication of diverse block co-polyesters or nano complex using the sustainable and renewable microbial cell factories that rival the chemical catalysis processes. However, it is important to note that the poor cell growth probably due to the intrinsic toxicity of 4HB-CoA synthesis pathway could be improved using static optimization approach, which has been previously proven in Halomonas spp.36.
活体内的生物大分子或纳米复合物(如块状淀粉样纤维)的有序组装(in vivo),是合成生物学(synthetic biology)中一个有趣但富有挑战性的主题,这需要对已表征的目标基因组进行严格且位置精确的控制。据报道,通过化学合成(chemosynthesis)制备的块状共聚酯,包括 PHA、PLGA 和其他种类的块状共聚物,显示出在材料加工和医疗用途方面的增强性能。此外,各种定制生物聚合物的制备尝试已被研究,用于高附加值应用。在此,我们使用 T-switch 动态操纵 3HB-CoA 和 4HB-CoA 合成的两个独立途径的表达,并实现了二嵌段共聚物 PHB-b-P4HB 的从头合成(de novo synthesis),机械性能有显著改善。本研究开辟了使用可持续和可再生的微生物细胞工厂(microbial cell factories)制造各种块状共聚酯或纳米复合物的可能性,这些工厂可与化学催化过程相媲美。然而,需要注意的是,细胞生长不良可能由于 4HB-CoA 合成途径的内在毒性,可以通过静态优化方法(static optimization approach)改善,这种方法先前已在 Halomonas spp. 中被证明。
In summary, the thermal switch based on temperature-dependent regulatory proteins enables a powerful, applicable and scalable strategy to modulate the expression of gene sets for dynamic control of cellular metabolism11,12. This study has demonstrated that a thermal switch of bifunction, termed T-switch, holds promise in cell morphology control, well-organized colony pattern, and in vivo ordered assembly of biomacromolecules in a chemosynthesis-like manner combined with metabolic engineering. These successes put programmable gene circuits into desirable and diverse functions efficiently triggered by temperature as a single input, as well as define a rational pipeline for the construction and utilization of gene circuits in living cells with well-prepared debugging measures using “Begin with the end in mind” design.
总之,基于温度依赖调控蛋白的热开关(thermal switch)启用了一种强大、可适用且可扩展的策略,来调节基因组的表达(gene sets),以实现细胞代谢的动态控制(cellular metabolism) 11,12 。本研究证明了双功能热开关(bifunction),称为 T-switch,在细胞形态控制、组织良好的菌落模式,以及在体内有序组装生物大分子(biomacromolecules)的方式类似于化学合成(chemosynthesis),并结合代谢工程,具有广阔的应用前景。这些成功使可编程基因回路(programmable gene circuits)实现多样化的理想功能,能够通过温度作为单一输入高效触发,同时定义了一个合理的流程,用于在活细胞中构建和利用基因回路,采用“Begin with the end in mind”设计以及完善的调试措施。
Methods 方法 (Methods)
Strains, plasmids, and media
菌株、质粒和培养基
The chemical competent cells of E. coli DH5α (Biomed, China) were used for plasmids construction in this study. And E. coli JM109SGL, namely E. coli JM109SGL derived from E. coli JM109SG by deleting lacI gene (see Supplementary Methods and Supplementary Fig. 1), was used as a start host for various assays, including circuit characterization by FACS, single-cell study on microfluids devices and agar plates, and PHA copolymer production, respectively, unless specifically noted. In particular, E. coli JM109SG was previously constructed by Li et al. by knocking out sad and gabD genes to block the effluxes of succinyl semi-aldehyde involved in P4HB synthesis from glucose only, and thus enhancing the molar fraction of 4HB component in P(3HB-co-4HB) production36. All plasmids were constructed by Gibson Assembly toolkits (NEB, USA) based on two expression vectors containing p15A origin of replication and Cm resistance, named p15a vector, and pSC101 origin of replication and kanamycin (Kan) resistance, named pSB4K5 vector, respectively. Designs and detailed information of these two mother-plasmids and their derivates are listed in Supplementary Table 1 and Supplementary File 9, respectively. Genetic design and sequence reading were performed by SnapGene v3.2.1. Primers used in this study are listed in Supplementary File 9. Target clones with correct sequences by Sanger sequencing (Ruibiotech, China) were extracted (plasmid mini-prep kit, Tiangen, China) and electro-transferred into recipient strain, E. coli JM109SGL for various assays.
本研究中使用 E. coli DH5α (Biomed, China) 的化学感受态细胞进行质粒构建。而且 E. coli JM109SGL,即通过删除 lacI 基因从 E. coli JM109SG 衍生而来的 E. coli JM109SGL(见补充方法和补充图 1),被用作各种测定的起始宿主,包括通过 FACS 进行的电路表征、微流控设备和琼脂平板上的单细胞研究,以及 PHA 共聚物生产,除非特别注明。特别地,E. coli JM109SG 之前由 Li 等人通过敲除 sad 和 gabD 基因来阻断仅从葡萄糖合成 P4HB 过程中涉及的琥珀酰半醛的外流(effluxes),从而增强 P(3HB-co-4HB) 生产中 4HB 成分的摩尔分数(molar fraction) 36 。所有质粒均使用 Gibson Assembly 工具包(NEB, USA)基于两种表达载体构建,这两种载体包含 p15A 复制起点和氯霉素(Cm)抗性,命名为 p15a 载体,以及 pSC101 复制起点和卡那霉素(Kan)抗性,命名为 pSB4K5 载体。两种母质粒及其衍生物的设计和详细信息的列表分别见补充表 1 和补充文件 9。遗传设计和序列读取由 SnapGene v3.2.1 软件进行。本研究中使用的引物列表见补充文件 9。通过 Sanger 测序(Ruibiotech, China)确认序列正确的目标克隆被提取(质粒微量提取试剂盒,Tiangen, China),并电转化入受体菌株 E. coli JM109SGL,用于各种测定。
A LB composed of 5 g L−1 yeast extract, 10 g L−1 tryptone, and 10 g L−1 NaCl was used for cell cultivation with antibiotics whatever necessary unless specifically noted. Electro-transferred cells were spread and grown on LB agar plate (LB media supplemented with 20 g L−1 agar) with relevant antibiotics for obtaining positive colonies. Stock solution of 50 mg mL−1 kanamycin (Kan) and 25 mg mL−1 chloramphenicol (Cm) was prepared for uses throughout the cultivation processes of recombinant E. coli JM109SGL.
