- Department of Ultrasound, Qilu Hospital of Shandong University (Qingdao), Qingdao, Shandong, China
山东大学齐鲁医院超声科
Purpose: Evaluating the performance of the Gallbladder Reporting and Data System (GB-RADS) combined with Color Doppler Flow Imaging (CDFI) for the diagnosis of gallbladder wall thickening disease in an Asian population.
目的:评价胆囊报告和数据系统(GB-RADS)结合彩色多普勒血流成像(CDFI)诊断亚洲人群胆囊壁增厚疾病的性能。
Methods: In this study, the lesions were classified and the actual incidence rate of malignant tumors was calculated for each GB-RADS category, following the guidelines provided by GB-RADS. To evaluate the diagnostic performance of GB-RADS and GB-RADS combined with CDFI, we plotted Receiver Operator Characteristic (ROC) curves. The sensitivity (SE), specificity (SP), positive predictive value (PPV), negative predictive value (NPV), and accuracy (AC) were also calculated. Inter-observer agreement (IRA) between the two observers was assessed using Kappa values.
方法:根据GB-RADS的分类标准,对病变进行分类,计算每种GB-RADS分类的恶性肿瘤的实际发生率。为了评价GB-RADS和GB-RADS结合CDFI的诊断性能,我们绘制了受试者工作特征(ROC)曲线。计算敏感性(SE)、特异性(SP)、阳性预测值(PPV)、阴性预测值(NPV)和准确性(AC)。使用Kappa值评估两名观察员之间的观察员间一致性(伊拉)。
Results: The incidence of malignancy risk for GB-RADS 2, 3, 4, and 5 was 9%, 12.5%, 72.2%, and 100%. The AUC for GB-RADS was 0.855 (95% CI: 0.800-0.900), with a sensitivity of 82.5%, a specificity of 84.6%, and an accuracy of 83.8%. The AUC of GB-RADS combined with CDFI was 0.965 (95% CI: 0.930-0.985), with a sensitivity of 96.2%, a specificity of 94.6%, and an accuracy of 95.2%. The AUC, sensitivity, specificity, and accuracy of GB-RADS combined with CDFI for diagnosing gallbladder malignancy were higher than those of GB-RADS alone, and the differences were statistically significant (all P < 0.05). The IRA was excellent between the two observers (Kappa = 0.870).
结果:GB-RADS 2、3、4和5的恶性风险发生率分别为9%、12.5%、72.2%和100%。GB-RADS的AUC为0.855(95%CI:0.800 - 0.900),敏感性为82.5%,特异性为84.6%,准确性为83.8%。GB-RADS结合CDFI的AUC为0.965(95%CI:0.930-0.985),敏感性为96.2%,特异性为94.6%,准确性为95.2%。GB-RADS联合CDFI诊断胆囊恶性肿瘤的AUC、敏感性、特异性和准确性均高于单独GB-RADS,差异均有统计学意义(均P<0.05)。两位观察者的IRA表现出色(Kappa = 0.870)。
Conclusions: GB-RADS combined with CDFI demonstrated excellent diagnostic accuracy when it comes to distinguishing various diseases that caused gallbladder wall thickening in the Asian population, which has good clinical value and can improve the detection rate of malignant tumors in patients with gallbladder wall thickening.
结论:GB-RADS结合CDFI对亚洲人群胆囊壁增厚的各种疾病的诊断准确率较高,具有较好的临床应用价值,可提高胆囊壁增厚患者恶性肿瘤的检出率。
1 Introduction 1引言
Gallbladder cancer is the most commonly observed tumor of the biliary system and is associated with poor prognosis and a low 5-year survival rate (1). In the early stages, gallbladder cancer does not present with any specific symptoms. By the time gallbladder cancer is diagnosed, the disease has often already progressed to an advanced stage, making it difficult to achieve the desired treatment outcomes. If gallbladder cancer is diagnosed correctly in the early stages, the 5-year survival rate will significantly improve (2, 3). Research reports indicate that the occurrence of gallbladder cancer demonstrates certain geographical variations, with high incidence rates observed in Asian populations (4). The most common imaging manifestation of gallbladder cancer is gallbladder wall thickening; however, benign gallbladder diseases such as adenomyosis and polyps can also cause gallbladder wall thickening (5, 6). Both benign and malignant gallbladder diseases may present with symptoms such as upper abdominal pain and jaundice, and distinguishing benign from malignant gallbladder diseases based solely on clinical symptoms is difficult (7). Accurate diagnosis of benign and malignant gallbladder wall thickening is particularly important for the appropriate treatment of patients, avoiding overtreatment of benign diseases and delays in the treatment of malignant tumors (8, 9).
胆囊癌是最常见的胆道系统肿瘤,预后差,5年生存率低(1)。在早期阶段,胆囊癌不存在任何特定症状。当胆囊癌被诊断出来时,疾病通常已经进展到晚期,因此很难达到预期的治疗效果。如果胆囊癌在早期得到正确诊断,5年生存率将显著提高(2,3)。研究报告表明,胆囊癌的发生表现出一定的地理差异,在亚洲人群中观察到高发病率(4)。胆囊癌最常见的影像学表现是胆囊壁增厚;然而,良性胆囊疾病如子宫腺肌病和息肉也可导致胆囊壁增厚(5,6)。 良性和恶性胆囊疾病都可能出现上腹痛和黄疸等症状,仅根据临床症状很难区分良性和恶性胆囊疾病(7)。良性和恶性胆囊壁增厚的准确诊断对于患者的适当治疗尤其重要,避免良性疾病的过度治疗和恶性肿瘤的治疗延误(8,9)。
Two-dimensional transabdominal ultrasonography is the most commonly employed imaging modality for gallbladder examination. Ultrasonography is characterized by real-time dynamics, accuracy (AC), economy, and the absence of radiological damage. Compared to computed tomography (CT), transabdominal ultrasound has the advantage of avoiding exposure to nuclear radiation. Compared to transabdominal ultrasound, magnetic resonance imaging studies can also be dynamic; however, their availability is limited and they are time-consuming (10). Although two-dimensional transabdominal ultrasound is considered the preferred screening method for gallbladder wall thickening, a certain degree of inter-examiner subjectivity and a lack of consensus exists, both domestically and internationally. With the continuous development of ultrasound technology, color Doppler flow imaging (CDFI) can display the blood flow status of lesions, improving the AC of gallbladder lesion diagnosis (6). Ultrasonographic features of gallbladder wall thickening in benign diseases usually exhibit diffuse symmetrical wall thickening, whereas ultrasonographic features of gallbladder wall thickening in malignant diseases usually involve focal asymmetrical thickening without a layered appearance, destruction of the gallbladder wall, or an indistinct interface with the adjacent liver parenchyma (9, 11). In February 2022, the American journal “Abdominal Radiology” published the Gallbladder Reporting and Data System (GB-RADS) for risk stratification of gallbladder wall thickening detected on ultrasonography. The system aims to assess the risk of malignancy in gallbladder wall thickening by applying consistent ultrasound terminology, which would allow for standardized and consistent descriptions of the lesion characteristics for consistent follow-up and management in clinical practice (12).
二维经腹超声检查是胆囊检查最常用的成像方式。超声检查的特点是实时动态,准确性(AC),经济,和没有放射性损伤。与计算机断层扫描(CT)相比,经腹超声具有避免暴露于核辐射的优点。与经腹超声相比,磁共振成像研究也可以是动态的;然而,它们的可用性有限并且耗时(10)。尽管二维经腹超声被认为是胆囊壁增厚的首选筛查方法,但在国内和国际上都存在一定程度的检查者间主观性和缺乏共识。随着超声技术的不断发展,彩色多普勒血流显像(CDFI)可以显示病变的血流状态,提高了胆囊病变诊断的AC(6)。 良性疾病胆囊壁增厚的超声特征通常表现为弥漫性对称性壁增厚,而恶性疾病胆囊壁增厚的超声特征通常包括局灶性不对称增厚,无分层外观,胆囊壁破坏,或与邻近肝实质交界不清(9,11)。2022年2月,美国《腹部放射学》杂志发表了胆囊报告和数据系统(GB-RADS),对超声检查发现的胆囊壁增厚进行风险分层。该系统旨在通过应用一致的超声术语来评估胆囊壁增厚的恶性风险,这将允许对病变特征进行标准化和一致的描述,以便在临床实践中进行一致的随访和管理(12)。
The purpose of this study was to explore the diagnostic value of GB-RADS in distinguishing benign from malignant gallbladder wall thickening in Asian populations and to evaluate whether incorporating CDFI to GB-RADS would improve the diagnostic performance of the system, thus providing valuable diagnostic methods for patients with gallbladder wall thickening.