一种由 5 g/L yeast extract、10 g/L tryptone 和 10 g/L NaCl 组成的 LB 被用于细胞培养(cell cultivation),并根据需要添加抗生素(antibiotics),除非特别注明。电转化细胞被涂布并在 LB 琼脂平板(LB 培养基中添加 20 g/L agar)上生长,并添加相关抗生素,以获得阳性菌落(positive colonies)。50 mg/mL kanamycin (Kan) 和 25 mg/mL chloramphenicol (Cm) 的储备溶液被制备,用于重组大肠杆菌 E. coli JM109SGL 的整个培养过程。
Plasmids transformation 质粒转化 (Plasmids transformation)
For circuit characterization, plasmids of interest were electro-transformed into competent cells of E. coli JM109SGL. For electroporation, cells were first made electrocompetent by concentrating 100-folds and washing twice with ice-cold 10% glycerol and stored in −80 °C freezer before uses. Then, 50 μL of competent cells were mixed with 30–50 ng of the PCR products, and then electroporated at 1.8 kV with around 50 mA in an ice-cold 0.1 cm cuvette (Bio-Rad, USA), followed by the addition of 1 mL LB medium. After incubation at 37 °C for 1 h, cells were spread on agar plates with relevant antibiotics and grown for 12 h for colony selection via PCR analysis and sequencing. The positive colonies were selected for further study. For plasmid constructions, chemical competent E. coli DH5α were used to screen positive constructs of PCR assembly products. Five microliters of PCR products were mixed with 50 μL of competent cells after 30 min on ice. Followed by 45 s heat shock, cells were placed on ice for 2 min, then mixed with 0.95 mL fresh LB medium for 1 h incubation at 37 °C. Finally, 50 μL of incubated cells were spread on LB agar plate with relevant antibiotics and grown for 12 h for colony selections. All of the competent cells were stored at −80 °C before uses.
为了表征电路,感兴趣的质粒被电转化进大肠杆菌 JM109SGL 的感受态细胞(competent cells)。对于电穿孔(electroporation),细胞首先通过 100 倍浓缩并用冰冷的 10% 甘油洗涤两次来制备成电感受态,并储存在-80 °C 冰箱中备用。然后,将 50 μL 感受态细胞与 30–50 ng 的 PCR 产物混合,随后在冰冷的 0.1 cm 比色皿(Bio-Rad, USA)中以 1.8 kV 和约 50 mA 的条件进行电穿孔,之后加入 1 mL LB 培养基。在 37 °C 下孵育 1 h 后,将细胞涂布在含有相关抗生素的琼脂平板上,培养 12 h,通过 PCR 分析和测序进行菌落筛选。筛选出阳性菌落用于进一步研究。对于质粒构建,使用化学感受态大肠杆菌 DH5α 来筛选 PCR 组装产物的阳性构建物。将 5 μL PCR 产物与 50 μL 感受态细胞在冰上放置 30 min 后混合。随后进行 45 s 热激处理,将细胞置于冰上 2 min,然后与 0.95 mL 新鲜 LB 培养基混合,在 37 °C 下孵育 1 h。最后,将 50 μL 孵育后的细胞涂布在含有相关抗生素的 LB 琼脂平板上,培养 12 h 进行菌落筛选。所有感受态细胞在使用前储存在-80 °C。
Temperature-response function characterization
温度响应函数表征
To characterize the performance of various designs of T-switch (Supplementary File 9), all measurements of fluorescence intensity were taken by cytometer of cells in the beginning of stationary phase cultured in 96-deep-well plates. Glycerol stocks of the start host, E. coli JM109SGL, containing target plasmids were streaked and activated on LB agar plates for 12-h incubation at 37 °C. Single colonies were inoculated into 1 mL LB medium, followed by 12 h pre-culture in 2 mL well in 96-deep-well plates (NEST, China) sealed with an air permeable film (Axygen, USA) at 1000 rpm at different temperatures (30, 31, 32, 33, 34, 35, 36, and 37 °C), respectively (Thermal Shaker, AOSHENG, China). Then, pre-cultures were 200-fold diluted into 1 mL fresh LB medium for 12-h growth under the same conditions. After growth, cell cultures were 100-fold diluted into 250 μL PBS supplemented with 2 mg mL−1 kanamycin to terminate the expression of proteins. Then, 0.1 vol% of 50 mg mL−1 Kan and 25 mg mL−1 Cm stock solution were added into the cultures for stabilizing plasmids of interest throughout the cultivation processes.
为了表征 T-switch 各种设计的性能(Supplementary File 9),所有荧光强度(fluorescence intensity)测量均在细胞进入静止期(stationary phase)初期时,使用细胞仪对培养在 96 深孔板中的细胞进行。甘油菌株(glycerol stocks)来源为起始宿主 E. coli JM109SGL,含有目标质粒(plasmids),被划线接种并活化于 LB agar 板上,在 37 °C 下孵育 12 小时。单克隆被接种到 1 mL LB 培养基中,随后在 96 深孔板(NEST, China)的 2 mL 孔中进行 12 小时预培养,使用透气膜(Axygen, USA)密封,在不同温度(30、31、32、33、34、35、36 和 37 °C)下以 1000 rpm 摇动(Thermal Shaker, AOSHENG, China)。然后,将预培养物 200 倍稀释到 1 mL 新鲜 LB 培养基中,在相同条件下生长 12 小时。生长后,将细胞培养物 100 倍稀释到 250 μL 补充有 2 mg/mL 卡那霉素(kanamycin)的 PBS 中,以终止蛋白表达。随后,加入 0.1 vol% 的 50 mg/mL Kan 和 25 mg/mL Cm 储备液,以在整个培养过程中稳定目标质粒。
On/off response during time course of cell growth (30–37 °C)
在细胞生长时间过程中的开关响应(on/off response)(30–37 °C)
To study the time course responses of T-switch designs, including the combinations of constructs 155+165, 147+167, and 163+166, recombinant E. coli JM109SGL harboring plasmids of interest were grown overnight on LB agar plates at 30 °C. After growth, single colonies were inoculated into 1 mL LB medium in 96-deep-well plates sealed with an air permeable film and grown for 12 h at 1000 rpm at 30 °C in a Thermal Shaker. Then, 5 μL of each culture was inoculated into 1 mL fresh LB medium for 12 h cultivation under the same conditions. Followed by 200-fold dilution in 1 mL fresh LB medium, cells were grown for 12 h at 1000 rpm at 37 °C. During the growth, 2–10 μL of each culture was sampled in every 1 h and mixed with 250 μL of PBS supplemented with 2 mg mL−1 Kan. Then, 0.1 vol% of Kan and Cm stock solution were added into the medium for stabilizing plasmids of interest throughout the cultivation processes. Fluorescence intensities of sfGFP and mRFP were measured by FACS.