本研究的目的是探讨GB-RADS在亚洲人群中鉴别胆囊壁良恶性增厚的诊断价值,并评估将CDFI与GB-RADS结合是否会提高系统的诊断性能,从而为胆囊壁增厚患者提供有价值的诊断方法。
2 Methods 2种方法
2.1 Materials 2.1材料
This retrospective study was approved by the hospital’s Institutional Health Research Ethics Committee, and the need for informed consent was waived (ethics number: KYLL-KS-2023148). Ultrasound images of all patients with gallbladder wall thickening from the hospital’s local picture archiving and communication system between February 2019 and May 2023 were retrospectively analyzed. The patient selection flowchart is displayed in Figure 1. The inclusion criteria were as follows: (1) availability of preoperative gallbladder ultrasonography and postoperative pathological results; (2) thickening of the gallbladder wall observed using abdominal ultrasound (gallbladder wall thickness > 3 mm); (3) no radiotherapy, chemotherapy, immunotherapy, or other related treatments before the examination; and (4) age > 18 years. The exclusion criteria were as follows: (1) pathological results not obtained; (2) evaluation impossible for various reasons (GB-RADS 0) and normal gallbladder (GB-RADS 1); (3) secondary changes in the gallbladder caused by systemic or hepatic diseases and acute cholecystitis; and (4) pregnant and lactating women.
这项回顾性研究得到了医院机构健康研究伦理委员会的批准,并且无需知情同意(伦理编号:KYLL-KS-2023148)。回顾性分析了2019年2月至2023年5月期间来自医院本地图像存档和通信系统的所有胆囊壁增厚患者的超声图像。患者选择流程图如图1所示。入选标准如下:(1)术前胆囊超声检查和术后病理结果的可用性;(2)腹部超声观察到胆囊壁增厚(胆囊壁厚度3 mm);(3)检查前未接受放疗、化疗、免疫治疗或其他相关治疗;(4)年龄18岁。 排除标准如下:(1)未获得病理结果;(2)由于各种原因无法评估(GB-RADS 0)和正常胆囊(GB-RADS 1);(3)全身或肝脏疾病和急性胆囊炎引起的胆囊继发性变化;(4)妊娠和哺乳期妇女。

Figure 1 Flowchart showing a selection of patients with gallbladder wall thickening.
图1显示胆囊壁增厚患者选择的流程图。
2.2 Methods 2.2方法
Diagnostic color ultrasound machines, such as PHILIPS IU22, TOSHIBA Aplio 500, and Canon Apilo i800, were used in this study. Gallbladder ultrasonography was performed after 6 hours of fasting. Ultrasonography of the left lateral position of the gallbladder was performed in both the sagittal and axial planes. The imaging provided a complete assessment of the gallbladder from multiple perspectives. The examination primarily focused on the following morphological characteristics for each evaluated gallbladder: the thickness of the gallbladder wall, extent, and symmetry of the gallbladder wall thickening, site of the gallbladder wall thickening, presence of contents in the gallbladder cavity, presence of a layering phenomenon in the gallbladder wall, intramural changes in the wall, clear interface with the liver, and distribution of CDFI in the gallbladder wall thickening. Two experts with over 10 years of experience in abdominal ultrasound diagnosis were trained in the GB-RADS. They classified each lesion under the assumption of unknown pathology, and when disagreements occurred, they were resolved through re-examination and discussion.
本研究中使用了诊断彩色超声机,如PHILIPS IU 22、TOSHIBA Aplio 500和Canon Apilo i800。禁食6小时后进行胆囊超声检查。在矢状面和轴向平面上对胆囊左侧位进行超声检查。成像从多个角度对胆囊进行了全面评估。检查主要集中于每个评价胆囊的以下形态学特征:胆囊壁的厚度、胆囊壁增厚的程度和对称性、胆囊壁增厚的部位、胆囊腔内是否存在内容物、胆囊壁是否存在分层现象、壁内变化、与肝脏的清晰界面,CDFI在胆囊壁增厚中的分布。两名在腹部超声诊断方面具有10年以上经验的专家接受了GB-RADS培训。 他们在未知病理的假设下对每个病变进行分类,当出现分歧时,通过重新检查和讨论解决。
The GB-RADS grading system was divided into six classes ranging from 0 to 5, including a range from assessable to high-risk. GB-RADS 0 signifies cases that cannot be evaluated due to technical reasons and patient-related factors such as obesity, porcelain gallbladder, and the presence of gas in the gallbladder lumen. GB-RADS 1 indicates a normal gallbladder, adequate gallbladder distension, and the gallbladder wall thickness ≤3 mm; GB-RADS 2 encompasses benign conditions (probability of malignancy < 2%), including (1) symmetric circumferential thickening with or without intramural changes or focal thickening with intramural changes, (2) layered appearance, (3) clear interface with the liver; GB-RADS 3 suggests a suspected malignant tumor (probability of malignancy 2%–50%), including (1) circumferential thickening without layered appearance, (2) no intramural features (localized thickening of cysts or echo lesions), (3) clear interface with the liver; GB-RADS 4 includes a lesion suggestive of malignant tumor (probability of malignant 50%–90%), presenting circumferential or focal thickening without stratification and with a vague interface with the life; GB-RADS 5: indicates a high suspicion of malignancy (probability of malignancy >90%), this included the features of GB-RADS 4, with the addition of definite extrahepatic invasion of the gallbladder wall. Moreover, invasion characteristics may involve the direct extension of the biliary or vascular structures by the mural thickening, as well as adjacent liver mass with the mural thickening (12). Color Doppler blood flow grading was divided into grades 0 to III according to Adler’s blood flow grading (13): 0, no flow signal; I, a small flow signal with one or two dots and short rods; II, moderate flow with three or four dots or one main blood vessel; and III, abundant flow with more than four dots or two main blood vessels. When the CDFI of the lesion in GB-RADS 3 was grade 0–I, the original grading remained unchanged and was represented as GB-RADS+CDFI 3. When the CDFI was grade II–III, it was adjusted upward to one grade, as demonstrated by GB-RADS+CDFI 4. When the CDFI of the lesion in GB-RADS 4 was grade 0–I, it was adjusted downward to one grade, as demonstrated by GB-RADS+CDFI 3. Furthermore, when the CDFI of the lesion in GB-RADS 4 was grade 0–I, a downward adjustment of one grade was represented by GB-RADS+CDFI 3. When the CDFI of the lesion in GB-RADS 4 was grades II–III, the original grading remained unchanged, as indicated by GB-RADS+CDFI 4. The lesions in GB-RADS 3 did not have a downward adjustment, as demonstrated by GB-RADS+CDFI 2, while the lesions in GB-RADS 5 did not have an upward adjustment, as indicated by GB-RADS+CDFI 5 (14, 15).