为了研究 T-开关设计 (T-switch designs) 的时间过程响应,包括构建体 (constructs) 155+165、147+167 和 163+166 的组合,携带感兴趣质粒 (plasmids) 的重组大肠杆菌 E. coli JM109SGL 在 30 °C 的 LB 琼脂平板上过夜培养。培养后,单个菌落被接种到 96 深孔板中 1 mL LB 培养基内,使用透气膜密封,并在 30 °C、1000 rpm 的条件下在热摇床上培养 12 小时。然后,取每个培养物的 5 μL 接种到 1 mL 新鲜 LB 培养基中,在相同条件下培养 12 小时。随后,在 1 mL 新鲜 LB 培养基中进行 200 倍稀释,细胞在 37 °C、1000 rpm 的条件下培养 12 小时。培养过程中,每隔 1 小时取 2–10 μL 的每个培养物,混入 250 μL 补充有 2 mg/mL Kan 的 PBS 中。然后,向培养基中加入 0.1 vol% 的 Kan 和 Cm 储备溶液,以在整个培养过程中稳定感兴趣的质粒。sfGFP 和 mRFP 的荧光强度 (fluorescence intensities) 通过 FACS 测量。
On/off response of dynamic control during different growth phases (30–37 °C)
动态控制 (dynamic control) 的开关响应在不同生长阶段期间(30–37℃)
The on/off performances of T-switch circuits in different growth phases were characterized by cytometer analysis. Single colonies of E. coli JM109SGL harboring plasmids of interest were grown on LB agar plates from glycerol stocks, and then inoculated into 1 mL LB medium for 12 h cultivation (1000 rpm, 30 °C, Thermal Shaker). After growth, 5 μL of each culture was transferred into 1 mL fresh LB media as inoculums. After 12-h growth under the same conditions, 5 μL cultures were diluted into 1 mL fresh LB medium and grown at 30 °C at 1000 rpm for 0, 2, 4, 6, 8, 10, and 12 h, respectively, then cultures were transferred to new shaker at 37 °C at 1000 rpm for a 12-h cultivation. Finally, cell cultures were 100-fold diluted into 250 μL PBS supplemented with 2 mg mL−1 Kan. Then, 0.1 vol% of Kan, and Cm stock solution were added into the medium for stabilizing plasmids of interest throughout the cultural processes. Fluorescence intensities of sfGFP and mRFP were measured by FACS.
不同生长阶段的 T-switch 电路的开/关性能通过流式细胞仪分析(cytometer analysis)进行了表征。单个 E. coli JM109SGL 菌落携带着感兴趣的质粒(plasmids),从甘油库存中在 LB 琼脂平板上生长,然后接种到 1 mL LB 培养基中进行 12 小时培养(1000 rpm,30 °C,Thermal Shaker)。生长后,将每个培养物的 5 μL 转移到 1 mL 新鲜 LB 培养基中作为接种物。在相同条件下生长 12 小时后,将 5 μL 培养物稀释到 1 mL 新鲜 LB 培养基中,并在 30 °C 下以 1000 rpm 生长 0、2、4、6、8、10 和 12 小时,分别,然后将培养物转移到新的摇床上,在 37 °C 下以 1000 rpm 培养 12 小时。最后,将细胞培养物 100 倍稀释到补充有 2 mg/mL Kan 的 250 μL PBS 中。然后,向培养基中添加 0.1 vol% 的 Kan 和 Cm 储备溶液,以在整个培养过程中稳定感兴趣的质粒。sfGFP(sfGFP)和 mRFP(mRFP)的荧光强度通过 FACS(FACS)测量。
Single-cell online recording on thermal responsive E. coli
单细胞(single-cell)在线记录热响应性(thermal responsive)E. coli
To online record the thermally responsive performances of engineered E. coli harboring T-switch of constructs 155+165, recombinant cells were incubated underneath a layer of solid 1.0% LB agarose (~1.5 mm) containing relevant antibiotics by placing 1 µL overnight incubated cell culture between a glass coverslip-bottomed 35 mm Petri dish with a glass diameter of 20 mm (Cellvis, USA). Cells were imaged on a Nikon A1RSi laser scanning confocal microscope equipped with a 100× (NA 1.40) oil-immersion lens. Images were obtained every 10 min and processed by NIS elements v4.60 in the end. Cells were maintained at required temperature during imaging with an active-control environmental chamber.
为了在线记录搭载 T-switch 的构建体 155+165 的工程化大肠杆菌(E. coli)的热响应性能(thermally responsive performances),重组细胞(recombinant cells)被置于含有相关抗生素的固体 1.0% LB agarose(约 1.5 mm)层下方,通过将 1 µL 过夜培养的细胞培养物置于玻璃盖玻片底的 35 mm 培养皿之间,该培养皿玻璃直径为 20 mm(Cellvis, USA)。细胞在配备 100× (NA 1.40) 油浸物镜(oil-immersion lens)的 Nikon A1RSi 激光扫描共聚焦显微镜(confocal microscope)上进行成像。图像每 10 分钟获取一次,并最终由 NIS elements v4.60 处理。在成像过程中,细胞通过主动控制环境控制室(environmental chamber)保持在所需温度。
Cytometry analysis Cytometry 分析
The cytometry analysis was carried out by a BD LSR Fortessa flow cytometer with HTS attachment (BD, USA). Fluorescence positive cells were captured under the excitation spectrum of 488 nm (FITC channel, 440 V, sfGFP) and 584 nm (PE-Texas Red channel, 580 V, mRFP), the channels of forward scatter (FSC, 440 V) and side scatter (SSC, 260 V). Furthermore, cells were first gated by FSC and SSC (varied by different temperature) to illuminate noise events. Subsequently, fluorescence positive events were determined by fluorescence channels of FITC and Texa-red, respectively, to remove the fluorescence negative cells. Finally, cytometer data were processed and analyzed by FlowJo software (v10.7) for generating the mean value of fluorescence intensity. All source data were modified by the subtraction of fluorescence levels of negative control groups, which were E. coli JM109SGL harboring null vectors, the constructs 196+197.