GB-RADS分级系统分为6个等级,从0到5,包括从可评估到高风险的范围。GB-RADS 0表示由于技术原因和患者相关因素(如肥胖、瓷化胆囊和胆囊腔内存在气体)而无法评价的病例。 GB-RADS 1表示胆囊正常,胆囊充分扩张,胆囊壁厚度≤3 mm; GB-RADS 2包括良性疾病(恶性概率< 2%),包括(1)对称性周向增厚伴或不伴壁内改变或局灶性增厚伴壁内改变,(2)分层外观,(3)与肝脏界面清晰; GB-RADS 3提示疑似恶性肿瘤(恶性概率2%-50%),包括(1)无分层外观的周向增厚,(2)无壁内特征(3)与肝脏分界清楚; GB-RADS 4包括提示恶性肿瘤的病变(恶性概率为50%-90%),表现为无分层的环形或局灶性增厚,与生活界面模糊; GB-RADS 5:表明高度怀疑恶性肿瘤(恶性肿瘤的概率为>90%),这包括GB-RADS 4的特征,例明确的胆囊壁肝外侵犯。此外,浸润特征可能包括胆管或血管结构的直接延伸,以及邻近肝肿块的壁增厚(12)。彩色多普勒血流分级根据Adler血流分级分为0 ~ III级(13):0,无流量信号; I,小血流信号,有一个或两个点和短杆; II,中等血流,有三个或四个点或一个主血管; III,丰富的血流,有四个以上的点或两个主血管。当GB-RADS 3中病变的CDFI为0-I级时,原始分级保持不变,并表示为GB-RADS+CDFI 3。当CDFI为II-III级时,向上调整一级,如GB-RADS+CDFI 4所示。当GB-RADS 4中病变的CDFI为0-I级时,将其向下调整为1级,如GB-RADS+CDFI 3所示。 此外,当GB-RADS 4中病变的CDFI为0-I级时,向下调整一级表示为GB-RADS+CDFI 3。当GB-RADS 4中病变的CDFI为II-III级时,原始分级保持不变,如GB-RADS+CDFI 4所示。如GB-RADS+CDFI 2所示,GB-RADS 3中的病变没有向下调整,而GB-RADS 5中的病变没有向上调整,如GB-RADS+CDFI 5所示(14,15)。
2.3 Statistical analysis 2.3统计分析
Data were analyzed using SPSS 26.0 and MedCalc 19.0. The age of the patients in the malignant and benign groups conformed to the normal distribution and was expressed as mean ± standard deviation, and comparisons were made using the independent samples t-test. The ultrasonographic features of the gallbladder and CDFI distributions of the gallbladder in the malignant and benign groups were expressed as numbers and percentages. Additionally, comparisons were made using the chi-square test. Sensitivity (SE), specificity (SP), positive predictive value, negative predictive value, AC, and Jordon indices were calculated for GB-RADS and GB-RADS combined with CDFI. For statistical convenience, intraepithelial neoplasia was classified as a malignant tumor in this study, and pathological findings were used as the gold standard to generate the receiver operating characteristic (ROC) curve, determine the optimal cutoff value, and calculate the area under the curve (AUC). Differences between the AUC values were analyzed using McNemar’s test. A P-value < 0.05 was considered statistically significant. Kappa values were used to assess inter-observer agreement (IRA) between the two observers for GB-RADS. Moreover, 0.21 ≤ Kappa ≤ 0.40 indicated general consistency, 0.41 ≤ Kappa ≤ 0.60 was suggestive of moderate consistency, 0.61 ≤ Kappa ≤ 0.80 signified good consistency, and 0.81 ≤ Kappa ≤ 1.00 was suggestive of excellent consistency.
采用SPSS 26.0和MedCalc 19.0进行数据分析。恶性组和良性组患者的年龄符合正态分布,以均数±标准差表示,采用独立样本t检验进行比较。用数字和百分比表示良、恶性组胆囊声像图特征和CDFI分布。此外,还使用卡方检验进行了比较。计算GB-RADS和GB-RADS联合CDFI诊断乳腺癌的敏感性(SE)、特异性(SP)、阳性预测值、阴性预测值、AC和Jordan指数。为了便于统计,本研究中将上皮内瘤变分类为恶性肿瘤,并将病理结果用作金标准以生成受试者工作特征(ROC)曲线,确定最佳截止值,并计算曲线下面积(AUC)。 使用McNemar检验分析AUC值之间的差异。认为P值< 0.05具有统计学显著性。Kappa值用于评估两名观察员之间GB-RADS的观察员间一致性(伊拉)。此外,0.21 ≤ Kappa ≤ 0.40表示一般一致性,0.41 ≤ Kappa ≤ 0.60表示中等一致性,0.61 ≤ Kappa ≤ 0.80表示良好一致性,0.81 ≤ Kappa ≤ 1.00表示良好一致性。
3 Results 3个结果
3.1 General characteristics
3.1一般特征
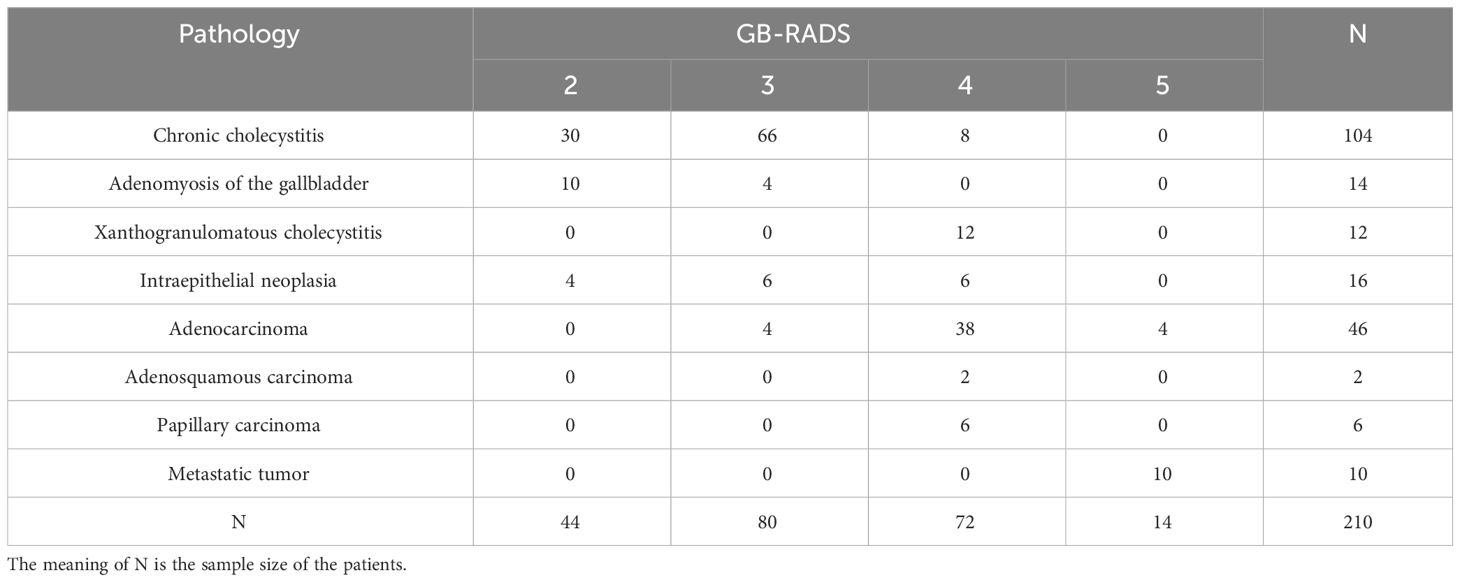
The study included 210 patients who underwent surgery and whose pathological results were obtained. The age range was between 29 and 84 years, with an average age of 59.66 ± 13.37. The average age in the benign group was 56.14 ± 13.43, while that in the malignant groups was 65.38 ± 11.18. According to the pathological results, the study included 130 cases of benign disease and 80 cases of malignant disease, with cholecystitis and adenocarcinoma being the most common benign and malignant diseases, respectively. The analysis encompassed 44 cases of GB-RADS 2, 76 cases of GB-RADS 3, 76 cases of GB-RADS 4, and 14 cases of GB-RADS 5. According to GB-RADS and CDFI, 44 cases of GB-RADS+CDFI 2, 85 cases of GB-RADS+CDFI 3, 67 cases of GB-RADS+CDFI 4, and 14 cases of GB-RADS+CDFI 5 were present. The specific pathological results and classifications are presented in Table 1.
该研究包括210例接受手术并获得病理结果的患者。年龄范围为29 - 84岁,平均年龄为59.66 ± 13.37岁。良性组平均年龄为56.14 ± 13.43岁,恶性组平均年龄为65.38 ± 11.18岁。根据病理结果,研究包括130例良性疾病和80例恶性疾病,胆囊炎和腺癌分别是最常见的良性和恶性疾病。分析包括44例GB-RADS 2、76例GB-RADS 3、76例GB-RADS 4和14例GB-RADS 5。根据GB-RADS和CDFI结果,GB-RADS+CDFI 2级44例,GB-RADS+CDFI 3级85例,GB-RADS+CDFI 4级67例,GB-RADS+CDFI 5级14例。具体病理结果和分类见表1。
3.2 Univariate and multivariate analysis of ultrasound characteristics
3.2超声特征的单变量和多变量分析
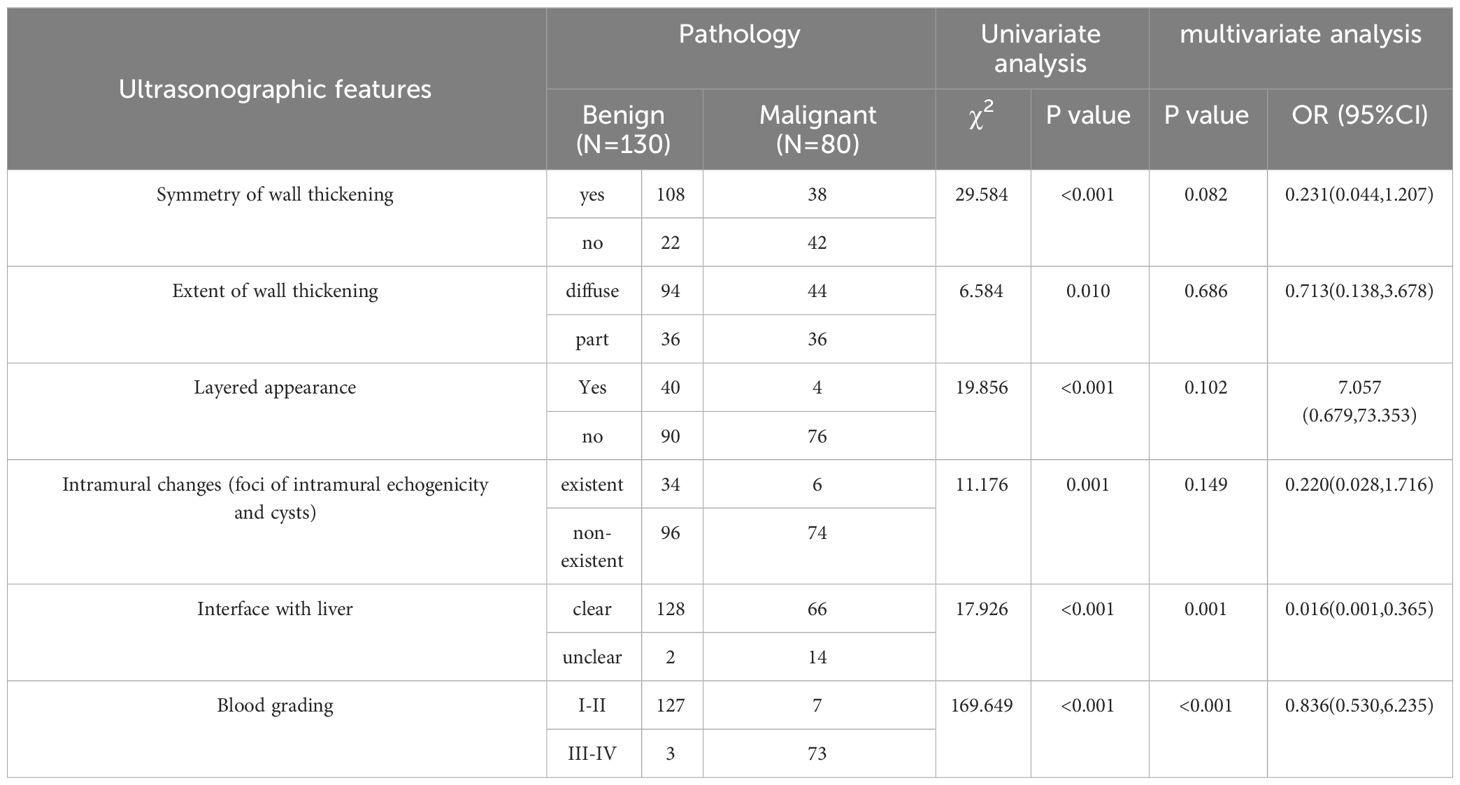
Table 2 displays the univariate and multivariate analyses of the ultrasound characteristics related to gallbladder wall thickening. In the univariate analysis, significant differences (P < 0.05) were observed between the benign and malignant groups in the extent of gallbladder wall thickening, symmetry of gallbladder wall thickening, the presence of layered appearance in the gallbladder wall, intramural changes in the gallbladder wall, whether the interface with the liver was clear, and the distribution of CDFI in the gallbladder wall thickening. Multivariate analysis of the aforementioned characteristics revealed that an unclear interface with the liver and grade III–IV of CDFI were associated with the development of malignant tumors of the gallbladder (all P < 0.05).
表2显示了与胆囊壁增厚相关的超声特征的单变量和多变量分析。单因素分析中,良恶性组在胆囊壁增厚程度、胆囊壁增厚对称性、胆囊壁内有无分层、胆囊壁内改变、与肝脏分界是否清晰、胆囊壁增厚内CDFI分布等方面差异有统计学意义(P<0.05)。多因素分析显示,胆囊与肝脏交界不清、CDFI Ⅲ ~ Ⅳ级与胆囊恶性肿瘤的发生有关(P均0.05)。
3.3 Diagnostic performance of GB-RADS, and GB-RADS combined with CDFI
3.3 GB-RADS及GB-RADS联合CDFI的诊断性能
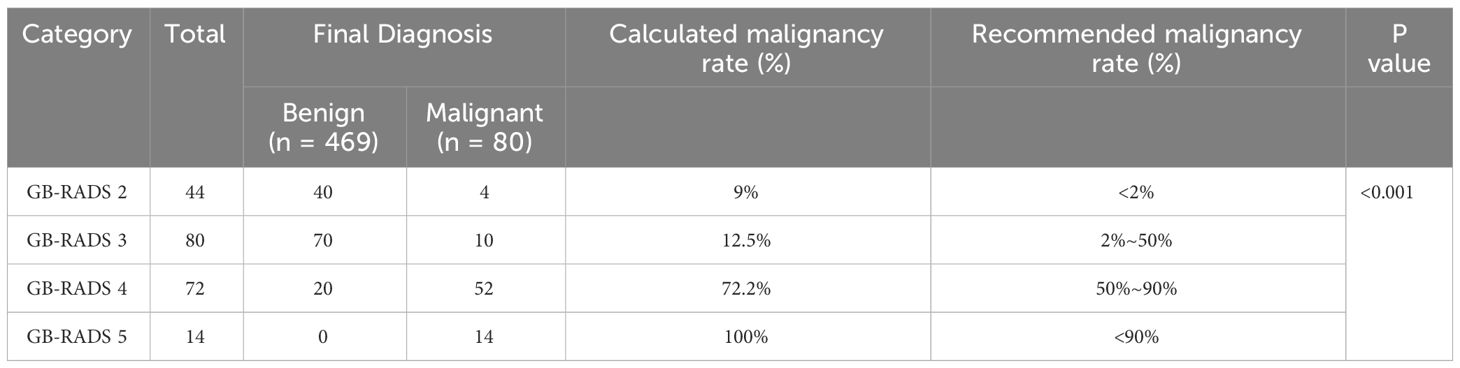
Table 3 demonstrates the incidence of malignancy in GB-RADS, and the risks of malignancy in GB-RADS 2, 3, 4, and 5 were 9%, 12.5%, 72.2%, and 100%, respectively, with statistically significant differences (P < 0.001). The ROC curves of the GB-RADS and GB-RADS combined with CDFI for the diagnosis of gallbladder malignancy are illustrated in Figure 2. The Jordon index of GB-RADS was 0.671; the AUC was 0.855 (95% CI: 0.800–0.900). The best cut-off value for predicting gallbladder malignancy was determined to be greater than GB-RADS 3. The Jordon index of GB-RADS combined with CDFI was 0.908 with an AUC of 0.965 (95% CI: 0.930–0.985), and the AUC of GB-RADS combined with CDFI for diagnosing gallbladder malignancy was higher than that of GB-RADS alone, with a statistically significant difference (P=0.0001). The diagnostic performances of GB-RADS and GB-RADS combined with CDFI for malignant gallbladder tumors are demonstrated in Table 4. The study identified that the SE, SP, and AC of the GB-RADS alone in diagnosing malignant gallbladder tumors were 82.5%, 84.6%, and 83.8%, respectively. However, when GB-RADS was combined with the CDFI, the SE, SP, and AC improved to 96.2%, 94.6%, and 95.2%, respectively. This indicated that the combination of the GB-RADS and CDFI was more effective in diagnosing malignant gallbladder tumors than the GB-RADS alone. Importantly, these differences in diagnostic AC were statistically significant (all P < 0.05).