细胞计量学分析由 BD LSR Fortessa 流式细胞仪(带 HTS attachment,BD, USA)进行。荧光阳性细胞在 488 nm 激发光谱(FITC channel,440 V,sfGFP)和 584 nm 激发光谱(PE-Texas Red channel,580 V,mRFP)下捕获,同时使用前向散射(FSC,440 V)和侧向散射(SSC,260 V)通道。随后,细胞首先通过 FSC 和 SSC(随温度变化)门控,以消除噪声事件。然后,荧光阳性事件分别通过 FITC 和 Texas Red 荧光通道确定,以去除荧光阴性细胞。最后,流式细胞仪数据由 FlowJo 软件(v10.7)处理和分析,以生成荧光强度的平均值。所有源数据均通过减去阴性对照组的荧光水平进行修正,阴性对照组为携带空载体的 E. coli JM109SGL 和构建体 196+197。
Tree ring-like colony assays on agar plates
类似于树轮的菌落测定(colony assays)在琼脂平板上
Assays were conducted on LB agar plates to generate tree ring-like colonies shown in Supplementary Figs. 9–11, respectively. Cell stocks of E. coli JM109SGL harboring T-switch circuits, including constructs 155+165, 147+167, and 147+168 groups, were streaked on LB agar plates and grown at 37 °C. After 12-h growth, single colony was picked and all were grown in a LB medium with relevant antibiotics for 12-h growth. Fifty microliters of cells cultures were spread on LB agar plate for x h (x = 6, 8, or 10) incubation, respectively, at 37 °C. Then the plates were moved to another incubator at 30 °C grown for (24–x) h, respectively. Finally, single-colony imaging was performed to generate tree ring-like patterns after three-time repetitive operations. Colonies of 155+165 group were recorded every 24 h of growth for tree ring-like formation analysis. Then, 0.1 vol% of Kan and Cm stock solution were added into the medium for stabilizing plasmids of interest throughout the assays.
实验在 LB agar plates 上进行,以生成如 Supplementary Figs. 9–11 所示的树轮状菌落。E. coli JM109SGL 细胞株携带有 T-switch circuits,包括 155+165、147+167 和 147+168 组构建体,被划线接种于 LB agar plates 上,并在 37 °C 下生长。生长 12 小时后,挑选单个菌落,并将所有菌落置于含相关抗生素的 LB 培养基中生长 12 小时。取 50 微升细胞培养物分别在 LB agar 平板上涂布,并在 37 °C 下培养(incubation)x 小时(x = 6、8 或 10)。随后,将平板转移至另一个 30 °C 培养箱中生长(24–x)小时。最终,进行单菌落成像(imaging),以在三次重复操作后生成树轮状图案。对 155+165 组菌落,每 24 小时记录一次生长情况,用于树轮状形成分析。然后,向培养基中添加 0.1 vol% 的 Kan 和 Cm 储备溶液,以在整个实验过程中稳定感兴趣的质粒(plasmid)。
Single colonies were photographed using Olympus SZX16 camera under Olympus U-RFL-T mercury lamp light combined with different filters (GFP: 436/20 EX filter and 480/40 EM filter; RFP: 572/35 EX filter and 645/75 EM filter). Images were taken at an aperture of f/2.8, and range of exposure times were typically between 0.01 and 5 s customized by optimizing the dynamic range of fluorescence of sfGFP and mRFP. Meanwhile, bright-field images of all colonies were photographed under ambient light exposure for comparative analysis. Photos were processed and adjusted by ImageJ (NIH, USA) with the size of 3.3 × 3.3 mm to generate figures.
单克隆体使用 Olympus SZX16 相机在 Olympus U-RFL-T 水银灯光下结合不同滤光片进行拍摄(GFP:436/20 EX 滤光片和 480/40 EM 滤光片;RFP:572/35 EX 滤光片和 645/75 EM 滤光片)。图像在 f/2.8 的光圈下拍摄,曝光时间范围通常在 0.01 和 5 s 之间,通过优化 sfGFP 和 mRFP 的荧光(fluorescence)动态范围(dynamic range)进行定制。同时,所有克隆体的明场图像(bright-field images)在环境光照射下进行拍摄,用于比较分析(comparative analysis)。照片由 ImageJ (NIH, USA) 处理和调整,尺寸为 3.3 × 3.3 mm,以生成图表。
Thermal control of cell morphology in cell growth
热控制(thermal control)在细胞生长(cell growth)中调节细胞形态(cell morphology)
For static control of cell morphology by T-switch, single colony of recombinant E. coli JM109SGL harboring double plasmids 145+221 was inoculated into 1 mL LB medium and grown 12 h in a 96-deep-well plate (1000 rpm, Thermal Shaker) at different temperatures (30, 33, 35, and 37 °C, respectively). Subsequently, 2 μL of cell cultures were sampled for cell shape recording by bright-field imaging using Nikon Eclipse Ti-E inverted fluorescence microscope after appropriate dilution to satisfy the single-cell analysis.
为了通过 T-switch 对细胞形态 (cell morphology) 进行静态控制,携带双质粒 145+221 的重组大肠杆菌 E. coli JM109SGL 的单个菌落被接种到 1 mL LB medium 中,并在 96 深孔板(1000 rpm,Thermal Shaker)中在不同温度(分别为 30、33、35 和 37 °C)培养 12 小时。随后,取样 2 μL 细胞培养物,经过适当稀释后,使用 Nikon Eclipse Ti-E 倒置荧光显微镜通过明场成像 (bright-field imaging) 记录细胞形状,以满足单细胞分析 (single-cell analysis)。
For dynamic control of cell shape, the recombinant E. coli used above was first grown at 30 °C and 33 °C, respectively, in 1 mL LB medium for 12 h (1000 rpm, Thermal Shaker). Then, cell cultures were injected into a microfluidic chip for single-cell capturing in specific chamber. Captured cells were grown at 30 °C initially, and temperature was changed to 33 °C in 1.5 h. Followed by 2.5-h growth, the temperature was modulated to 35 °C. Cell growth during the time course was recorded by bright-field imaging every 5 min. In contrast, captured cells from both 30 and 33 °C were grown at 35 °C constantly as comparative groups to study the thermal responsive morphology change in time course. Imaged with microscopy to measure cell shapes and cell lengths.