表3显示了GB-RADS中恶性肿瘤的发生率,GB-RADS 2、3、4和5中恶性肿瘤的风险分别为9%、12.5%、72.2%和100%,具有统计学显著性差异(P % 3 C 0.001)。GB-RADS和GB-RADS联合CDFI诊断胆囊恶性肿瘤的ROC曲线如图2所示。GB-RADS的Jordan指数为0.671; AUC为0.855(95%CI:0.800-0.900)。预测胆囊恶性肿瘤的最佳截止值被确定为大于GB-RADS 3。GB-RADS联合CDFI的Jordan指数为0.908,AUC为0.965(95%CI:0.930-0.985),诊断胆囊恶性肿瘤的AUC高于单独使用GB-RADS,差异有统计学意义(P=0.0001)。表4显示了GB-RADS和GB-RADS结合CDFI对恶性胆囊肿瘤的诊断性能。 本研究确定,单独使用GB-RADS诊断恶性胆囊肿瘤的SE、SP和AC分别为82.5%、84.6%和83.8%。而当GB-RADS与CDFI结合时,SE、SP和AC分别提高到96.2%、94.6%和95.2%。结果表明,GB-RADS与CDFI联合应用对胆囊恶性肿瘤的诊断优于单独应用GB-RADS。重要的是,诊断AC的这些差异具有统计学显著性(所有P % 3 C 0. 05)。
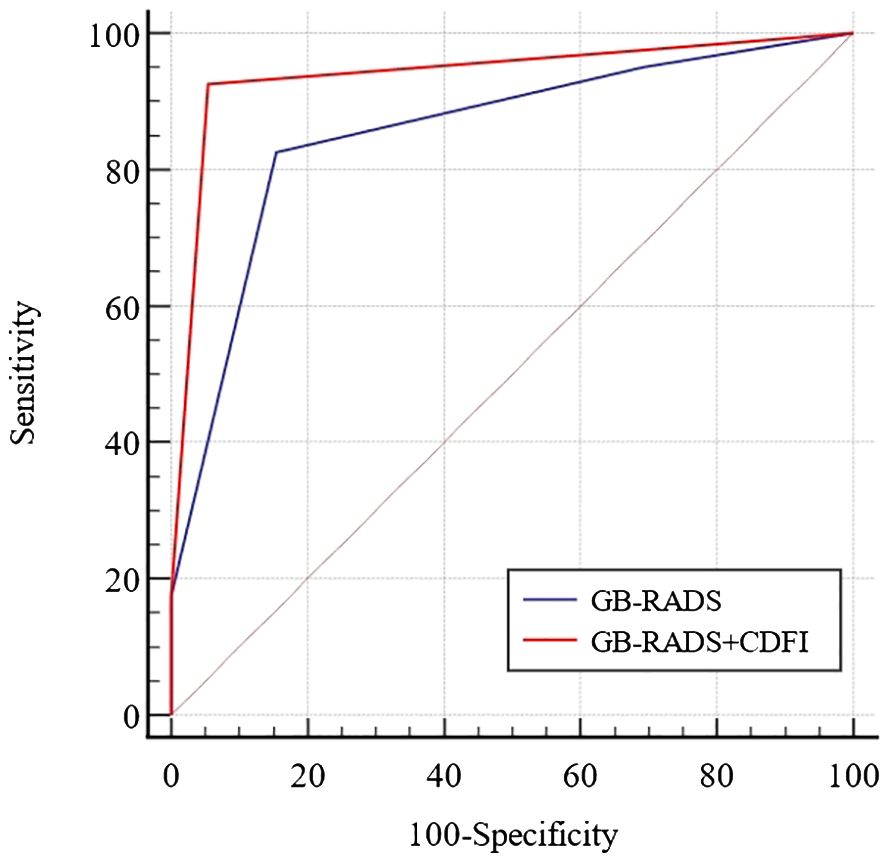
Figure 2 Receiver-operator characteristic curves for diagnosis of benign and malignant gallbladder wall thickening by GB-RADS and GB-RADS combined with CDFI.
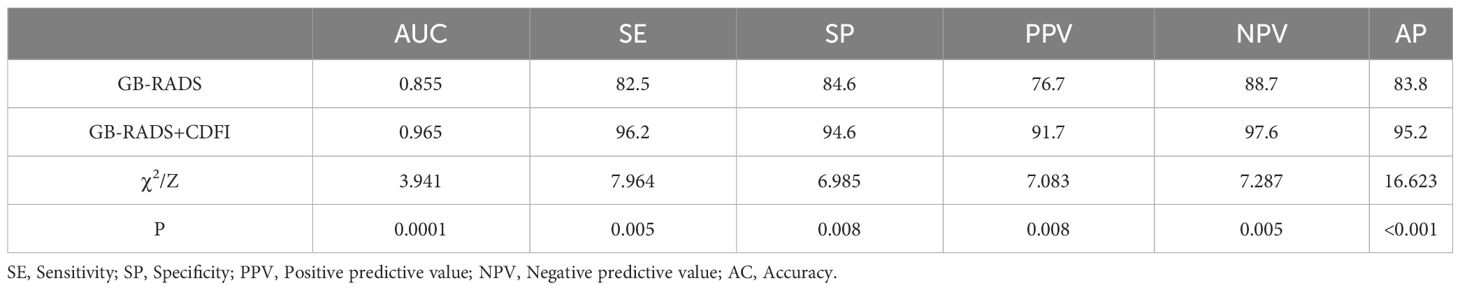
Table 4 Diagnostic performance of GB-RADS and GB-RADS combined with CDFI for benign and malignant gallbladder wall thickening.
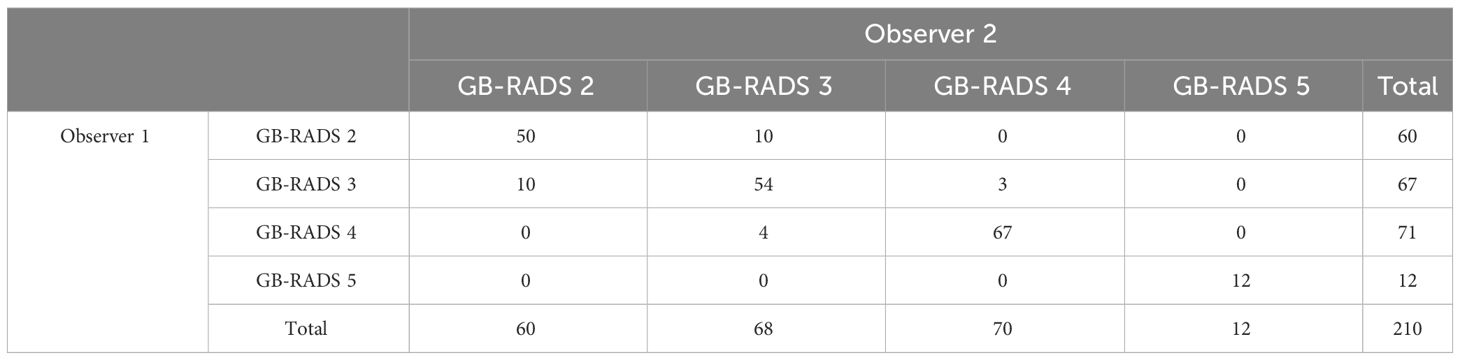
3.4 IRA between the two observers
Two observers independently analyzed the ultrasound images using the GB-RADS, and their detailed categorization results are presented in Table 5. The Kappa value was 0.870 (95% CI: 0.823–0.917), with P < 0.001, indicating an excellent level of agreement between the observers.
Representative cases from our study are displayed in Figures 3–5.