为了动态控制细胞形状,上文中使用的重组大肠杆菌(recombinant E. coli)首先分别在 30 °C 和 33 °C 条件下,在 1 mL LB 培养基中培养 12 小时(1000 rpm,Thermal Shaker)。然后,细胞培养物被注入微流控芯片(microfluidic chip)中,用于在特定腔室中捕获单细胞。捕获的细胞最初在 30 °C 下生长,温度在 1.5 小时内改变到 33 °C。随后经过 2.5 小时生长,温度被调节到 35 °C。在整个时间过程中,细胞生长每 5 分钟通过明场成像(bright-field imaging)记录一次。相比之下,从 30 °C 和 33 °C 捕获的细胞在 35 °C 下恒温生长,作为对照组,以研究温度响应形态变化(morphology change)的时间过程。用显微镜成像来测量细胞形状和细胞长度。
Cell growth imaging were generated using a Nikon Eclipse Ti-E inverted fluorescence microscope equipped with a 100× (NA 1.40) oil-immersion lens, together with an Andor DU885 EMCCD and/or Neo 5.5 sCMOS camera (Andor Technology, USA). Cells were maintained at needed temperature during imaging with an active-control environmental chamber. Images were collected using μManager v1.446 software to generate videos, then NIS elements v4.60 was used for adjusting the video. Cell lengths were measured by ImageJ manually. All assays are shown in Fig. 4 and Supplementary materials.
细胞生长成像使用 Nikon Eclipse Ti-E 倒置荧光显微镜生成,该显微镜配备了 100× (NA 1.40) 浸油透镜,以及 Andor DU885 EMCCD 和/或 Neo 5.5 sCMOS 相机(Andor Technology, USA)。在成像过程中,细胞通过主动控制环境室保持在所需的温度。图像使用 μManager v1.446 软件收集以生成视频,然后使用 NIS elements v4.60 软件调整视频。细胞长度通过 ImageJ 手动测量。所有实验(assays)显示在 Fig. 4 和补充材料中。
Debugging of PHB and P4HB synthesis pathways in shake flask studies
PHB 和 P4HB 合成途径(synthesis pathways)的调试在摇瓶研究(shake flask studies)中进行。
The recombinant cells of E. coli JM109SGL harboring construct 434 (PHB) and 435+68 (P4HB), respectively, were pre-cultured in 15 mL tubes containing 5 mL LB for 12 h at 200 rpm at 37 °C. Then seed cultures were 5 vol% inoculated into 100 mL LB medium supplemented with 20 g L−1 glucose for 24 h cultivation. Then, 0.1 vol% of Kan and/or Cm stock solution were added into the medium for stabilizing plasmids of interest throughout the cultural processes. IPTG was added after 4 h cultivation whatever necessary. Then, 30 mL of fermentation broths were sampled for further analysis.
E. coli JM109SGL 的重组细胞(recombinant cells),分别携带 construct 434 (PHB) 和 435+68 (P4HB),在 15 mL 管中含有 5 mL LB,37 °C、200 rpm 下预培养 12 小时。然后,种子培养物以 5 vol% 接种到补充了 20 g L −1 葡萄糖的 100 mL LB 培养基中,培养 24 小时。随后,向培养基中添加 0.1 vol% 的 Kan 和/或 Cm 储备溶液,以在整个培养过程中稳定感兴趣的质粒。必要时,在培养 4 小时后添加 IPTG。然后,采样 30 mL 发酵液(fermentation broths)进行进一步分析。
Fed-batch studies for copolymer productions of 3HB and 4HB, namely, PHB-b-P4HB and P(3HB-co-4HB)
fed-batch 研究用于 3HB 和 4HB 的共聚物生产,即 PHB-b-P4HB 和 P(3HB-co-4HB)
Single colonies of recombinant cells containing target plasmids, constructs 169+170 for PHB-b-P4HB production and 149+150 for P(3HB-co-4HB) production, were grown in 5 mL LB medium as inoculums for 12 h at 30 °C at 200 rpm. Then, these pre-cultures were 1 vol% inoculated into 500 mL shake flasks carrying 100 mL LB medium and grown under the same condition for 12 h for seed culture preparation. Subsequently, 300 mL of seed cultures were inoculated into 2.7 L LB medium supplemented with 20 g L−1 glucose. All fed-batch studies were carried out for 32 h. Agitation speed was coupled with dissolved oxygen (~30%) to reach 800 rpm from 200 rpm during the fermentation process. Particularly, 100 mL of feeding solution I, Feed-I (g L−1) containing 300 glucose, 22.5 NH4Cl, and 80 Yeast Extract, was added in a feeding rate of 0.5 mL min−1 when the residual glucose decreased to lower than 5 g L−1. Once Feed-I exhausted, the feeding solution II, Feed-II (g L−1) consisting of 600 glucose, should be added to maintain the concentration of residual glucose at 50–70 mmol L−1 (9–12.6 g L−1) by real-time monitoring of glucose measured using Clinistix paper (SANNUO, China). The pH was automatically adjusted at 7.0 using 5 mol L−1 NaOH solution. Then, 20 mL of fermentation broth was sampled for further analysis during the bioprocessing whatever necessary.
含有目标质粒的重组细胞单克隆,用于 PHB-b-P4HB 生产的 constructs 169+170 和用于 P(3HB-co-4HB) 生产的 149+150,在 5 mL LB medium 中作为 inoculum (inoculum) 培养 12 小时,条件为 30 °C 和 200 rpm。然后,这些预培养物以 1 vol% 体积百分比接种到装有 100 mL LB medium 的 500 mL 摇瓶中,在相同条件下培养 12 小时以制备种子培养物。随后,将 300 mL 种子培养物接种到补充有 20 g/L 葡萄糖的 2.7 L LB medium 中。所有 fed-batch (fed-batch) 研究均进行 32 小时。搅拌速度与溶解氧(约 30%)耦合,在发酵 (fermentation) 过程中从 200 rpm 增加到 800 rpm。特别地,当残留葡萄糖降至低于 5 g/L 时,以 0.5 mL/min 加料速率加入 100 mL 的 Feed-I(g/L 单位),Feed-I 含有 300 g/L 葡萄糖、22.5 g/L NH4Cl 和 80 g/L 酵母提取物。一旦 Feed-I 用尽,应加入 Feed-II(g/L 单位),Feed-II 含有 600 g/L 葡萄糖,通过实时监测使用 Clinistix paper (SANNUO, China) 测量的葡萄糖,将残留葡萄糖浓度维持在 50–70 mmol/L (9–12.6 g/L)。pH 值使用 5 mol/L NaOH 溶液自动调整至 7.0。然后,在生物加工过程中必要时取 20 mL 发酵液进行进一步分析。
Assays of dry cell mass and PHA contents
干细胞质量(dry cell mass)和 PHA 含量的测定(assays)
Cell growth and PHA content analyses were carried out to study the dynamic performances of copolymer production controlled by T-switch during fed-batch fermentations. First, 20 or 30 mL of fermentation broth of each time point was harvested by centrifugation at 12,000 rpm for 10 min, and washed by distilled water twice. Then, the bacterial precipitates were freeze-dried using a vacuum lyophilizer (LGJ‐10 C, Beijing Sihuan, China) under −80 °C, and weighted for calculating the cell dry weights (CDW, g L−1). Second, 20–40 mg lyophilized cells in powder forms were sampled for methanolysis in a 15-mL tube containing 2 mL chloroform and 2 mL methanolysis solution (85 wt% methanol, 15 wt% H2SO4, and 1 g L−1 benzonic acid), followed by extraction mixed with 1 mL distilled water in the cooled methanolysis solution, approximately 1 mL of extract liquor was used for gas chromatography analysis (GC-2014, SHIMADZU, Japan) to measure PHA content and monomer compositions36. About 10 mg of PHB standard (Sigma-Aldrich) and 10 mg of γ-butyrolactone (4HB standard, Sigma-Aldrich) were used as standards here.