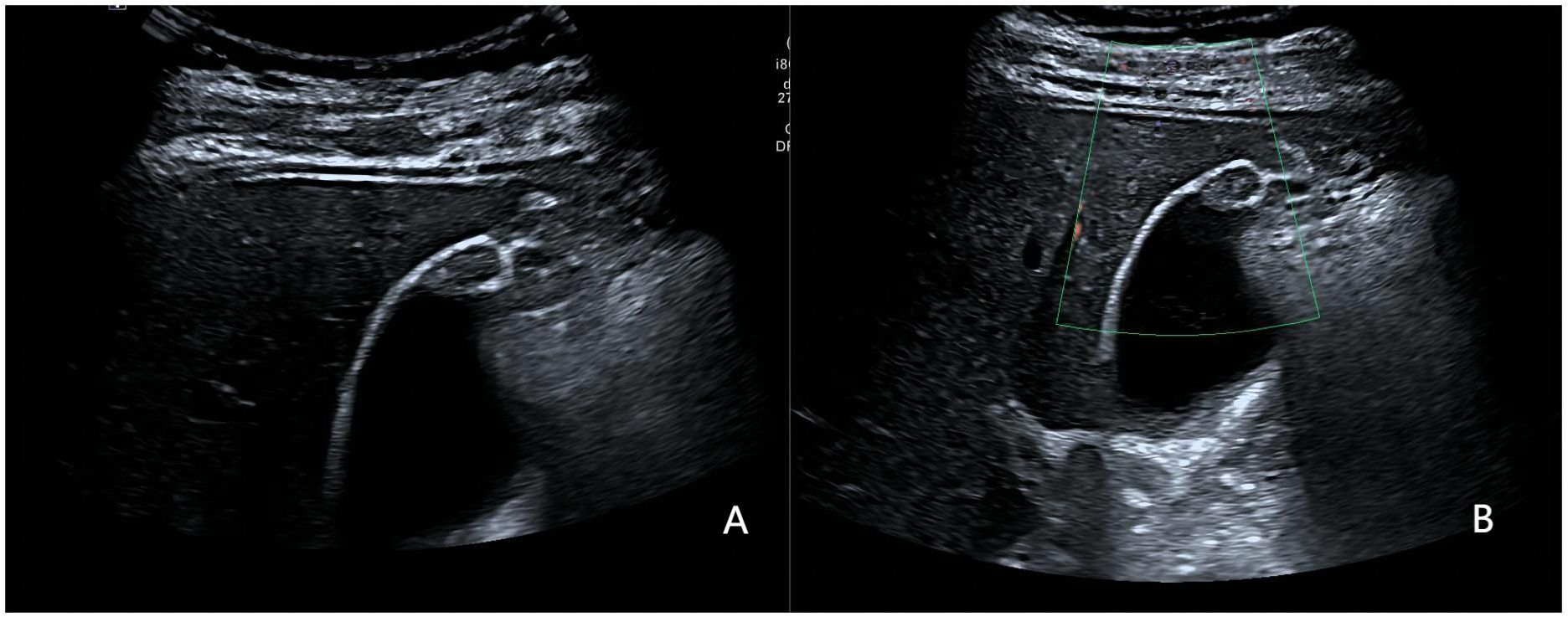
Figure 3 A 36-year-old woman with pathologically proven xanthogranulomatous cholecystitis. (A) Focak thickening of the base of the gallbladder with layering; (B) CDFI=0.Based on a consensus review of the sonographic findings, the lesion was categorized as GB-RADS 2, GB-RADS+CDFI 2.
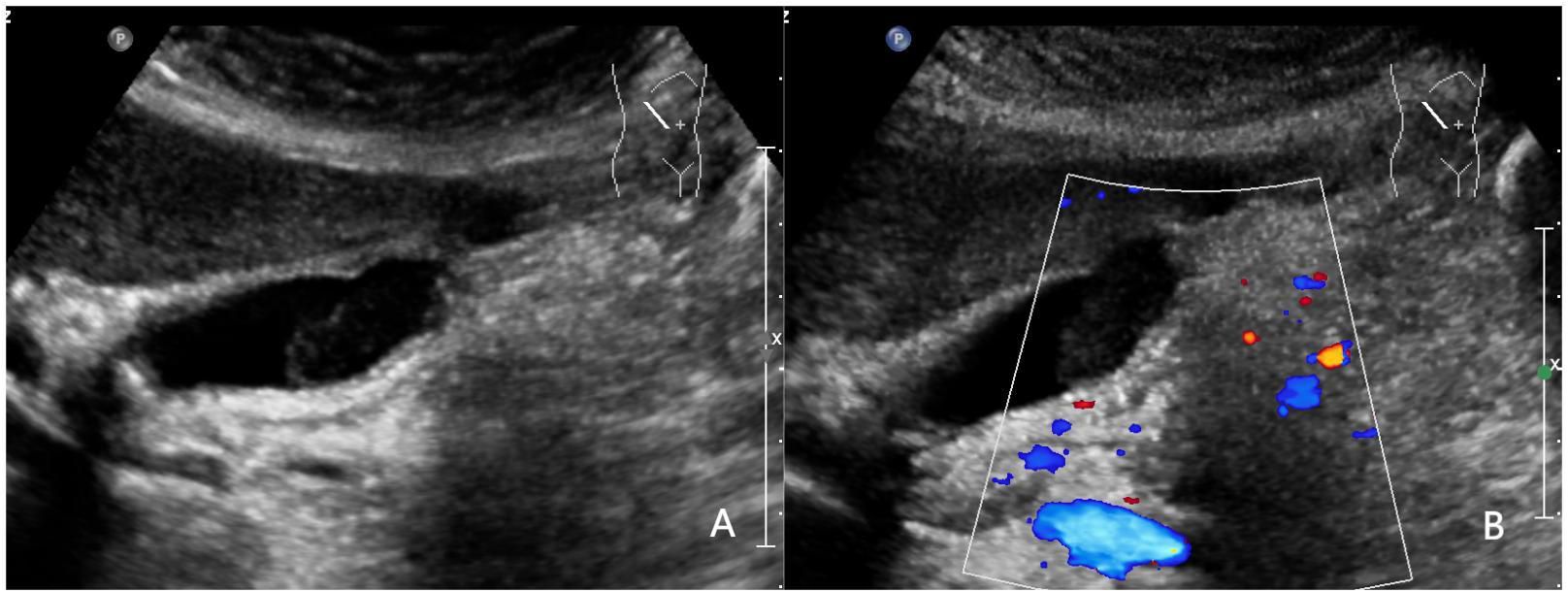
Figure 4 A 52-year-old woman with pathologically proven chronic cholecystitis. (A) Focal thickening of the base of the gallbladder, with no stratified appearance, and loss of interface with the liver.; (B) CDFI=0.Based on a consensus review of the sonographic findings, the lesion was categorized as GB-RADS 4, GB-RADS+CDFI 3.
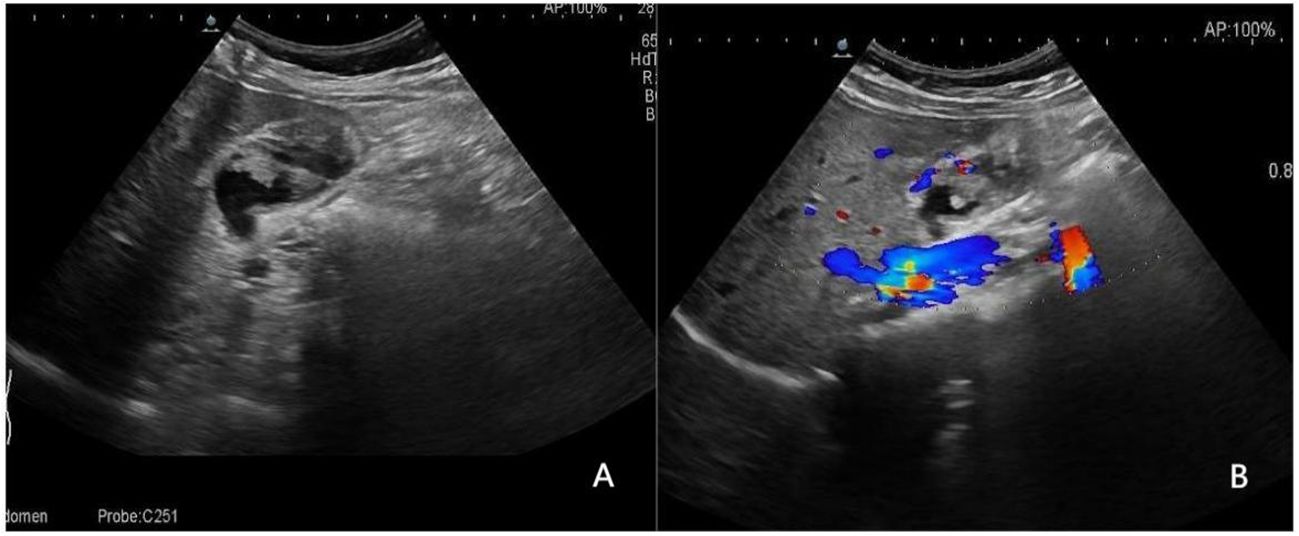
Figure 5 A 52-year-old man with a pathologically proven adenocarcinoma. (A) Focal thickening of the base of the gallbladder, with no stratified appearance, and loss of interface with the liver.; (B) CDFI=II. Based on a consensus review of the sonographic findings, the lesion was categorized as GB-RADS 4, GB-RADS+CDFI 4.
4 Discussion
Thickening of the gallbladder wall is a common manifestation of biliary system diseases. In the presence of a history of right upper abdominal pain and inflammatory findings, thickening of the gallbladder wall usually is associated with acute cholecystitis. However, in the absence of clinical symptoms of cholecystitis, consideration should be given to various perspectives, especially gallbladder cancer (16). Gallbladder cancer presents with no obvious symptoms during the early stages, progresses rapidly, and is prone to recurrence and metastasis (17). These characteristics have a significant impact on a patient’s treatment and lead to an extremely poor overall prognosis. Therefore, an early and accurate diagnosis of gallbladder wall thickening is extremely important for the effective management and favorable prognosis of patients. Conventional ultrasound is currently considered the preferred imaging modality for the clinical diagnosis of gallbladder diseases (18). However, no consensus exists on how to differentiate between benign and malignant gallbladder wall thickening. The GB-RADS is a risk stratification system that categorizes gallbladder wall thickening based on ultrasound characteristics and adopts different management measures for each category, helping to improve diagnostic AC (12). To date, the performance of GB-RADS has not been validated. This study analyzed the diagnostic value of GB-RADS in diagnosing gallbladder wall thickening in an Asian population and evaluated whether the addition of CDFI can improve the diagnostic performance of GB-RADS.
This study discovered that the ROC of GB-RADS for the diagnosis of malignant gallbladder cancer was 0.855. When using a GB-RADS score > 3 as a predictive factor for malignant tumors, the SE was 82.5% and the SP was 84.6%, indicating good diagnostic performance. In routine ultrasound diagnosis of malignant gallbladder cancer, the SE was 65.3%–82.5%, with significant variation. Some researchers have explored the value of high-frame-rate contrast-enhanced ultrasound for gallbladder wall thickening in nonacute settings (19). The study determined that the SE, SP, and AC of the GB-RADS for diagnosing malignant gallbladder cancer were only 68.75%, 73.33%, and 71.74%, respectively, which were lower than those in our study. The reason for this difference may be that, in the study by Zhu et al., the sample size consisted of 46 cases, whereas in our study, the sample size comprised 210. Despite being derived from routine transabdominal ultrasonography, the GB-RADS identifies key ultrasonographic features for each risk category (20–23). The ultrasonic features adopt standard terminology and describe the meanings of these terms. Management strategies for malignant potential were proposed for each risk category. This may explain why GB-RADS exhibits high SE and SP. When the GB-RADS was combined with the CDFI for the diagnosis of gallbladder cancer, the AUC, SE, and SP were 0.965, 96.2%, and 94.6%, respectively. The AUC, SE, and SP for differentiating benign and malignant gallbladder wall thickening using the GB-RADS combined with CDFI were all higher than those obtained when the GB-RADS was used alone. Additionally, CDFI can distinguish between benign and malignant disease by assessing blood supply status at the lesion site (6, 24). Studies have indicated that most malignant lesions exhibit blood flow signals, whereas in benign lesions they are hardly observed (25). The pathological basis of gallbladder cancer with abundant blood flow is the abnormal proliferation of blood vessels within the tumor, thickening, and dilation of the gallbladder artery and its branches, and a tortuous and disorderly blood vessel shape, which in turn increases blood flow (25, 26). Some researchers have reported elevated SE and SP in detecting blood flow signals, particularly in distinguishing early malignant lesions of the gallbladder from benign gallbladder bulge lesions. This underscores the effectiveness of CDFI as a method for determining the benign or malignant nature of gallbladder bulge lesions (27). Combining CDFI with the GB-RADS enhances the diagnostic AC in differentiating between benign and malignant gallbladder wall thickening disease in Asian populations. This approach evaluates ultrasound characteristics using two-dimensional ultrasound alongside CDFI, significantly improving diagnostic performance (28).
We analyzed the ultrasonographic features associated with gallbladder wall thickening. The results demonstrated that an unclear interface with the liver, grades III–IV of CDFI, was associated with gallbladder malignancy (P < 0.05), confirming the role of key ultrasound terms in the GB-RADS. Our findings suggest that only three of the 76 cases with pathologic grades III–IV were benign, and grades III–IV of the CDFI were associated with gallbladder malignancy in both univariate and multivariate analyses. Therefore, the addition of CDFI to GB-RADS may improve the diagnostic performance of the system. In the current study, we evaluated the incidence of malignant tumors across different GB-RADS categories and discovered that the incidence of malignant tumors in GB-RADS 3, 4, and 5 aligned with the recommended rate, whereas the incidence of malignant tumors in G-RADS 2 exceeded the recommended rate. A possible explanation for this observation could be that in the cases included in this study, the ultrasound findings of intraepithelial neoplasia did not display any malignant features. Therefore, based on the GB-RADS, they were classified as GB-RADS 2. However, in the present study, intraepithelial neoplasia was classified as a malignant tumor. Therefore, further research is required to assess more reasonable risks. False-positive cases mainly occurred in GB-RADS 4, including eight cases of chronic cholecystitis and 12 cases of xanthogranulomatous cholecystitis. All ultrasounds demonstrated diffuse thickening of the gallbladder wall with loss of normal structure, inhomogeneous internal echoes, and an unclear interface with the liver, These findings were indicative of a gallbladder tumor invading the liver, leading to classification as GB-RADS 4. When CDFI was added for diagnosis, the blood flow signals within most lesions were insignificant and could be downgraded.
The consistency and reproducibility of GB-RADS are also very important. During our analysis, we evaluated the IRA between the two experts using the kappa statistic. The obtained kappa value of 0.870 revealed a high level of agreement and excellent consistency in the application of the GB-RADS. These results demonstrate that the GB-RADS can be reliably and consistently implemented by experienced professionals in the field of diagnostic ultrasound.
Our study has some limitations. First, this study was retrospective in nature; we analyzed static images retained in the system, which may resulted in some errors in diagnostic AC. Furthermore, ultrasound images are manipulated by different operators using different ultrasound machinery with varying image qualities, which may have caused selection bias. Therefore, future multicenter, large-sample prospective studies are needed to validate the diagnostic performance of GB-RADS. Furthermore, several studies have demonstrated that the implementation of Artificial Intelligence (AI) has the potential to enhance the AC and precision of ultrasound in distinguishing between benign and malignant tumors. Combining AI with the GB-RADS holds great promise for improving the overall diagnostic performance in detecting and diagnosing gallbladder cancer (29).
In conclusion, GB-RADS combined with CDFI has high SE and SP to distinguish between benign and malignant gallbladder wall thickening in Asian populations, indicating the potential of the system to significantly enhance the AC of diagnosing gallbladder diseases. Moreover, the GB-RADS displayed good consistency in its findings, further validating its clinical utility. To ensure the widespread applicability and reliability of this approach, future research should focus on conducting multicenter, large-sample prospective studies that would assist healthcare professionals in effectively assessing and managing gallbladder diseases, leading to improved patient outcomes and enhanced medical decision-making.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Qilu Hospital of Shandong University (Qingdao). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The study involved only ultrasound images of patients. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because The study involved only ultrasound images of patients.