进行了细胞生长和 PHA 含量分析,以研究 T-switch 控制下的共聚物生产的动态性能,在 fed-batch 发酵(fed-batch fermentations)过程中。首先,在每个时间点采集 20 或 30 mL 发酵液,通过 12,000 rpm 离心(centrifugation)10 min 收获,并用蒸馏水洗涤两次。然后,细菌沉淀物使用真空冻干机(LGJ‐10 C, Beijing Sihuan, China)在 −80 °C 下冻干(freeze-dried),并称重以计算细胞干重(CDW, g L⁻¹)。其次,取 20–40 mg 冻干细胞粉末,在一个 15-mL 管中进行甲醇解(methanolysis),管中含有 2 mL 氯仿和 2 mL 甲醇解溶液(85 wt% 甲醇, 15 wt% H₂SO₄, 和 1 g L⁻¹ 苯甲酸),随后在冷却的甲醇解溶液中加入 1 mL 蒸馏水进行提取,大约 1 mL 提取液用于气相色谱(gas chromatography)分析(GC-2014, SHIMADZU, Japan),以测量 PHA 含量和单体组成。这里使用了约 10 mg PHB 标准品(Sigma-Aldrich)和 10 mg γ-丁内酯(4HB 标准品, Sigma-Aldrich)作为标准。
NMR and D-value NMR 和 D-value
3HB and 4HB copolymers were extracted using chloroform solvent in a Soxhlet extractor (Soxtec 2050, Foss, Denmark) from lyophilized cells harvested from fermentation broth. Subsequently, the extracted PHA was dissolved in chloroform and then precipitated with ten-fold volume of ethanol. After centrifugation at 12,000 rpm for 10 min, the resulted PHA was oven dried at 65 °C for 12 h prior to the subsequent studies. All fractionated polymers were analyzed by 13C NMR (Nuclear Magnetic Resonance) (Oxford-600, UK) for identifying block copolymer and/or random copolymer of 3HB and 4HB49. MestReNova12 (Mestrelab Research, Spain) was used for spectra analysis. Bernoullian statistics method50 was employed to calculate the D-value of block copolymer and random copolymer (Supplementary Figs. 17, 19, and 21).
3HB 和 4HB 共聚物使用氯仿溶剂在 Soxhlet 提取器(Soxtec 2050, Foss, 丹麦)中从发酵液(fermentation broth)收获的冻干细胞(lyophilized cells)中提取。随后,提取的 PHA 在氯仿中溶解,然后用十倍体积的乙醇沉淀。经 12,000 rpm 离心(centrifugation)10 分钟后,所得 PHA 在 65 °C 烘箱中干燥 12 小时,以便进行后续研究。所有分级聚合物通过 13 C NMR(Nuclear Magnetic Resonance)(Oxford-600, 英国)分析,以鉴定 3HB 和 4HB 的嵌段共聚物和/或无规共聚物 49 。MestReNova12(Mestrelab Research, 西班牙)用于谱图分析。Bernoullian statistics method 50 被用于计算嵌段共聚物和无规共聚物的 D-value(补充图 17、19 和 21)。
DSC analysis DSC 分析
DSC was used to measure crystallinity degree, melting temperature, and glass-transition temperature of PHB, P4HB, and their copolymers, respectively, via a TA Instrument (DSC-Q20, TA, USA). A sample of 3–10 mg was compressed in an aluminum-sealed pan. Then the pan was cooled to −80 °C, then heated from −80 to 180 °C at a rate of 10 °C min−1. The sample was maintained at 180 °C for 2 min under a nitrogen atmosphere of 50 mL min−1. Then, the pan was quenched to −80 °C and reheated from −80 to 180 °C at a rate of 10 °C min−1. Data were collected during the second heating run49. P4HB purchased from Tepha (USA) and PHB from Sigma-Aldrich were used as standards for comparative analysis.
DSC 用于测量 PHB、P4HB 及其共聚物的结晶度(crystallinity degree)、熔融温度和玻璃化转变温度(glass-transition temperature),分别通过 TA 仪器(DSC-Q20, TA, USA)进行。一份 3-10 mg 的样品被压缩在铝密封盘中。然后,该盘被冷却至 -80 °C,随后以 10 °C/min 的速率加热至 180 °C。样品在 180 °C 下保持 2 min,在 50 mL/min 的氮气氛围中。然后,该盘被淬火至 -80 °C,并以 10 °C/min 的速率重新加热至 180 °C。数据在第二次加热运行过程中被收集。P4HB 从 Tepha(USA)购买,PHB 从 Sigma-Aldrich 获得,用作比较分析的标准。
Figures generation 图表生成 (figures generation)
Figures were generated through Adobe Illustrator CC2017, Prism v8 (GraphPad), Microsoft Office 2016 (Power Point, Excel, and Word), and ImageJ whatever necessary. Figures of temperature-response performance on LB agar plates were generated by using color palettes of ImageJ. FI of agar plate experiments were normalized by the maximum FI value of sfGFP and mRFP measured from every single image, ranging from 0 to 100.
图表是通过 Adobe Illustrator CC2017、Prism v8 (GraphPad)、Microsoft Office 2016 (Power Point、Excel 和 Word) 以及 ImageJ 视情况而定生成的。LB 琼脂平板上温度响应性能的图表是通过使用 ImageJ 的颜色调色板生成的。琼脂平板实验的 FI 通过每个图像中测量的 sfGFP 和 mRFP 的最大 FI 值进行归一化,范围从 0 到 100。
Reporting summary 报告摘要
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
关于研究设计(research design)的更多信息可在链接到本文的 Nature Research Reporting Summary 中找到。
Data availability 数据可用性 (Data availability)
The authors declare that source data processed for figure generation in this study are available within the paper and its Supplementary Information files. Plasmids used in this study, OD600 measured in fermentation experiments are deposited in Source Data file. The datasets generated and analyzed during the current study are available from the corresponding authors upon request. Any other relevant data are available from the authors upon reasonable request. Source Data are provided with this paper.
作者声明,本研究中用于图表生成(figure generation)的源数据(source data)可在本论文及其补充信息文件(Supplementary Information)中获得。本研究中使用的质粒,以及发酵实验中测量的 OD600,已存放在源数据文件中。当前研究中生成和分析的数据集(datasets)可应要求从相应作者处获得。任何其他相关数据可应合理要求从作者处获得。源数据已随本论文提供。
References
Moser, F. et al. Dynamic control of endogenous metabolism with combinatorial logic circuits. Mol. Syst. Biol. 14, 1–18 (2018).
Moser, F. et al. 动态调控内源代谢的组合逻辑电路. Mol. Syst. Biol. 14, 1–18 (2018)。Potvin-Trottier, L., Lord, N. D., Vinnicombe, G. & Paulsson, J. Synchronous long-term oscillations in a synthetic gene circuit. Nature 538, 514–517 (2016).
Potvin-Trottier, L., Lord, N. D., Vinnicombe, G. & Paulsson, J. 合成基因电路中的同步长期振荡 (synthetic gene circuit)。 Nature 538, 514–517 (2016).Dahl, R. H. et al. Engineering dynamic pathway regulation using stress-response promoters. Nat. Biotechnol. 31, 1039–1046 (2013).
Dahl, R. H. 等. Engineering dynamic pathway regulation using stress-response promoters. Nat. Biotechnol. 31: 1039–1046, 2013。Xu, X. et al. Pyruvate-responsive genetic circuits for dynamic control of central metabolism. Nat. Chem. Biol. 16, 1261–1268 (2020).
Xie, M. & Fussenegger, M. Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nat. Rev. Mol. Cell Biol. 19, 507–525 (2018).
Ro, D. K. et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943 (2006).
Durante-Rodríguez, G., De Lorenzo, V. & Nikel, P. I. A post-translational metabolic switch enables complete decoupling of bacterial growth from biopolymer production in engineered Escherichia coli. ACS Synth. Biol. 7, 2686–2697 (2018).
Fang, M. et al. Intermediate-sensor assisted push-pull strategy and its application in heterologous deoxyviolacein production in Escherichia coli. Metab. Eng. 33, 41–51 (2016).
Xu, P., Li, L., Zhang, F., Stephanopoulos, G. & Koffas, M. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc. Natl Acad. Sci. USA 111, 11299–11304 (2014).
Niu, T. et al. Engineering a glucosamine-6-phosphate responsive glmS ribozyme switch enables dynamic control of metabolic flux in Bacillus subtilis for overproduction of N-Acetylglucosamine. ACS Synth. Biol. 7, 2423–2435 (2018).
Harder, B. J., Bettenbrock, K. & Klamt, S. Temperature-dependent dynamic control of the TCA cycle increases volumetric productivity of itaconic acid production by Escherichia coli. Biotechnol. Bioeng. 115, 156–164 (2018).
Zhou, L. et al. Genetically switched d-lactate production in Escherichia coli. Metab. Eng. 14, 560–568 (2012).
Aparicio, T., de Lorenzo, V. & Martínez-García, E. Improved thermotolerance of genome-reduced Pseudomonas putida EM42 enables effective functioning of the PL/cI857 system. Biotechnol. J. 14, 1–8 (2019).
Zhao, E. M. et al. Optogenetic regulation of engineered cellular metabolism for microbial chemical production. Nature 555, 683–687 (2018).
Chen, X. et al. An extraordinary stringent and sensitive light-switchable gene expression system for bacterial cells. Cell Res. 109, 854–857 (2016).
Ding, Q. et al. Light-powered Escherichia coli cell division for chemical production. Nat. Commun. 11, 2262 (2020).
Gupta, A. et al. Dynamic regulation of metabolic flux in engineered bacteria using a pathway-independent quorum-sensing circuit. Nat. Biotechnol. 35, 273–279 (2017).
Lalwani, M. A. et al. Optogenetic control of the lac operon for bacterial chemical and protein production. Nat. Chem. Biol. 17, 71–79 (2021).
Dinh, C. V. & Prather, K. L. J. Development of an autonomous and bifunctional quorum-sensing circuit for metabolic flux control in engineered Escherichia coli. Proc. Natl Acad. Sci. USA 116, 25562–25568 (2019).
Yang, Y. et al. Sensor-regulator and RNAi based bifunctional dynamic control network for engineered microbial synthesis. Nat. Commun. 9, 3043 (2018).
Solomon, K. V., Sanders, T. M. & Prather, K. L. J. A dynamic metabolite valve for the control of central carbon metabolism. Metab. Eng. 14, 661–671 (2012).
Luo, N., Wang, S. & You, L. Synthetic pattern formation. Biochemistry 58, 1478–1483 (2019).
Majerle, A., Schmieden, D. T., Jerala, R. & Meyer, A. S. Synthetic biology for multiscale designed biomimetic assemblies: from designed self-assembling biopolymers to bacterial bioprinting. Biochemistry 58, 2095–2104 (2019).
Cao, Y. et al. Collective space-sensing coordinates pattern scaling in engineered bacteria. Cell 165, 620–630 (2016).
Liu, C. et al. Sequential establishment of stripe patterns in an expanding cell population. Science 334, 238–241 (2011).
Curatolo, A. I. et al. Cooperative pattern formation in multi-component bacterial systems through reciprocal motility regulation. Nat. Phys. 16, 1152–1157 (2020).
Chen, A. Y. et al. Synthesis and patterning of tunable multiscale materials with engineered cells. Nat. Mater. 13, 515–523 (2014).
Lalwani, M. A., Zhao, E. M. & Avalos, J. L. Current and future modalities of dynamic control in metabolic engineering. Curr. Opin. Biotechnol. 52, 56–65 (2018).
Piraner, D. I. et al. Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat. Chem. Biol. 13, 75–80 (2017).