Author contributions
RW: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. LLv: Writing – review & editing. LLi: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the aut.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. (2001) 51:349–64. doi: 10.3322/canjclin.51.6.349
2. Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol. (2017) 23:3978–98. doi: 10.3748/wjg.v23.i22.3978
3. Gupta P, Kumar M, Sharma V, Dutta U, Sandhu MS. Evaluation of gallbladder wall thickening: A multimodality imaging approach. Expert Rev Gastroenterol Hepatol. (2020) 14:463–73. doi: 10.1080/17474124.2020.1760840
4. Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. (2003) 4:167–76. doi: 10.1016/s1470-2045(03)01021-0
5. Yu MH, Kim YJ, Park HS, Jung SI. Benign gallbladder diseases: imaging techniques and tips for differentiating with Malignant gallbladder diseases. World J Gastroenterol. (2020) 26:2967–86. doi: 10.3748/wjg.v26.i22.2967
6. Zhu L, Han P, Jiang B, Zhu Y, Li N, Fei X. Value of micro flow imaging in the prediction of adenomatous polyps. Ultrasound Med Biol. (2023) 49:1586–94. doi: 10.1016/j.ultrasmedbio.2023.03.004
7. Chatterjee A, Lopes Vendrami C, Nikolaidis P, Mittal PK, Bandy AJ, Menias CO, et al. Uncommon intraluminal tumors of the gallbladder and biliary tract: spectrum of imaging appearances. Radiographics. (2019) 39:388–412. doi: 10.1148/rg.2019180164
8. Kim SJ, Lee JM, Lee JY, Kim SH, Han JK, Choi BI, et al. Analysis of enhancement pattern of flat gallbladder wall thickening on Mdct to differentiate gallbladder cancer from cholecystitis. AJR Am J Roentgenol. (2008) 191:765–71. doi: 10.2214/ajr.07.3331
9. Soundararajan R, Marodia Y, Gupta P, Rana P, Chhabra M, Kalage D, et al. Imaging patterns of wall thickening type of gallbladder cancer. Clin Exp Hepatol. (2022) 8:255–66. doi: 10.5114/ceh.2022.122285
10. Cheng Y, Wang M, Ma B, Ma X. Potential role of contrast-enhanced ultrasound for the differentiation of Malignant and benign gallbladder lesions in east asia: A meta-analysis and systematic review. Med (Baltimore). (2018) 97:e11808. doi: 10.1097/md.0000000000011808
11. Rana P, Gupta P, Kalage D, Soundararajan R, Kumar MP, Dutta U. Grayscale ultrasonography findings for characterization of gallbladder wall thickening in non-acute setting: A systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. (2022) 16:59–71. doi: 10.1080/17474124.2021.2011210
12. Gupta P, Dutta U, Rana P, Singhal M, Gulati A, Kalra N, et al. Gallbladder reporting and data system (Gb-rads) for risk stratification of gallbladder wall thickening on ultrasonography: an international expert consensus. Abdom Radiol (NY). (2022) 47:554–65. doi: 10.1007/s00261-021-03360-w
13. Adler DD, Carson PL, Rubin JM, Quinn-Reid D. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol. (1990) 16:553–9. doi: 10.1016/0301-5629(90)90020-D
14. Chen L, Zhan J, Diao XH, Liu YC, Shi YX, Chen Y, et al. Additional value of superb microvascular imaging for thyroid nodule classification with the thyroid imaging reporting and data system. Ultrasound Med Biol. (2019) 45:2040–8. doi: 10.1016/j.ultrasmedbio.2019.05.001
15. Zheng X, Li F, Xuan ZD, Wang Y, Zhang L. Combination of shear wave elastography and bi-rads in identification of solid breast masses. BMC Med Imaging. (2021) 21:183. doi: 10.1186/s12880-021-00702-4
16. Jenssen C, Lorentzen T, Dietrich CF, Lee JY, Chaubal N, Choi BI, et al. Incidental findings of gallbladder and bile ducts-management strategies: general aspects, gallbladder polyps and gallbladder wall thickening-a world federation of ultrasound in medicine and biology (Wfumb) position paper. Ultrasound Med Biol. (2022) 48:2355–78. doi: 10.1016/j.ultrasmedbio.2022.06.016
17. Miranda-Filho A, Piñeros M, Ferreccio C, Adsay V, Soerjomataram I, Bray F, et al. Gallbladder and extrahepatic bile duct cancers in the americas: incidence and mortality patterns and trends. Int J Cancer. (2020) 147:978–89. doi: 10.1002/ijc.32863
18. Gerstenmaier JF, Hoang KN, Gibson RN. Contrast-enhanced ultrasound in gallbladder disease: A pictorial review. Abdom Radiol (NY). (2016) 41:1640–52. doi: 10.1007/s00261-016-0729-4
19. Zhu L, Li N, Zhu Y, Han P, Jiang B, Li M, et al. Value of high frame rate contrast enhanced ultrasound in gallbladder wall thickening in non-acute setting. Cancer Imaging. (2024) 24:7. doi: 10.1186/s40644-023-00651-x
20. Kong WT, Shen HY, Qiu YD, Han H, Wen BJ, Wu M. Application of contrast enhanced ultrasound in gallbladder lesion: is it helpful to improve the diagnostic capabilities? Med Ultrason. (2018) 20:420–6. doi: 10.11152/mu-1626
21. Zhang HP, Bai M, Gu JY, He YQ, Qiao XH, Du LF. Value of contrast-enhanced ultrasound in the differential diagnosis of gallbladder lesion. World J Gastroenterol. (2018) 24:744–51. doi: 10.3748/wjg.v24.i6.744
22. Zheng SG, Xu HX, Liu LN, Lu MD, Xie XY, Wang WP, et al. Contrast-enhanced ultrasound versus conventional ultrasound in the diagnosis of polypoid lesion of gallbladder: A multi-center study of dynamic microvascularization. Clin Hemorheol Microcirc. (2013) 55:359–74. doi: 10.3233/ch-121651
23. Wang W, Fei Y, Wang F. Meta-analysis of contrast-enhanced ultrasonography for the detection of gallbladder carcinoma. Med Ultrason. (2016) 18:281–28. doi: 10.11152/mu.2013.2066.183.wei
24. Sun Y, Yang Z, Lan X, Tan H. Neoplastic polyps in gallbladder: A retrospective study to determine risk factors and treatment strategy for gallbladder polyps. Hepatobiliary Surg Nutr. (2019) 8:219–27. doi: 10.21037/hbsn.2018.12.15
25. Boddapati SB, Lal A, Gupta P, Kalra N, Yadav TD, Gupta V, et al. Contrast enhanced ultrasound versus multiphasic contrast enhanced computed tomography in evaluation of gallbladder lesions. Abdom Radiol (NY). (2022) 47:566–75. doi: 10.1007/s00261-021-03364-6
26. Zhou W, Li G, Ren L. Triphasic dynamic contrast-enhanced computed tomography in the differentiation of benign and Malignant gallbladder polypoid lesions. J Am Coll Surg. (2017) 225:243–8. doi: 10.1016/j.jamcollsurg.2017.04.014
27. Kim SY, Cho JH, Kim EJ, Chung DH, Kim KK, Park YH, et al. The efficacy of real-time colour doppler flow imaging on endoscopic ultrasonography for differential diagnosis between neoplastic and non-neoplastic gallbladder polyps. Eur Radiol. (2018) 28:1994–2002. doi: 10.1007/s00330-017-5175-3
28. Yang L, Lin N, Wang M, Chen G. Diagnostic efficiency of existing guidelines and the ai-sonic™ Artificial intelligence for ultrasound-based risk assessment of thyroid nodules. Front Endocrinol (Lausanne). (2023) 14:1116550. doi: 10.3389/fendo.2023.1116550
Keywords: GB-RADS, gallbladder cancer, ultrasound, CDFI, diagnostic performance
Citation: Wang R, Lv L and Li L (2024) Diagnostic performance of the gallbladder reporting and data system combined with color doppler flow imaging for gallbladder cancer in the Asian population. Front. Oncol. 14:1367351. doi: 10.3389/fonc.2024.1367351
Received: 18 January 2024; Accepted: 27 March 2024;
Published: 15 April 2024.
Edited by:
Pankaj Gupta, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Raghuraman Soundararajan, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaPratyaksha Rana, U.N. Mehta Institute of Cardiology and Research Centre, India
Copyright © 2024 Wang, Lv and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Li, ll028007@qlyyqd.com