Abedi, M. H., Lee, J., Piraner, D. I. & Shapiro, M. G. Thermal control of engineered T-cells. ACS Synth. Biol. 9, 1941–1950 (2020).
Piraner, D. I., Wu, Y. & Shapiro, M. G. Modular thermal control of protein dimerization. ACS Synth. Biol. 8, 2256–2262 (2019).
Elvin, C. M. et al. Modified bacteriophage lambda promoter vectors for overproduction of proteins in Escherichia coli. Gene. 87, 123–126 (1990).
Chen, G. Q. & Hajnal, I. The ‘PHAome’. Trends Biotechnol. 33, 559–564 (2015).
Lorenzo, V. et al. The power of synthetic biology for bioproduction, remediation and pollution control. EMBO Rep. 19, 4–9 (2018).
Stanton, B. C. et al. Genomic mining of prokaryotic repressors for orthogonal logic gates. Nat. Chem. Biol. 10, 99–105 (2014).
Li, Z. J. et al. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab. Eng. 12, 352–359 (2010).
Fang, Y. et al. Rebalancing microbial carbon distribution for L-threonine maximization using a thermal switch system. Metab. Eng. 61, 33–46 (2020).
Jiang, X. R. & Chen, G. Q. Morphology engineering of bacteria for bio-production. Biotechnol. Adv. 34, 435–440 (2016).
Guo, L. et al. Engineering Escherichia coli lifespan for enhancing chemical production. Nat. Catal. 3, 307–318 (2020).
Volke, D. C. & Nikel, P. I. Getting bacteria in shape: synthetic morphology approaches for the design of efficient microbial cell factories. Adv. Biosyst. 2, 1–21 (2018).
Lv, Y. et al. Coupling feedback genetic circuits with growth phenotype for dynamic population control and intelligent bioproduction. Metab. Eng. 54, 109–116 (2019).
Gu, F. et al. Quorum sensing-based dual-function switch and its application in solving two key metabolic engineering problems. ACS Synth. Biol. 9, 209–217 (2020).
Burg, J. M. et al. Large-scale bioprocess competitiveness: the potential of dynamic metabolic control in two-stage fermentations. Curr. Opin. Chem. Eng. 14, 121–136 (2016).
Wei, R. et al. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat. Catal. 3, 867–871 (2020).
Ye, J. et al. Engineering of Halomonas bluephagenesis for low cost production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose. Metab. Eng. 47, 143–152 (2018).
Ye, J. et al. Stimulus response-based fine-tuning of polyhydroxyalkanoate pathway in Halomonas. Metab. Eng. 57, 85–95 (2020).
Tripathi, L. et al. Pseudomonas putida KT2442 as a platform for the biosynthesis of polyhydroxyalkanoates with adjustable monomer contents and compositions. Bioresour. Technol. 142, 225–231 (2013).
Wang, Q. et al. Production of block copolymer poly(3-hydroxybutyrate)-block-poly(3-hydroxypropionate) with adjustable structure from an inexpensive carbon source. ACS Macro. Lett. 2, 996–1000 (2013).
Tripathi, L., Wu, L.-P., Meng, D., Chen, J. & Chen, G.-Q. Biosynthesis and characterization of diblock copolymer of p(3-hydroxypropionate)-block-p(4-hydroxybutyrate) from recombinant Escherichia coli. Biomacromolecules 14, 862–870 (2013).
Hu, D. et al. Biosynthesis and characterization of polyhydroxyalkanoate block copolymer P3HB-b-P4HB. Biomacromolecules 12, 3166–3173 (2011).
Pederson, E. N., McChalicher, C. W. J. & Srienc, F. Bacterial synthesis of PHA block copolymers. Biomacromolecules 7, 1904–1911 (2006).
Tang, X., Westlie, A. H., Watson, E. M. & Chen, E. Y. X. Stereosequenced crystalline polyhydroxyalkanoates from diastereomeric monomer mixtures. Science 366, 754–758 (2019).
Kapoor, D. N. et al. PLGA: a unique polymer for drug delivery. Ther. Deliv. 6, 41–58 (2015).
Gao, J., Dutta, K., Zhuang, J. & Thayumanavan, S. Cellular- and subcellular- targeted delivery using a simple all‐in‐one polymeric nanoassembly. Angew. Chemie Int. Ed. 59, 23466–23470 (2020).
Halmschlag, B., Steurer, X., Putri, S. P., Fukusaki, E. & Blank, L. M. Tailor-made poly-γ-glutamic acid production. Metab. Eng. 55, 239–248 (2019).
Moradali, M. F. & Rehm, B. H. A. Bacterial biopolymers: from pathogenesis to advanced materials. Nat. Rev. Microbiol. 18, 195–210 (2020).
Rehm, B. H. A. Synthetic biology towards the synthesis of custom-made polysaccharides. Microb. Biotechnol. 8, 19–20 (2015).
Acknowledgements
This research was financially supported by grants from the Ministry of Science and Technology of China (Grant No. 2018YFA0900200; No. 2016YFB0302500), National Natural Science Foundation of China (Grant No. 21761132013; No. 31870859; No. 32001029), and Tsinghua University-INDITEX Sustainable Development Fund (Grant No. TISD201907). This project is also funded by the National Natural Science Foundation of China (Grant No. 31961133017, No. 31961133018, No. 31961133019). These grants are part of MIX-UP, a joint NSFC and EU H2020 collaboration. In Europe, MIX-UP has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 870294. The PhlF repressor encoded gene was donated by Professor Chunbo Lou from SIAT in Shenzhen, China. The PHA mechanical property analysis was carried out with the help of Professor Jun Xu from Department of Chemical Engineering, Tsinghua University.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Pablo Ivan Nikel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Han, JN., Zhang, X. et al. Reversible thermal regulation for bifunctional dynamic control of gene expression in Escherichia coli. Nat Commun 12, 1411 (2021). https://doi.org/10.1038/s41467-021-21654-x
This article is cited by
-
Accelerating the Understanding of Biosensors Through the Lens of Cells: State of the Field, Emerging Directions, Advances, and Challenges
Applied Biochemistry and Biotechnology (2025)
-
Engineered autonomous dynamic regulation of metabolic flux
Nature Reviews Bioengineering (2023)
-
A synthetic population-level oscillator in non-microfluidic environments
Communications Biology (2023)
-
Advances in biosynthesis of higher alcohols in Escherichia coli
World Journal of Microbiology and Biotechnology (2023)
-
A novel co-production of cadaverine and succinic acid based on a thermal switch system in recombinant Escherichia coli
Microbial Cell Factories (2022)